Abstract
Coatomer protein I (COPI) is well known as the protein coat surrounding vesicles involved in returning endoplasmic reticulum (ER)-resident proteins to the ER. COPI coats are also found in vesicles involved in other trafficking processes including endocytosis, autophagy and anterograde transport in the secretory pathway. In view of the diverse functions of COPI proteins, it is expected that they will affect virus replication, and many reports of such COPI involvement have now appeared. The experimental approaches most often employ specific siRNA to deplete COPI subunits or brefeldin A to block COPI activation. Here we briefly describe the results obtained with viruses in which COPI is found to have a role in replication. The results demonstrate that COPI affects viruses quite differently with effects observed in processes such as entry, RNA replication, and intracellular transport of viral proteins.
Keywords: Coatomer, COPI, virus replication, Golgi, Arf1
COPI function and structure
Cytoplasmic vesicles containing a COPI coat are best known for their involvement in retrograde transport of cargo from the Golgi apparatus to the endoplasmic reticulum (ER). Resident ER proteins able to escape to the Golgi during anterograde transport of membrane glycoproteins are returned to the ER by way of COPI-coated vesicles (illustrated in Fig. 1). This sorting step contributes to the ability of the ER and Golgi to maintain their distinct identities. Cell biological studies have demonstrated that COPI function is not limited to Golgi-to-ER transport; several other functions have been defined (Fig. 1). For example, COPI has some functions required for anterograde transport at the Golgi [1,2]. COPI subunits have been identified in endosomal compartments suggesting they may have a role in endocytosis or in the maintenance of endocytic compartments [3–6]. Coatomer’s functions in early endosomes are required for the maturation of autophagic vacuoles [7]. COPI also plays a role in expression of cell surface receptors, as well as in modulation of the plasma membrane lipid composition. For example, depletion of COPI alters the distribution of cholesterol, the sphingolipid GM1 and the Rho GTPases Cdc42 and Rac1 at the plasma membrane [8]. The diverse roles of coatomer provide opportunities for viruses to exploit this important protein complex.
Figure 1.
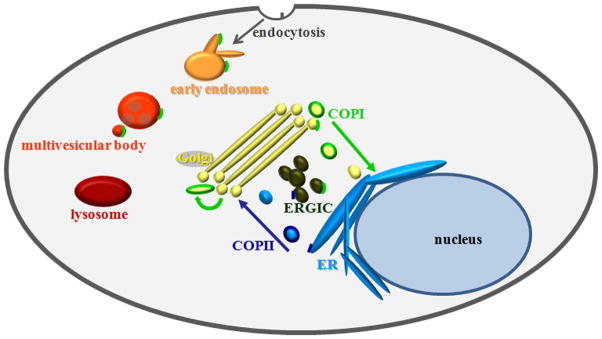
Drawing of the known functional locations of coatomer. COPI, indicated in bright green, is present at many locations including the ERGIC, Golgi, early endosomes, and multi-vesicular bodies. Note that these locations correspond to the many functions of COPI in a typical cell including facilitating retrograde transport from the Golgi to the ERGIC and ER, anterograde transport among Golgi stacks, and transport among compartments in the endosomal pathway.
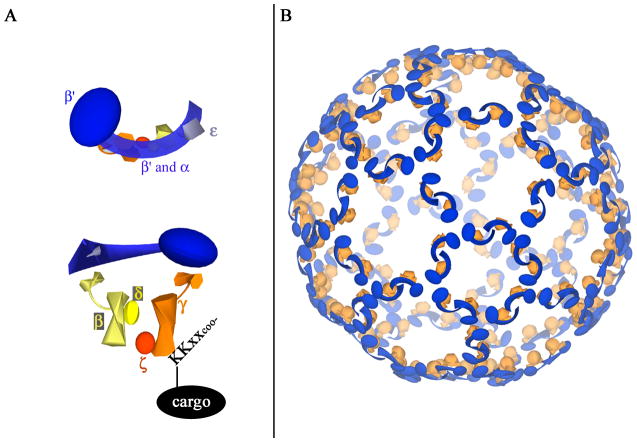
The COPI complex is composed of seven subunits (α, β, β′, γ, δ, ε, and ζ) that form a cage-like structure expected to be similar to the clathrin coat [9–12]. Like the clathrin coat, the COPI coat consists of subcomplexes linked with three-fold symmetry and organized into rings with six-fold and five-fold symmetry (Fig. 2). The αβ′ε subcomplex forms the backbone of the COPI cage and associates at the vertices to form a triskelion structure [11]. The remaining subunits are located beneath the αβ′ε backbone, close to the lipid membrane, where they interact with vesicle cargo [13–17]. COPI can mediate the formation of both vesicles and tubular structures, with vesicles formed primarily for retrograde transport and tubules for anterograde transport among Golgi cisternae [2].
Figure 2.
Illustration of COPI subcomplexes (A) and a potential structure of the COPI cage around a spherical lipid vesicle (B). (A) As revealed by X-ray crystallography and electron microscopy, the backbone of the COPI cage is formed by the αβ′ε subcomplex (top) [11,16], which is on the cytoplasmic face of the vesicle. The βδγζ subcomplex structure (bottom) is similar to that of the adaptor protein complex, which is important for formation of clathrin-coated vesicles. [12,14,15,17]. The δ subunit interacts with the KKXX domain on ER resident cargo proteins [13]. (B) In this model of the COPI lattice, the αβ′ε and βδγζ subcomplexes assemble in a manner similar to clathrin and adaptor protein complexes, respectively. The βδγζ subcomplex is located between the αβ′ε backbone and the lipid vesicle; it interacts with cargo in the vesicle membrane. Note that this is only one possible structure of a COPI cage; COPI coats can form tubular structures and vesicles of varying sizes. This structure is based on work by Lee and Goldberg [11] and Yip and Walz [16] and was created using Google SketchUp8.
For retrograde transport, vesicle formation begins when lumenal proteins associate with the Golgi membrane. First, soluble proteins containing the ER localization signal KDEL interact with the membrane bound KDEL receptor (KDELR) [18]. Arf-GEF then associates with the KDELR activating Arf1-GDP. Arf1-GTP now associates with the Golgi membrane and recruits COPI, which in turn recruits Arf-GAP [10, 19–21]. For cargo sorting, KKXX domains on the cytoplasmic tails of membrane proteins associate with the gamma subunit of COPI [13]. Then COPI forms a cage around the membrane, inducing membrane curvature and forming a vesicle. Shortly after vesicle budding, the COPI coat is lost. Arf1-GTP is hydrolyzed by Arf-GAP, which releases Arf1-GDP, Arf-GAP, and COPI from the membrane [22]. For a diagram of COPI assembly see Fig. 2 of Nickel et al [23]. Finally, the uncoated vesicle completes its journey to the ER, where it fuses with the membrane and unloads its cargo. The formation of COPI coated vesicles is inhibited by brefeldin A (BFA), a small molecule that prevents Arf1 activation [24].
COPI and virus replication
In view of the several functions of COPI in uninfected cells, it is reasonable to expect COPI would be involved in virus replication. Recent studies have shown this is the case. Most experiments use siRNA to deplete cells of COPI subunits or BFA to block Arf1activation. Depleted or inhibited cells are then tested for their ability to support virus replication. For instance, siRNA depletion studies have documented the involvement of COPI in replication of vaccinia, drosophila C, polio and influenza viruses [25–27]. Further research has indicated that COPI has a variety of roles in the lifecycles of different viruses including involvement in virus entry, RNA replication, and intracellular transport.
In the remainder of this review, we describe studies that have documented involvement of COPI subunits in specific aspects of virus growth including the processes mentioned above. The studies reviewed were published prior to June 2012. The results are summarized in Table I. For comparison, a description has been included of the role of COPI in Salmonella typhimurium infection. Overlapping material has recently been covered by Yang and Zhang [28].
Table 1.
Viruses shown to require COPI for infection.
| Virus | Process | Evidence |
|---|---|---|
| HPV16 | Entry – COPI | BFA [34] |
| Entry – Caveolin-1 | sh caveolin-1; co-localization IF [34] | |
| Influenza | Entry | si α-, β′-, γ-, δ-COP [27] |
| VSV | Entry | Ts eCOPI [29, 3] si α-, β-, β′-, γ-, δ-, ζ-COP [29] |
| Semiliki Forest Virus | Entry | Ts eCOPI [3,56] |
| SV40 | Entry | BFA; Arf1 and Sar1 mutants; neutralizing antibodies against βCOP [32] |
| Entry | BFA [33] | |
| Drosophila C | Virus replication | si α-, β-, β′-, γ-, δ-, ζ-COP [26] |
| Polio | Virus replication | si α-COP [26] |
| RNA replication | BFA, microscopy [37] | |
| Echovirus 11 | Virus replication | BFA [36] |
| RNA replication | Microscopy [36] | |
| Coxsackie B3 | RNA replication | si GBF1 [57] |
| Potyvirus | RNA replication | Dom. Neg. Arf1 [58] |
| Vaccinia | Virus replication | si β-COP and Ts ε-COP [25] |
| Hepatitis C | Vesicle organization | si α-, β-, β′-, γ-, δ-, ζ-COP [38] |
| Early lifecycle | BFA [38] | |
| RNA replication | si Arf1 and si GBF1 [39, 41] | |
| Virus replication | si Arf1 [39] | |
| Ebola | VP40 VLP formation | Dom. Neg. Arf1, GBF1 [43,42] |
| SARS | Virus budding at ERGIC | In vitro binding [45] |
| HIV-1 | CD4 down regulation | GST pull down [53] |
COPI and virus entry
In several viruses, entry into the host cell has been found to involve COPI. Entry sometimes involves endosomes, where COPI is known to play a role in uninfected cells. This may account for the high proportion of viruses using COPI for entry. For example, four COPI subunits were identified in an siRNA screen for host factors important for influenza virus replication [27]. In order to identify the step(s) in the influenza lifecycle for which COPI is important, cells were treated with siRNA specific for δ-COP (si δ-COP). Cells were then infected with influenza virus-like particles (VLPs) containing reporter proteins. Results showed there was a two thirds reduction in the percentage of reporter positive cells from the si δ-COP sample, suggesting COPI is important for influenza virus entry [27].
Vesicular stomatitis (VSV) and Semliki Forest viruses showed reduced infectivity in cells deficient in ε-COP [3]. In order to further define the role of COPI, cells were treated with siRNA specific for coatomer subunits, and infected with VSV encoding GFP. siRNA treatment was found to cause a 50–90% reduction in the number of cells expressing GFP, without generalized cytotoxic effects [29]. Fluorescence microscopy of VSV infected cells lacking εCOP showed a reduction in the number of parental virions attached to cells as well as a lower percentage of internalized virions. The results suggest COPI is required early in the VSV lifecycle, steps that include virus attachment and entry. Since VSV entry is clathrin-dependent, Cureton et al [29] posit that coatomer knockdown causes a block in entry indirectly, perhaps by altering receptor expression at the cell surface.
Previous studies identified other COPI-dependent steps in the VSV lifecycle. For example, BFA treatment of VSV infected cells resulted in a two thirds reduction of viral RNA synthesis [30]. The same treatment also led to a decrease in translation of virus mRNA’s, but only if BFA was administered early in infection. The results indicate COPI is not directly involved in viral translation, but is important for RNA synthesis. In addition, VSV-G protein glycosylation was inhibited by BFA [30]. This was suggested to result from failure of the Golgi secretory system to process glycoproteins, an effect known to follow COPI depletion [31]. The importance and versatility of COPI are highlighted by its functions at several steps in the VSV lifecycle.
A role for coatomer in simian virus 40 (SV40) entry has also been demonstrated. Microscopic analysis of infected, BFA-treated cells showed an accumulation of parental virions at the cell periphery instead of in the cytoplasm [32]. The percentage of infected cells was greatly reduced when neutralizing antibodies specific for β-COP were introduced before or after SV40 exposure. The results indicate COPI is important for entry as well as for later steps in SV40 replication [32].
Time-course experiments were carried out to further define the steps at which COPI is involved in SV40 infection. At 3–5 hours post infection (hpi) the SV40 capsid protein VP-1 was found to co-localize with β-COP and caveolin-1 [33]. By 10 hpi VP-1 co-localized with the ER marker PD-1. In contrast, when cells were treated with BFA, VP-1 did not co-localize with PD-1 at 10 hpi, but was found adjacent to the ER. The results suggest that after endocytosis, SV40 is trafficked to an intermediate compartment containing β-COP, and coatomer is required for its subsequent transport to the ER [33].
Similar time-course experiments were also performed with human papilloma virus 16 (HVP16). HPV16 was found to co-localize with the early endosome marker EEA1 shortly after clathrin-mediated endocytosis [34]. Twenty minutes to 2 hpi HPV16 also co-localized with caveolin-1. Later, at four hpi HPV16 co-localized with the ER markers Erp29 and calnexin. This progression suggests that HPV16 traffics similarly to SV40, from early endosomes to caveosomes to the ER. Treatment with BFA blocked virus transport to the ER and led to the accumulation of virions in caveosomes, indicating that COPI is important for virion transport from caveosomes to the ER [34].
Recent work by the group that first identified the caveosome has questioned its existence [35]. The results suggest the compartment that had been known as the caveosome is actually a modified late endosome/lysosome. Regardless of whether or not the caveosome exists, the results are clear that both SV40 and HPV16 are trafficked through an intermediate compartment in route to the ER, and that this trafficking requires COPI.
COPI and virus RNA replication
Many RNA viruses form membranous replication compartments in the cytoplasm. Picornaviruses, for example, carry out RNA synthesis on membrane-bound structures containing the virus-encoded RNA-dependent RNA polymerase. COPI has been shown to be involved in the formation of such structures. For instance, BFA treatment of cells infected with echo virus 11 (EV11) resulted in inhibition of virus replication and RNA synthesis. β-COP co-localized with sites of EV11 RNA synthesis early in infection, providing further evidence that COPI is important for formation of replication complexes [36].
Five of the seven COPI subunits were identified in an siRNA screen for host cell components required for replication of Drosophila C virus (DCV), a picorna-like virus [26]. In contrast, depletion of COPII did not affect DCV replication. However, depletion of β-COP inhibited formation of DCV replication compartments. Similar experiments with polio virus showed the same result, with COPI, but not COPII, required for virus replication [26]. Arf1 co-localized with polio virus RNA replication centers, further suggesting a role for COPI in the formation of these structures [37].
An siRNA screen for cellular factors required for hepatitis C virus (HCV) replication identified six coatomer subunits [38]. This intriguing observation was followed by a study in which the inhibitory effects of BFA were measured as a function of time after infection. The results showed an inhibitory effect of BFA up to 8 hpi, suggesting a role for COPI early in HCV infection [38]. In a similar study, BFA treatment of HCV-infected cells was found to cause decreased RNA production, but no decrease in viral protein synthesis [39]. BFA treatment also resulted in the mis-localization of HCV nonstructural protein 5A, a component of the replication complex [40]. Depletion of Arf1 and GBF1 by siRNA greatly reduced HCV replication and RNA synthesis [40, 41]. The results suggest Arf1 and/or COPI are important for the formation or maintenance of HCV replication complexes.
Coatomer and intracellular transport
Roles for both COPI and COPII in Ebola virus infection have been suggested by the results of experiments in which cells were transfected with a gene encoding the Ebola matrix protein VP40 [42,43]. Transfected cells extend thin, cylindrical protrusions containing VP40 and release VLPs resembling the mature virus. Two observations support a link involving VP40, coatomer proteins, and VLP formation. First, VP40 expressed as a result of transfection is found to interact with Sec24C, a component of the COPII coat. Depletion of Sec24C by siRNA resulted in reduced VLP release, suggesting a role for Sec24C in VLP formation [42]. The second observation has to do with Rab1b, a small GTPase that activates GBF1. GBF1 is an Arf-GEF that regulates Arf1 activity. It was observed that a dominant negative Rab1b antagonized VP40-induced VLP release, supporting the idea that COPI is also involved in VLP formation [43].
Most viruses requiring COPI for replication appear to require it for entry, transport, RNA replication, or membrane morphogenesis. However, coronaviruses and human imunodeficiency virus type 1 (HIV-1) use COPI in a unique way; dibasic motifs in viral proteins bind to coatomer. For instance, the cytoplasmic tail of the spike (S) protein of the coronavirus infectious bronchitis virus (IBV) contains a KKXX motif that functions as a targeting signal, directing S protein to the endoplasmic reticulum-Golgi intermediate complex (ERGIC) near sites of virus replication [44]. A similar dibasic motif, KXHXX, was identified on the cytoplasmic tail of severe acute respiratory syndrome-coronavirus (SARS-CoV) S protein and also localizes S to the ERGIC [44]. GST pull down assays showed that SARS-CoV S protein binds to COPI and this binding requires the KXHXX motif [45]. It is suggested, therefore, that COPI is involved in directing S proteins to the ERGIC.
HIV-1 Nef is important for the downregulation of several cell surface proteins including CD4, CD8, CD28, and major histocompatibility complex class 1 (MHC-1), [46, 47, 48]. These proteins are targeted to endosomal compartments upon interacting with Nef. Nef binds to MHC1via the adaptor protein AP-1, and this interaction requires Arf1 activation [49]. Nef binds to the cytoplasmic tail of CD4 to mediate its downregulation [50]. This process requires binding of the cytoplasmic tail of Nef with β-COP [41]. Nef contains two binding sites for β-COP [51,52]. Recent studies suggest that β-COP is important for the transport of CD4, CD8, and MHC1 into lysosomes for degradation [48,52,53].
COPI and membrane lipid composition
COPI is important for the attachment, entry, and RNA replication of VSV, as described above. In VSV-infected cells depleted of γ-COP, replenishment of both GM1 and cholesterol partially rescued the infection rate [8]. While COPI depletion may affect virus entry due endocytic functions, the above results suggest it may also be important for entry by maintaining the plasma membrane lipid composition and the availability of receptors.
COPI and bacterial invasion
Viruses are not the only microbes to require COPI for infection. An siRNA screen for host factors important for invasion of Salmonella typhimurium identified five COPI subunits [8]. To initiate infection, S. typhimurium first binds to a host cell and then injects effectors via a type III secretion system. Effector injection stimulates actin rearrangements in host cells, resulting in the visually stunning phenomenon of membrane ruffling [54, 55]. Treatment of host cells with si β or γ-COP did not affect S. typhimurium attachment, but effector injection was greatly impaired. COPI subunit depletion also resulted in reduced invasion and decreased membrane ruffling [8]. Membrane lipid composition is known to be important for distribution of cell surface receptors. In COPI-depleted cells cholesterol, GM1, Cdc42 and Rac1 were mis-localized. This is noteworthy because Cdc42 and Rac1 are important for membrane ruffling. Cholesterol replenishment partially rescued membrane ruffling [8]. The results suggest maintenance of the plasma membrane composition by COPI is important for S. typhimurium invasion.
Conclusion
Several screens for cellular factors important for virus replication identified COPI subunits. It is intriguing that these screens identify COPI so frequently as opposed to COPII, because both are important for the function and maintenance of the secretory pathway. If viruses required COPI simply to facilitate the production of viral proteins, then COPII should also be required. However, COPI is specifically required for replication by a wide variety of viruses. This suggests the functions of COPI other than retrograde transport are important for infection by several viruses and at least one bacterium. For example, coatomer’s roles in the endocytic pathway may be important for virus entry and its transport functions are used for the localization of coronavirus S proteins. The membrane morphogenesis capabilities of COPI are important for bacteria and virus entry, as well as required for the formation of RNA replication centers. COPI is a versatile complex with several important functions in uninfected cells, and these have been exploited for virus replication.
Acknowledgments
We thank William Newcomb for productive discussions about coatomer function. This work was supported by NIH award R01 AI41644.
Abbreviations
- COPI
Coatomer protein I
- ER
endoplasmic reticulum
- VLP
virus-like particle
- BFA
brefeldin A
- hpi
hours post infection
References
- 1.Orci L, Ravazzola M, Volchuk A, Engel T, Gmachl M, et al. Anterograde flow of cargo across the Golgi stack potentially mediated via bidirectional “percolating” COPI vesicles. Proc Natl Acad Sci. 2002;97:10400–10405. doi: 10.1073/pnas.190292497. http://www.ncbi.nlm.nih.gov/pubmed/10962035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JS, Valente C, Polishchuk RS, Turacchio G, Layre E, et al. COPI acts in both vesicular and tubular transport. Nat Cell Biol. 2011;13:996–1003. doi: 10.1038/ncb2273. http://www.ncbi.nlm.nih.gov/pubmed/21725317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daro E, Sheff D, Gomez M, Kreis T, Mellmanm I. Inhibition of endosome function in CHO cells bearing a temperature-sensitive defect in the coatomer (COPI) component ε-COP. J Cell Biol. 1997;139:1747–1759. doi: 10.1083/jcb.139.7.1747. http://www.ncbi.nlm.nih.gov/pubmed/9412469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabriely G, Kama R, Gerst JE. Involvement of specific COPI subunits in protein sorting from the late endosome to the vacuole in yeast. Mol Cell Biol. 2007;27:526–540. doi: 10.1128/MCB.00577-06. http://www.ncbi.nlm.nih.gov/pubmed/17101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu F, Aniento F, Parton RG, Gruenburg J. Functional dissection of COP-I subunits in the biogenesis of multivesicular endosomes. J Cell Biol. 1997;139:1183–1195. doi: 10.1083/jcb.139.5.1183. http://www.ncbi.nlm.nih.gov/pubmed/9382865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Styers ML, O’Connor AK, Grabski R, Cormet-Boyaka E, Sztul E. Depletion of beta-COP reveals a role for COP-I in compartmentalization of secretory compartments and in biosynthetic transport of caveolin-1. Am J Physiol Cell Physiol. 2008;294:1485–1498. doi: 10.1152/ajpcell.00010.2008. http://www.ncbi.nlm.nih.gov/pubmed/18385291. [DOI] [PubMed] [Google Scholar]
- 7.Razi M, Chan EYW, Tooze Sharon A. Early endosomes and endosomal coatomer are required for autophagy. J Cell Biol. 2009;185:305–321. doi: 10.1083/jcb.200810098. http://www.ncbi.nlm.nih.gov/pubmed/19364919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Misselwitz B, Dilling S, Vonaesch P, Sacher R, Snijder B, et al. RNAi screen of Salmonella invasion shows role of COPI in membrane targeting of cholesterol and CDC42. Mol Sys Biol. 2011;7:474. doi: 10.1038/msb.2011.7. http://www.ncbi.nlm.nih.gov/pubmed/21407211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehm M, Bonifacino JS. Adaptins: the final recount. Mol Biol Cell. 2001;12:2907–2920. doi: 10.1091/mbc.12.10.2907. http://www.ncbi.nlm.nih.gov/pubmed/11598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eugster A, Frigerio G, Dale M, Duden R. COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 2000;19:3905–3917. doi: 10.1093/emboj/19.15.3905. http://www.ncbi.nlm.nih.gov/pubmed/10921873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee C, Goldberg J. Structure of coatomer cage proteins and the relationship among COPI, COPII, and clathrin vesicle coats. Cell. 2010;142:123–132. doi: 10.1016/j.cell.2010.05.030. http://www.ncbi.nlm.nih.gov/pubmed/20579721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schledzewski K, Brinkmann H, Mendell RR. Phylogenetic analysis of components of the eukaryotic vesicle transport system reveals a common origin of adaptor protein complexes 1, 2, and 3 and the F subcomplex of the coatomer COPI. J Mol Evol. 1999;48:770–778. doi: 10.1007/pl00006521. http://www.ncbi.nlm.nih.gov/pubmed/10229581. [DOI] [PubMed] [Google Scholar]
- 13.Harter C, Pavel J, Coccia F, Draken E, Wegehingel S, et al. Nonclathrin coat protein gamma, a subunit of coatomer, binds to the cytoplasmic dilysine motif of membrane proteins of the early secretory pathway. Proc Natl Acad Sci. 1996;93:1902–1906. doi: 10.1073/pnas.93.5.1902. http://www.ncbi.nlm.nih.gov/pubmed/8700856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman GR, Rahl PB, Collins RN, Cerione RA. Conserved structural motifs in intracellular trafficking pathways: structure of the γCOP appendage domain. Mol Cell. 2003;12:615–625. doi: 10.1016/j.molcel.2003.08.002. http://www.ncbi.nlm.nih.gov/pubmed/14527408. [DOI] [PubMed] [Google Scholar]
- 15.Takatsu H, Futatsumori M, Yoshino K, Yoshida Y, Shin HW, Nakayama K. Similar subunit interactions contribute to assembly of clathrin adaptor complexes and COPI complex: analysis using yeast three hybrid system. Biochem Biophys Res Comm. 2001;284:1083–1089. doi: 10.1006/bbrc.2001.5081. http://www.ncbi.nlm.nih.gov/pubmed/11409905. [DOI] [PubMed] [Google Scholar]
- 16.Yip CK, Walz T. Molecular structure and flexibility of the yeast coatomer as revealed by electron microscopy. J Mol Biol. 2011;408:825–831. doi: 10.1016/j.jmb.2011.03.029. http://www.ncbi.nlm.nih.gov/pubmed/21435344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu W, Lin J, Jin C, Xia B. Solution structure of human ζ-COP: direct evidences for structural similarity between COP I and clathrin-adaptor coats. J Mol Biol. 2009;386:903–912. doi: 10.1016/j.jmb.2008.12.083. http://www.ncbi.nlm.nih.gov/pubmed/19167404. [DOI] [PubMed] [Google Scholar]
- 18.Lewis MJ, Pelham HRB. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell. 1992;68:353–364. doi: 10.1016/0092-8674(92)90476-s. http://www.ncbi.nlm.nih.gov/pubmed/1310258. [DOI] [PubMed] [Google Scholar]
- 19.Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-Ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein beta-COP to Golgi membranes. Proc Natl Acad Sci. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. http://www.ncbi.nlm.nih.gov/pubmed/1631136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cukierman E, Huber I, Rotman M, Cassel D. The Arf1 GTPase-activating protein: zinc finger motif and Golgi complex location. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. http://www.ncbi.nlm.nih.gov/pubmed/8533093. [DOI] [PubMed] [Google Scholar]
- 21.Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, et al. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;76:239–253. doi: 10.1016/0092-8674(91)90176-y. http://www.ncbi.nlm.nih.gov/pubmed/1680566. [DOI] [PubMed] [Google Scholar]
- 22.Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, et al. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. http://www.ncbi.nlm.nih.gov/pubmed/8253837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nickel W, Brugger B, Wielan FT. Vesicular transport: the core machinery of COPI recruitment and budding. J Cell Sci. 2002;115:3235–3240. doi: 10.1242/jcs.115.16.3235. http://www.ncbi.nlm.nih.gov/pubmed/12140255. [DOI] [PubMed] [Google Scholar]
- 24.Peyroche A, Antonny B, Robineau S, Acker J, Cherfils J, et al. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell. 1999;3:275–285. doi: 10.1016/s1097-2765(00)80455-4. http://www.ncbi.nlm.nih.gov/pubmed/10198630. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Lee SY, Beznoussenko GV, Peters PJ, Yang JS, et al. A role for the host coatomer and KDEL receptor in early vaccinia biogenesis. Proc Natl Acad Sci. 2009;106:163–168. doi: 10.1073/pnas.0811631106. http://www.ncbi.nlm.nih.gov/pubmed/19109439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherry S, Kunte A, Wang H, Coyne C, Rawson RB, et al. COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLOS Pathogens. 2006;2:900–912. doi: 10.1371/journal.ppat.0020102. http://www.ncbi.nlm.nih.gov/pubmed/17040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konig R, Stertz S, Zhou Y, Inoue A, Hoffman HH, et al. Human host factors required for Influenza virus replication. Nature. 2010;463:813–817. doi: 10.1038/nature08699. http://www.ncbi.nlm.nih.gov/pubmed/20027183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang GB, Zhang LL. Roles of COPI related proteins during virus replication. Bing Du Xue Bao. 2012;28:185–189. http://www.ncbi.nlm.nih.gov/pubmed/22519182. [PubMed] [Google Scholar]
- 29.Cureton DK, Burdeinick-Kerr R, Whelan SPJ. Genetic inactivation of COPI coatomer separately inhibits Vesicular Stomatitis Virus entry and gene expression. J Virol. 2012;86:655–666. doi: 10.1128/JVI.05810-11. http://www.ncbi.nlm.nih.gov/pubmed/22072764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irurzun A, Perez L, Carrasco L. Brefeldin A blocks protein glycosylation and RNA replication of vesicular stomatitis virus. FEBS Lett. 1993;336:496–500. doi: 10.1016/0014-5793(93)80863-p. http://www.ncbi.nlm.nih.gov/pubmed/8282118. [DOI] [PubMed] [Google Scholar]
- 31.Pringle JR, Broach JR, Jones EW. The molecular and cellular biology of the yeast saccharomyces: cell cycle and cell biology. Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- 32.Richards AA, Stang E, Pepperkok R, Parton RG. Inhibitors of COP-mediated transport and cholera toxin action inhibit simian virus 40 infection. Mol Biol Cell. 2002;13:1750–1764. doi: 10.1091/mbc.01-12-0592. http://www.ncbi.nlm.nih.gov/pubmed/12006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A. Caveolar endocytosis of Simian Virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J Virol. 2002;76:5156–5166. doi: 10.1128/JVI.76.10.5156-5166.2002. http://www.ncbi.nlm.nih.gov/pubmed/11967331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laniosz V, Dabydeen SA, Havens MA, Meneses PI. Human papillomavirus type 16 infection of human keratinocytes requires clathrin and caveolin-1 and is brefeldin A sensitive. J Virol. 2009;83:8221–8232. doi: 10.1128/JVI.00576-09. http://www.ncbi.nlm.nih.gov/pubmed/19494002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayer A, Stoeber M, Ritz D, Engel S, Meyer HH, et al. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J Cell Biol. 2010;191:615–629. doi: 10.1083/jcb.201003086. http://www.ncbi.nlm.nih.gov/pubmed/21041450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gazina EV, Mackenzie JM, Gorrell RJ, Anderson DA. Differential requirements for COPI coats in formation of replication complexes among three genera of Picornaviridae. J Virol. 2002;76:11113–11122. doi: 10.1128/JVI.76.21.11113-11122.2002. http://www.ncbi.nlm.nih.gov/pubmed/12368353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belov GA, Fogg MH, Ehrenfeld E. Poliovirus proteins induce membrane association of GTPase ADP-ribosylation factor. J Virol. 2005;79:7207–7216. doi: 10.1128/JVI.79.11.7207-7216.2005. http://www.ncbi.nlm.nih.gov/pubmed/15890959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, et al. A functional genomic screen identifies cellular cofactors of Hepatitis C Virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. http://www.ncbi.nlm.nih.gov/pubmed/19286138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matto M, Sklan EH, David N, Melamed-Book N, Casanova JE, et al. Role for ADP ribosylation factor 1 in the regulation of Hepatitis C Virus replication. J Virol. 2011;85:946–956. doi: 10.1128/JVI.00753-10. http://www.ncbi.nlm.nih.gov/pubmed/21068255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gosert R, Egger D, Lohmann V, Bartenschlager R, Blum HE, et al. Identification of the Hepatitis C Virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J Virol. 2003;77:5487–5492. doi: 10.1128/JVI.77.9.5487-5492.2003. http://www.ncbi.nlm.nih.gov/pubmed/12692249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L, Hong Z, Lin W, Shao RX, Goto K, et al. ARF1 and GBF1 generate a P14P-enriched environment supportive of hepatitis C virus replication. PloS One. 2012;7:e32135. doi: 10.1371/journal.pone.0032135. http://www.ncbi.nlm.nih.gov/pubmed/22359663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamayoshi S, Noda T, Ebihara H, Goto H, Morikawa Y, et al. Ebola virus matrix VP40 protein uses the COPII transport system for its intracellular transport. Cell Host Microbe. 2008;3:168–177. doi: 10.1016/j.chom.2008.02.001. http://www.ncbi.nlm.nih.gov/pubmed/18329616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamayoshi S, Neumann G, Kawaoka Y. Role of the GTPase Rab1b in Ebolavirus particle formation. J Virol. 2010;84:4816–4820. doi: 10.1128/JVI.00010-10. http://www.ncbi.nlm.nih.gov/pubmed/20164217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lontok E, Corse E, Machamer CE. Intracellular targeting signals contribute to localization of coronavirus spike proteins near the virus assembly site. J Virol. 2004;78:5913–5922. doi: 10.1128/JVI.78.11.5913-5922.2004. http://www.ncbi.nlm.nih.gov/pubmed/15140989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McBride CE, Li J, Machamer CE. The cytoplasmic tail of the Severe Acute Respiratory Syndrome Coronavirus spike protein contains a novel endoplasmic reticulum retrieval signal that binds COPI and promotes interaction with membrane protein. J Virol. 2007;81:2418–2428. doi: 10.1128/JVI.02146-06. http://www.ncbi.nlm.nih.gov/pubmed/17166901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia J, Miller A. Serine phosphorylation-independent downregulation of cell-surface CD4 by Nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. http://www.ncbi.nlm.nih.gov/pubmed/2014052. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz O, Marechal V, LeGall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. http://www.ncbi.nlm.nih.gov/pubmed/8612235. [DOI] [PubMed] [Google Scholar]
- 48.Leonard JA, Filzen T, Carter CC, Schaefer M, Collins KL. HIV-1 Nef disrupts intracellular trafficking of major histocompatibility complex class I, CD4, CD8, and CD28 by distinct pathways that share common elements. J Virol. 2011;85:6867–6887. doi: 10.1128/JVI.00229-11. http://www.ncbi.nlm.nih.gov/pubmed/21543478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wonderlich ER, Leonard JA, Kulpa DA, Leopold KE, Norman JM, et al. ADP ribosylation factor 1 activity is required to recruit AP-1 to the major histocompatibility complex class I (MHC-1) cytoplasmic tail and disrupt MHC-1 trafficking in HIV-1-infected primary T cells. J Virol. 2011;85:12216–12226. doi: 10.1128/JVI.00056-11. http://www.ncbi.nlm.nih.gov/pubmed/21917951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. http://www.ncbi.nlm.nih.gov/pubmed/8124721. [DOI] [PubMed] [Google Scholar]
- 51.Aiken C, Krause L, Chen YL, Trono D. Mutational analysis of HIV-1 Nef: Identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology. 1996;217:293–300. doi: 10.1006/viro.1996.0116. http://www.ncbi.nlm.nih.gov/pubmed/8599214. [DOI] [PubMed] [Google Scholar]
- 52.Schaefer MR, Wonderlich ER, Roeth JF, Leonard JA, Collins KL. HIV-1 Nef targets MHC-1 and CD4 for degradation via a final common beta-COP-dependent pathway in T cells. PloS Pathog. 2008;4:e1000131. doi: 10.1371/journal.ppat.1000131. http://www.ncbi.nlm.nih.gov/pubmed/18725938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, et al. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97:63–73. doi: 10.1016/s0092-8674(00)80715-1. http://www.ncbi.nlm.nih.gov/pubmed/10199403. [DOI] [PubMed] [Google Scholar]
- 54.Francis CL, Ryan TA, Jones BD, Smith SJ, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. http://www.ncbi.nlm.nih.gov/pubmed/8350922. [DOI] [PubMed] [Google Scholar]
- 55.Finlay BB, Ruschkowski S. Cytoskeletal rearrangements accompanying Salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. http://www.ncbi.nlm.nih.gov/pubmed/1909337. [DOI] [PubMed] [Google Scholar]
- 56.Vonderheit A, Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of Semliki Forest Virus to late endosomes. PLoS Biol. 2005;3:1225–1238. doi: 10.1371/journal.pbio.0030233. http://www.ncbi.nlm.nih.gov/pubmed/15954801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanke KHW, van der Shaar HM, Belov GA, Feng Q, Duijsings D, et al. GBF1, a guanine nucleotide exchange factor for Arf, is crucial for Coxsackievirus B3 RNA replication. J Virol. 2009;83:11940–11949. doi: 10.1128/JVI.01244-09. http://www.ncbi.nlm.nih.gov/pubmed/19740986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei T, Wang A. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J Virol. 2008;82:12252–12264. doi: 10.1128/JVI.01329-08. http://www.ncbi.nlm.nih.gov/pubmed/18842721. [DOI] [PMC free article] [PubMed] [Google Scholar]