Abstract
During natural infection with the agent of Lyme disease, Borrelia burgdorferi, spirochetes are delivered with vector saliva, which contains anti-inflammatory and antihemostatic activities. We show here that the saliva of ixodid ticks reduces polymorphonuclear leukocyte (PMN) adhesion via downregulation of β2-integrins and decreases the efficiency of PMN in the uptake and killing of spirochetes. Inhibition of integrin adhesion and signaling reduces anti-inflammatory functions of PMN. These effects may favor the initial survival of spirochetes in vivo.
Lyme disease is caused by infection with the spirochete Borrelia burgdorferi and is characterized by the skin lesion erythema migrans, which may be followed by carditis, neurological symptoms, and arthritis (12). In a natural infection, spirochetes are delivered via an ixodid tick vector in the presence of saliva and are contained in a cement plug at the inoculation site (1). Arthropod saliva contains potent anti-inflammatory and antihemostatic activities and promotes a higher spirochete burden in the host (7, 21, 29).
Polymorphonuclear leukocytes (PMN) are the initial responding cells after a tick bite (2). They are effective in eliminating spirochetes by a variety of pathways but are more efficient in the presence of opsonizing antisera (10, 11, 17). The saliva of Ixodes ticks is known to inhibit in some way both phagocytosis and superoxide production by PMN in vitro (24). Ixodes tick saliva also inhibits T-cell proliferation (27), perhaps due to an interleukin-2 binding protein (6); inhibits complement lysis of erythrocytes (28); and reduces the production of cytokines and nitric oxide (NO) and the killing of spirochetes by macrophages (9, 20, 27). We undertook the present study to determine whether saliva inhibits PMN chemotaxis and adherence, basic functions that are likely to be important for the clearance of spirochetes.
MATERIALS AND METHODS
Cell isolation and imaging.
PMN were isolated from the heparinized blood of healthy volunteers by 3% dextran sedimentation, and red blood cells were removed by hypotonic lysis (10). Informed consent was obtained from all blood donors in accordance with the guidelines of the Human Investigations Committee of Yale University. For immunofluorescent staining, PMN were plated at a concentration of 5 × 105 cells per 12-mm round glass coverslip in Krebs Ringer phosphate buffer with 5.4 mM glucose (KRPG) containing 10% heat-inactivated pooled human serum. Adherent PMN samples were incubated with spirochetes at a 100:1 ratio in KRPG containing 10% heat-inactivated pooled normal human serum as described previously (17). At times up to 60 min, samples were fixed in 4% paraformaldehyde and blocked in phosphate-buffered saline (PBS) containing 10% goat serum and 0.01% saponin. PMN were labeled with primary antibodies directed against the PMN azurophilic granule component myeloperoxidase (DAKO Corp., Carpinteria, Calif.). Rabbit polyclonal anti-B. burgdorferi serum, a kind gift from Fred Kantor, Yale University School of Medicine, was used to label spirochetes (18). Antibodies were detected with appropriate fluorescein isothiocyanate- or tetramethyl rhodamine isocyanate-conjugated secondary antibodies (Tago Immunologicals, Biosource Intl., Camarillo, Calif.), and samples were imaged by using multitracking with a Zeiss LSM 510 scanning laser confocal microscope equipped with an argon-krypton laser (17).
For measurement of cell area, freshly isolated PMN in PBS containing Ca2+, Mg2+, and 0.1% bovine serum albumin (BSA) were plated at a concentration of 1 × 105 cells per glass coverslip and incubated for 1 h at 37°C to allow adherence. Adherent cells were then incubated for an additional 1 h in a humidified chamber either alone or in dilutions of saliva (1:10, 1:20, and 1:100). Cells were fixed in 4% paraformaldehyde in PBS, mounted in Mowiol, and examined with a Zeiss Axiovert 200 M microscope (Carl Zeiss Microimaging, Inc., Thornwood, N.Y.). Digital images were taken from 100 to 200 cells under each condition at a magnification of ×40, and the diameter of each cell (in arbitrary units) was determined from a print of the digital image.
For videomicroscopy, 5-μl portions of cells in PBS-2% BSA were sealed under glass coverslips with a mean final thickness of 5.7 μm so that the cells were compressed between the slides and coverslips (13, 15). PMN were examined by phase-contrast microscopy by using a ×40 objective on a 33°C stage. The orientation and trajectory of PMN was recorded before, during, and after their response to a chemotactic stimulus created by ruby laser microbeam destruction of an erythrocyte (wavelength, 694.3 min; duration of flash, 0.5 ms) (13). Freeze-frame images were collected with a Hamamatsu C2400 microscope video camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan) and a Panasonic AG6720 time-lapse video recorder (Matsushita Electric Industrial Co., Osaka, Japan). Reagents for treatment of cells were obtained from Sigma-Aldrich Fine Chemicals (St. Louis, Mo.)
Spirochete culture.
A low-passage clonal isolate of B. burgdorferi strain N40 was cultivated in Barbour-Stoenner-Kelly (BSK) II medium at 33°C as described previously (10). Spirochetes were opsonized for 30 min with 1 to 10% heat-inactivated serum from a well-characterized Lyme disease patient or a normal volunteer in PBS; donor serum reactivities were documented by an enzyme-linked immunosorbent assay and Western blotting against B. burgdorferi lysate.
Saliva preparation.
Adult Ixodes scapularis ticks were allowed to feed on naïve rabbits for 5 to 7 days until they were engorged. Saliva was harvested from the ticks following pilocarpine stimulation as described previously (28) and was stored at −80°C until it was used.
Detection of killing of spirochetes.
B. burgdorferi was quantified by using a modified regrowth assay which measures spirochete uptake of [3H]adenine over 48 h (10). PMN in these experiments did incorporate some [3H]adenine, but as they did not divide, it was a small amount. Briefly, 5 × 106 B. burgdorferi cells per ml were incubated with PMN for 1 h at 37°C with agitation. Triplicate aliquots (50 μl) plated in 96-well plates in the presence of 200 μl of BSK II medium containing 5 μCi of [3H]adenine were incubated for 48 h at 33°C. Spirochetes and cells were harvested with a semiautomated cell harvester (Skatron Instruments, Inc., Sterling, Va.), and the incorporated [3H]adenine was counted with an LKB/Wallac 1205 Betaplate liquid scintillation counter (LKB/Wallac, Gaithersburg, Md.). Viability was determined by measuring incorporation of [3H]adenine by B. burgdorferi alone compared to incorporation of [3H]adenine by B. burgdorferi incubated with PMN. The results were expressed as the increase in the percentage of viable spirochetes recovered after incubation with saliva-treated PMN compared with the results obtained with control PMN.
FACS analysis of PMN.
Freshly isolated PMN in PBS containing Ca2+, Mg2+, and 0.1% BSA were preincubated in dilutions of saliva before stimulation with tumor necrosis factor alpha (TNF-α) (15 ng/ml; R & D Systems, Minneapolis, Minn.). Cells were labeled with specific antibodies (DAKO Corp.) in PBS-BSA for 1 h at 4°C, washed, fixed in 0.5% paraformaldehyde in PBS, and stored at 4°C until fluorescence-activated cell sorter (FACS) analysis. Fluorescein isothiocyanate-conjugated CD4 (T-cell marker) served as a negative control. Pilocarpine treatment did not change integrin expression.
Statistical analysis.
Significance was assessed by analysis of variance and paired, one-tailed Student's t tests.
RESULTS
Saliva treatment reduces attachment of B. burgdorferi by PMN.
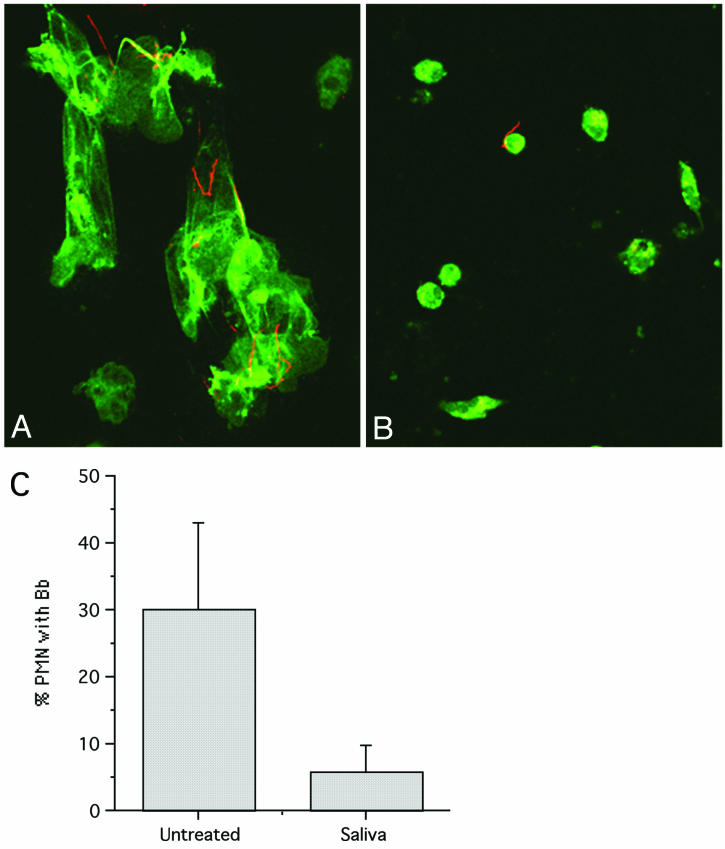
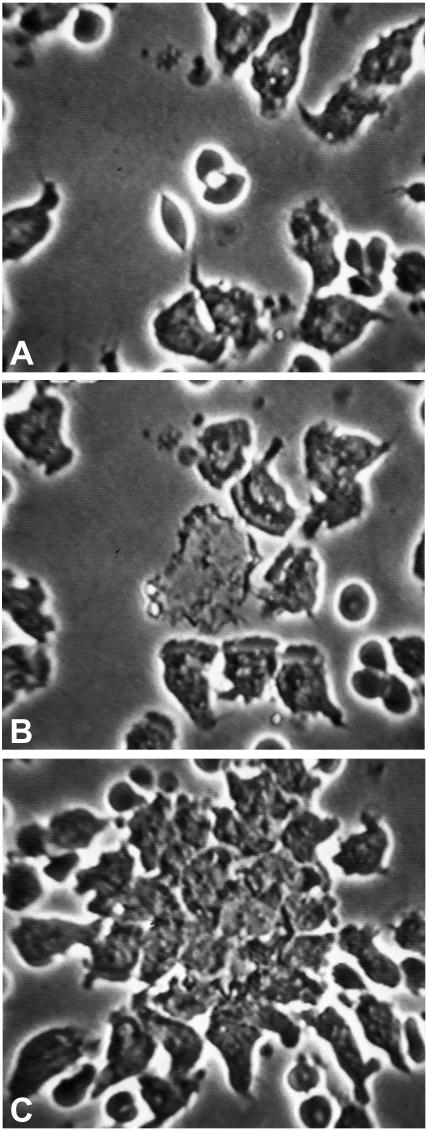
We have recently shown that PMN are inefficient at binding spirochetes in the absence of specific antibody (17). In our examination of PMN efficiency, we noted that saliva-treated PMN appeared to be less well attached to coverslips, which suggested that they might have an impaired ability to bind spirochetes. When control PMN on coverslips were incubated with opsonized spirochetes, a procedure that generally results in efficient binding, we observed well-spread cells with numerous attached spirochetes (Fig. 1A). In contrast, saliva-treated PMN were significantly less well spread and bound fewer spirochetes, even when the spirochetes had been opsonized with specific antibody (Fig. 1B). A fivefold reduction in the number of PMN with attached B. burgdorferi cells was noted when saliva-treated PMN were compared to control cells (P = 0.09; n = 3) (Fig. 1C). Reduced binding was observed both in the presence of serum and under serum-free conditions (data not shown).
FIG. 1.
Saliva reduces adherence of PMN and attachment to spirochetes. Fresh human PMN on coverslips were incubated in KRPG containing 10% human serum with opsonized B. burgdorferi. Samples were fixed after 5 min of incubation at 37°C and double labeled with antibodies specific for myeloperoxidase (green) and spirochetes (red). Images were recorded at a magnification of ×63. (A) Control PMN are adherent and spread. (B) PMN treated with saliva spread less and bind few spirochetes. (C) Reduced percentage of PMN with attached B. burgdorferi after saliva treatment (P = 0.09). The number of attached B. burgdorferi (Bb) cells per 100 cells was determined (n = 3).
Saliva reduces the cellular area of treated PMN.
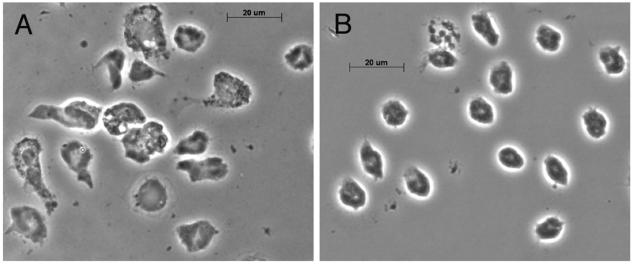
When we examined coverslips with adherent PMN that had been incubated for 1 h in the presence of saliva, we observed an apparent reduction in the number of cells. While counts of cells on grid coverslips indicated that there was a modest reduction in the number of cells after saliva treatment (data not shown), there was a dramatic difference in the area of the remaining cells (Fig. 2). We quantified the cellular areas of untreated PMN and PMN incubated with dilutions of saliva (1:10, 1:20, and 1:100) from printed digital images of 100 to 200 fixed cells per condition (n = 2). As shown in Table 1, there was a dose-dependent reduction in the cellular area after treatment of PMN with saliva for 1 h; the difference is statistically significant (P = 0.0001). We found a similar reduction in the cellular area of PMN treated with saliva in suspension for 1 h prior to plating on glass coverslips (data not shown).
FIG. 2.
Saliva reduces PMN spreading. Adherent PMN on glass coverslips were incubated alone (A) or with a 1:10 dilution of saliva (B) for 1 h at 37°C before fixation. Cell spreading was examined at a magnification of ×63 by phase-contrast microscopy. Note the reduction in cell area after saliva treatment (n = 4).
TABLE 1.
Saliva reduces PMN cell area and PMN β2-integrin (CD18) expression
| Treatment | Cell areaa | CD18 expression withb:
|
|
|---|---|---|---|
| No stimulation | TNF-α | ||
| None | 1.0 ± 0.04 | 68.2 ± 15.1 | 110.3 ± 38.1 |
| Saliva (1:10) | 0.48 ± 0.02c | 37.6 ± 15.7d | 42.1 ± 14.5f |
| Saliva (1:20) | 0.67 ± 0.03c | 43.4 ± 15.0e | 57.9 ± 20.4 |
| Saliva (1:100) | 0.69 ± 0.03c | 60.7 ± 17.0 | 97.1 ± 37.2 |
Adherent PMN on glass coverslips were treated with no saliva or different dilutions of saliva for 1 h at 37°C. Fixed cells were examined, and the area of 100 to 200 cells was determined from digital images.
The data are the average mean fluorescent intensity ± standard error of the mean (n = 4). The mean fluorescent intensity values for CD15, a PMN marker, were not changed by TNF-α stimulation or by saliva treatment (36.2 without treatment, 40.0 with TNF-α alone, 33.9 with saliva alone, and 45.7 with TNF-α and saliva). The value for negative control (CD4) antibody labeling was 21.
The relative area value is significantly different from the value for untreated PMNs (P = 0.0001; n = 2).
The value is significantly different from the untreated PMN value at a P value of 0.0004.
The value is significantly different from the untreated PMN value at a P value of 0.0015.
The value is significantly different from the untreated PMN value at a P value of 0.08.
Saliva reduces expression of PMN β2-integrins.
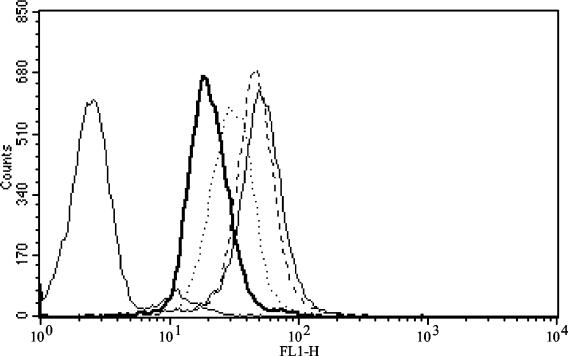
The reduced attachment and spreading of PMN in the presence of saliva suggested that there was altered expression of PMN β2-integrins, the molecules that mediate adhesion of the cells. To examine this, we incubated PMN with saliva and quantified expression of several surface antigens. As expected, PMN stimulated in suspension with 15 ng of TNF-α per ml for 30 min showed significant upregulation of CD18 integrin expression (Table 1) (16). In contrast, a dramatic decrease in CD18 (β2-integrin) expression was detected in saliva-treated cells. This reduction was dose dependent, and higher concentrations of saliva resulted in more profound reductions (Fig. 3). The reduction in CD18 expression on saliva-treated PMN was apparent in the presence or absence of TNF-α activation and was statistically different from CD18 expression by PMN not exposed to saliva (for 1:10 dilution of saliva, P = 0.0004 without TNF-α and P = 0.08 in the presence of TNF-α; n = 4) (Table 1). Expression of the PMN surface marker CD15 (siayl Lewis X antigen) was not appreciably changed by saliva treatment or by TNF-α stimulation.
FIG. 3.
Saliva downregulates integrin expression on PMN. Freshly isolated PMN were incubated with dilutions of saliva for 60 min at 37°C prior to stimulation by 15 ng of TNF-α per ml for 30 min. Cells were stained before fixation for FACS analysis. The data are representative of four separate experiments; unstained cells are at the far left. Light solid line, untreated PMN; heavy solid line, PMN treated with saliva at a dilution of 1:10; dotted line, PMN treated with saliva at a dilution of 1:20; dashed line, PMN treated with saliva at a dilution of 1:100.
We examined PMN surface marker expression after treatment with reagents similar to components of tick saliva. No significant differences from untreated PMN were detected for PMN expression of the integrin molecules Cd11a, CD11b, CD11c, and CD18 or of surface protein CD15 after 1 h of incubation in the presence of the reagent apyrase (10 U/ml), prostaglandin E2 (PGE2) (20 μg/ml), or calcitonin gene-related peptide (1 × 10−8 M) at concentrations reported to be effective for activity (22, 23, 26, 27). As expected, TNF-α produced a statistically significant increase in expression of all integrins tested (P = 0.04), regardless of treatment, but not of CD15 (n = 3) (data not shown).
Saliva treatment impairs spirochete killing in suspension.
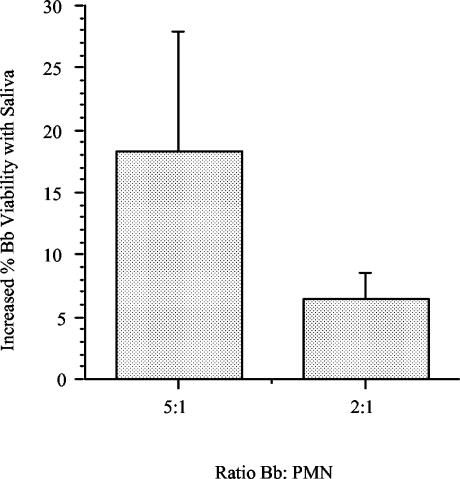
Saliva-treated PMN were dramatically less adherent (Fig. 1) and thus were not suitable for direct microscopic observation of spirochete killing by vital staining. To measure killing, we quantified the recovery of spirochetes in suspension using a well-characterized [3H]adenine regrowth assay (10). PMN were preincubated in suspension with saliva at a 1:10 dilution for 1 h before addition of spirochetes at a B. burgdorferi/PMN ratio of 5:1 or 2:1. In three experiments in which PMN killed unopsonized spirochetes, a modest increase in the level of viability was noted in saliva-treated PMN (Fig. 4); the reduction was significant at a B. burgdorferi/PMN ratio of 2:1 (P = 0.04). While this suspension assay does not require adherence of the PMN to a surface, the increased survival of spirochetes in the presence of treated PMN is in keeping with the observed decrease in attachment of spirochetes to PMN.
FIG. 4.
Saliva reduces killing of spirochetes by PMN. Freshly isolated PMN were preincubated with saliva at a 1:10 dilution for 60 min at 37°C prior to incubation with 5 × 106 B. burgdorferi (Bb) cells for 1 h at 37°C. The data show the increase in the percentage of viable spirochetes recovered after incubation with saliva-treated PMN compared with the results obtained with control PMN after 48 h of regrowth in BSK II medium at 33°C (n = 3). Increases in the percentage of spirochetes recovered were statistically significant at the 2:1 ratio (P = 0.04).
Tick saliva does not affect PMN orientation or chemotaxis.
In preparations in which PMN have access to only one surface and adhesive properties are important, the absence of integrins greatly reduces locomotion (13). We have found that compressed PMN in very thin slide preparations exhibit normal random locomotion and chemotaxis even in the presence of antibodies that disable their integrins. Normal locomotion was also noted in compressed PMN from a patient whose PMN lack β2-integrins (13, 14). In the present study, as expected, control PMN were well attached to the glass slides and locomoted flat on the slide before and after stimulation. In contrast, saliva-treated PMN were less adherent and often rounded up (data not shown). Saliva-treated cells resembled cells treated with EDTA or anti-CD18 (β2-integrin) antibodies, conditions that disable integrins (13, 14). To determine which aspects of chemotaxis were affected by saliva, we examined saliva-treated PMN in compressed slide preparations, in which integrins were not required for a PMN response to a chemotactic target. Compressed PMN showed no effect of tick saliva at a dilution of 1:10 and responded appropriately to the chemotactic gradient created by laser irradiation of erythrocytes (Fig. 5), indicating that orientation and directed locomotion were intact.
FIG. 5.
Saliva does not affect orientation or chemotaxis of PMN. Freshly isolated PMN were incubated with saliva (1:10 dilution) and examined live by videomicroscopy at a magnification of ×40. (A) PMN in the field, compressed to minimize the need for adhesion molecules, move randomly. The white dot indicates two erythrocytes targeted for laser destruction. (B) Twenty-two seconds after the laser flash, leading fronts of nearby PMN orient toward the newly created chemoattractant source. (C) By 3 min 25 s later, nearby PMN and other PMN from outside the field arrive at the target. This response is indistinguishable from that of control PMN (data not shown). No effect of saliva was noted.
In the presence of saliva, both in pretreatment and throughout the interaction, we saw the disaggregation of platelets described previously (24). As noted above for surface marker integrin expression as assessed by FACS, apyrase (1 to 10 U/ml) and PGE2 (2 to 20 μg/ml), reagents similar to salivary components, had no effect on random locomotion or chemotaxis in thin or thick preparations (data not shown). PMN responded normally to the chemotactic stimulus even in the presence of 200 μg of PGE2 per ml.
DISCUSSION
We found that PMN are less adherent, bind fewer spirochetes, and have a reduced cellular footprint after saliva treatment. The mechanism for reduced spirochete attachment after saliva treatment is unclear and may include the reduced surface area or the absence or masking of a specific receptor. FACS analysis of saliva-treated PMN revealed a dose-dependent decrease in expression of CD18, the common monomer of the three β2-integrins, evident in the presence or absence of stimulation with TNF-α. Moreover, killing of spirochetes was reduced in the presence of saliva, in keeping with evidence that integrins mediate binding of unopsonized spirochetes to leukocytes (3, 4). Although we did not distinguish between decreased appearance and increased removal of CD18 from the cell surface, the behavior of saliva-treated PMN during chemotaxis suggests that there is aggregation of integrin molecules that are swept to the rear of moving PMN. In compressed slide preparations, in which adhesion was less important for locomotion (13), tick saliva had no effect on the random locomotion of PMN or on their orientation or chemotaxis.
After a tick bite, a local cement plug or absorbent reservoir forms, which contains high concentrations of saliva that are replenished by intermittent bursts of salivation during feeding (1). Generally, attachment of ticks for 48 h is necessary for efficient transmission of B. burgdorferi to a host (25), which provides a critical window of time for saliva to exert its modulatory effects on the host immune response.
Besides their apparent contribution to the adhesion of PMN to B. burgdorferi and killing of spirochetes as demonstrated here, integrins are known to be important in adherence and locomotion and other aspects of phagocyte function; the absence of these molecules results in severe complications of infectious diseases and wound healing (8). For example, masking of CD18 blocked cytokine stimulation of H2O2 production by PMN (19) and delayed apoptosis (5). The downregulation of PMN integrins by saliva compromises the abilities of the responding PMN and provides spirochetes with critical time to establish themselves in situ before they disseminate to cause systemic illness.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (grants AI 43558, AR 10493, AR 48513, and AR 07107), from the Eshe Fund, from the Mathers Foundation, and initially from the Centers for Disease Control and Prevention. D.L. was a fellow of the Arthritis Foundation.
We are grateful for ticks supplied by John Anderson and Durland Fish and for the technical assistance of Rita Palmarozza and Deborah Beck.
Editor: D. L. Burns
REFERENCES
- 1.Alekseev, A. N., E. A. Arumova, and I. S. Vasilieva. 1995. Borrelia burgdorferi sensu lato in female cement plug of Ixodes persulcatus ticks (Acari, Ixodiae). Exp. Appl. Acarol. 19:519-522. [DOI] [PubMed] [Google Scholar]
- 2.Barthold, S. W., M. de Souza, E. Fikrig, and D. H. Persing. 1992. Lyme borreliosis in the laboratory mouse, p. 223-242. In S. E. Schutzer (ed.), Lyme disease: molecular and immunologic approaches. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 3.Cinco, M., R. Murgia, G. Presani, and S. Perticarari. 1997. Integrin CR3 mediates the binding of nonspecifically opsonized Borrelia burgdorferi to human phagocytes and mammalian cells. Infect. Immun. 65:4784-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coburn, J., J. M. Leong, and J. K. Erban. 1993. Integrin αIIbβ3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc. Natl. Acad. Sci. 90:7059-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coxon, A., P. Rieu, F. J. Barkalow, S. Askari, A. H. Sharpe, U. H. von Andrian, M. A. Arnaout, and T. N. Mayadas. 1996. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 5:653-666. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie, R. D., M. C. Dolan, J. Piesman, and R. G. Titus. 2001. Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J. Immunol. 166:4319-4326. [DOI] [PubMed] [Google Scholar]
- 7.Gillespie, R. D., M. L. Mbow, and R. G. Titus. 2000. The immunomodulatory factors of bloodfeeding arthropod saliva. Parasite Immunol. 22:319-331. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto, T. K., E. T. Baldwin, and D. C. Anderson. 1999. The role of β2 integrins in inflammation, p. 537-569. In J. I. Gallin and R. Snyderman (ed.), Inflammation: basic principals and clinical correlates, 3rd ed. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 9.Kuthejlova, M., J. Kopecky, G. Stepanova, and A. Macela. 2001. Tick salivary gland extract inhibits killing of Borrelia afzelii spirochetes by mouse macrophages. Infect. Immun. 69:575-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lusitani, D., S. E. Malawista, and R. R. Montgomery. 2002. Borrelia burgdorferi are susceptible to killing by a variety of PMN components. J. Infect. Dis. 185:797-804. [DOI] [PubMed] [Google Scholar]
- 11.Lusitani, D. L., S. E. Malawista, and R. R. Montgomery. 2003. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect. Immun. 71:4711-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malawista, S. E. Lyme disease. In W. J. Koopman (ed.), Arthritis and allied conditions. A textbook of rheumatology, in press. Lippincott, Williams & Wilkins, Philadelphia, Pa.
- 13.Malawista, S. E., and A. de Bloisfleury Chevance. 1997. Random locomotion and chemotaxis of human blood polymorphonuclear leukocytes in the presence of EDTA: PMN in close quarters require neither leukocyte integrins nor external divalent cations. Proc. Natl. Acad. Sci. 94:11577-11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malawista, S. E., A. de Bloisfleury Chevance, and L. A. Boxer. 2000. Random locomotion and chemotaxis of human blood polymorphonuclear leukocytes from a patient with leukocyte adhesion deficiency-1: normal displacement in close quarters via chimneying. Cell Motil. Cytoskel. 46:183-189. [DOI] [PubMed] [Google Scholar]
- 15.Malawista, S. E., A. de Boisfleury Chevance, E. J. Brown, L. A. Boxer, and S. K. Law. 2003. Chemotaxis of non-compressed blood polymorphonuclear leukocytes from an adolescent with severe leukocyte adhesion deficiency. Am. J. Hematol. 73:115-120. [DOI] [PubMed] [Google Scholar]
- 16.Miller, L. J., D. F. Bainton, N. Borregaard, and T. A. Springer. 1987. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J. Clin. Investig. 80:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery, R. R., D. L. Lusitani, A. de Boisfleury Chevance, and S. E. Malawista. 2002. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 185:1773-1779. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery, R. R., M. H. Nathanson, and S. E. Malawista. 1993. The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages: destruction, survival, recovery. J. Immunol. 150:909-915. [PubMed] [Google Scholar]
- 19.Nathan, C., S. Srimal, C. Farber, E. Sanchez, L. Kabbash, A. Asch, J. Gailit, and S. D. Wright. 1989. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J. Cell Biol. 109:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramachandra, R. N., and S. K. Wikel. 1992. Modulation of host-immune responses by ticks (Acari:Ixodidae): effect of salivary gland extracts on host macrophages and lymphocyte cytokine production. J. Med. Entomol. 29:818-826. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro, J. M., and I. M. Francischetti. 2003. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 48:73-88. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro, J. M. C., G. T. Makoul, J. Levine, D. R. Robinson, and A. R. Spielman. 1985. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J. Exp. Med. 161:332-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro, J. M. C., A. Vachereau, G. B. Modi, and R. B. Tesh. 1989. A novel vasodilatory peptide from the salivary glands of the sand fly Lutzomyia longipalpis. Science 243:212-214. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro, J. M. C., J. J. Weis, and S. R. Telford III. 1990. Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp. Parasitol. 70:382-388. [DOI] [PubMed] [Google Scholar]
- 25.Shih, C.-M., R. J. Pollack, S. R. Telford III, and A. Spielman. 1992. Delayed dissemination of Lyme disease spirochetes from the site of deposition in the skin of mice. J. Infect. Dis. 166:827-831. [DOI] [PubMed] [Google Scholar]
- 26.Titus, R. G., and J. M. C. Ribeiro. 1990. The role of vector saliva in transmission of arthropod-borne disease. Parasitol. Today 6:157-160. [DOI] [PubMed] [Google Scholar]
- 27.Urioste, S., L. R. Hall, S. R. Telford III, and R. G. Titus. 1994. Saliva of the Lyme disease vector, Ixodes dammini, blocks cell activation by a nonprostaglandin E2-dependent mechanism. J. Exp. Med. 180:1077-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valenzuela, J. G., R. Charlab, T. N. Mather, and J. M. Ribeiro. 2000. Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J. Biol. Chem. 275:18717-18723. [DOI] [PubMed] [Google Scholar]
- 29.Zeidner, N. S., B. S. Schneider, M. S. Nuncio, L. Gern, and J. Piesman. 2002. Coinoculation of Borrelia spp. with tick salivary gland lysate enhances spirochete load in mice and is tick species-specific. J. Parasitol. 88:1276-1278. [DOI] [PubMed] [Google Scholar]