Abstract
Alzheimer’s disease (AD) is characterized by neuronal loss and the accumulation of β-amyloid plaques and neurofibrillary tangles in the brain. Cerebrospinal fluid (CSF) levels of β-amyloid and tau/ptau181 are also associated with AD. We have previously demonstrated that a single nucleotide polymorphism in calcineurin is associated with CSF ptau181 levels and AD progression. In this study, we demonstrate that calcineurin protein levels are inversely correlated with dementia severity and Braak tangle stage in AD brains, and calcineurin activity is globally reduced in AD brains. We then sought to model the observed changes in CSF tau by measuring extracellular tau in cultured cells. SH-SY5Y cells treated with calcineurin inhibitors produced reduced calcineurin activity and a corresponding increase in extracellular ptau181. These findings are consistent with our observations in AD patients, who have elevated CSF ptau181 and reduced calcineurin activity in brain extracts. Thus, we have identified a gene that contributes to AD pathology and has functional consequences on tau metabolism in cultured cells.
Keywords: Calcineurin, tau, Alzheimer’s disease, phosphorylation
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by extensive neuronal loss and the accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles in the brain. Intracellular neurofibrillary tangles are primarily composed of hyperphosphorylated tau proteins.
Tau is an intracellular, microtubule associated protein, which functions to stabilize and facilitate the assembly of microtubules. The phosphorylation state of tau is a critical regulator of microtubule binding. Tau that is phosphorylated in the microtubule-binding region is unable to interact with microtubule subunits or stabilize the structure. Tau phosphorylation is tightly regulated by several kinases and phosphatases. Disturbances in microtubule-binding equilibrium could contribute to tau aggregation in that aberrant, hyperphosphorylated tau is unlikely to bind to and stabilize microtubules, which increases the proportion of cytosolic, unbound tau available to misfold and aggregate (Ballatore, et al., 2007, Mandelkow and Mandelkow, 1998).
Cerebrospinal fluid (CSF) levels of soluble total tau/ptau181 are associated with clinical AD (reviewed in (Craig-Schapiro, et al., 2009)) and are used as a biomarker for AD (Galasko, et al., 1998, Vigo-Pelfrey, et al., 1995). CSF total and ptau181 levels are significantly elevated in clinically demented individuals compared with non-demented controls (Galasko, et al., 1998, Vigo-Pelfrey, et al., 1995). In stroke (Hesse, et al., 2001) and traumatic brain injury (Ost, et al., 2006), CSF total tau levels are briefly elevated after initial insult, reflective of tau release due to neuronal loss; however, CSF phospho-tau levels remain unchanged. Thus, AD is unique in that both total and phosphorylated forms of tau are elevated in the CSF.
We have previously used CSF tau/ptau181 levels as a quantitative endophenotype to identify genes that influence tau levels and contribute to AD progression (Cruchaga, et al., 2010, Kauwe, et al., 2008). We have demonstrated that the minor allele of a single nucleotide polymorphism (SNP) in the regulatory subunit of calcineurin (PPP3R1- rs1868402) is associated with elevated levels of CSF ptau181 and accelerated disease progression (Cruchaga, et al., 2010). Calcineurin (PP3, previously PP2B) is a heterodimeric calcium/calmodulin-dependent phosphatase composed of a 60 kDa catalytic subunit (PPP3CA) and a 19 kDa calcium binding regulatory subunit (PPP3R1) (reviewed in (Rusnak and Mertz, 2000)). The 60 kDa catalytic subunit possesses a catalytic domain, regulatory subunit binding domain, calmodulin binding domain, and autoinhibitory domain at the C-terminus (Yu, et al., 2006a). Calcineurin is abundantly expressed in the cytosol of neuronal cells in the brain (Dawson, et al., 1994, Goto, et al., 1986, Polli, et al., 1991) where it directly dephosphorylates tau at Thr181, Thr231, and Ser396 (Garver, et al., 1999).
Calcineurin dysregulation has been proposed to contribute to AD pathology in several ways: inhibition of synaptic function (Kayyali, et al., 1997); modification of learning and memory pathways (Kayyali, et al., 1997, Malleret, et al., 2001); PKA-dependent long-term potentiation (Malleret, et al., 2001); and mediation of dendritic spine density and dendritic arbor length/branching (Spires-Jones, et al., 2011). Hyperphosphorylated tau is detectable in mice in which calcineurin is knocked-down by anti-sense oligonucleotides (Garver, et al., 1999) or completely knocked out (Kayyali, et al., 1997). Furthermore, calcineurin knockout mice perform poorly in learning and memory tasks, providing strong evidence for a role of calcineurin in AD.
In this study, we examined the contribution of calcineurin subunits to AD pathology in human brains and in a cell model of tau metabolism. We determined that PPP3R1 and PPP3CA protein levels are inversely correlated with clinical and pathologic measures of AD. Calcineurin activity levels were significantly lower in AD brains compared with age-matched, cognitively normal controls after correcting for total cell loss. Thus, calcineurin expression and function are altered in AD brains. To model the observed effects in AD brains and on CSF ptau181 levels, we utilized a cell culture system in which extracellular tau is measured in immortalized cells. We demonstrate that treatment of SH-SY5Y cells with calcineurin inhibitors, cyclosporin A and FK506, results in reduced calcineurin activity and a corresponding increase in extracellular ptau181. These findings are consistent with our observation in AD patients, who have elevated CSF tau and significantly lower calcineurin activity in brain extracts.
2. Methods
2.1 Brain samples
Parietal lobes from autopsy-confirmed AD (N=73) and age-matched, cognitively normal control (N=39) brains were obtained from the Charles F. and Joanne Knight Alzheimer’s Disease Research Center (Table 1). AD pathology was measured using Braak and Braak staging (Alafuzoff, et al., 2008, Braak, et al., 2006, Braak and Braak, 1991).
Table 1.
Summary of brain samples
| Sample | N | Age (yrs)* | Male (%) | ApoE4+ (%) |
|---|---|---|---|---|
| Case | 73 | 87 ± 7 | 42 | 41 |
| Control | 39 | 86 ± 9 | 44 | 23 |
Mean +/− SD
2.2 Antibodies
Antibodies used in this study include: Tau12, Tau1, Tau7 (generously provided by Dr. Lester Binder), BT2 (Thermo-Pierce), total calcineurin (Millipore), calcineurin regulatory subunit (Millipore), calcineurin catalytic subunit (Millipore), Tuj1 (Abcam), and β-tubulin (Sigma).
2.3 Cell lines and transient transfection
Undifferentiated SH-SY5Y cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine, and penicillin/streptomycin. Human embryonic kidney (HEK293-T) cells were cultured in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% FBS, 1% L-glutamine, and penicillin/streptomycin. For transient transfection, HEK293-T cells were cultured in 6 well lysine-coated plates. Upon reaching 90% confluence, cells were transfected with Lipofectamine 2000 (Invitrogen) and harvested after 24 hours. Human wild-type MAPT (the longest isoform, 2N4R) cDNA was expressed in pcDNA3.1 vector (generously provided by Dr. Virginia Lee), human PPP3R1 and human PPP3CA were expressed in the pCMVsport6 vector (OriGene).
2.4 Enzyme-linked immunosorbent assay (ELISA)
Media was collected and centrifuged at 3000xg at 4°C for 10 minutes to remove cell debris. Cell lysates were extracted in lysis buffer (50mM Tris, pH 7.6, 1mM EDTA, 150mM NaCl, 1% TritonX-100, phosphatase and protease inhibitors). Media and cell lysates were analyzed using commercially available ELISA kits (Invitrogen) specific to human total tau, ptau181 and ptau396. To account for protein concentration, total protein in cell lysates were measured by BCA assay according to manufacturer’s protocol (Thermo Scientific). ELISA values were obtained (pg/mL) and corrected for total intracellular protein (ug/mL), resulting in a final value measured in pg/ug.
2.5 Immunoprecipitation
Media and cell lysates were pre-cleared with Protein G beads (Thermo-Pierce). Pre-cleared supernatants were incubated overnight at 4°C with the antibodies indicated (5ug). Supernatant-antibody complexes were then incubated with Protein G beads at room temperature for 2 hours. After washing, proteins were dissociated from the Protein G beads with incubation in Laemmli sample buffer (Laemmli, 1970) supplemented with 5% βME at 95°C for 10 minutes.
2.6 Immunoblotting
Standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in 4–20% Criterion Tris-HCl gels (Bio-Rad). Samples were boiled for 5 minutes in Laemmli sample buffer (Laemmli, 1970) prior to electrophoresis. Quantification of calcineurin subunits, Tuj1, and β-tubulin was performed by measuring the band intensity of the protein bands in each lane using a Syngene imaging system.
2.7 Lactate Dehydrogenase (LDH) assay
Cytotoxicity was measured in SH-SY5Y cells using an LDH assay kit (Clontech) according to manufacturer’s protocol. SH-SY5Y cells (2×105) were grown in a 96-well plate. Cells were then incubated with untreated LDH assay media (low control) or with LDH assay media supplemented with 1% Triton X-100 (high control), DMSO (Sigma 1:1000), cyclosporin A (Sigma 10 uM), or FK506 (LC Lab 10 uM) for 6 hours. Media from untreated and treated cells were then incubated with tetrazolium salt. Samples were measured at 490nm. Cytotoxicity was expressed relative to maximal cell lysis.
2.8 Brain extraction
Brains were homogenized in lysis buffer on ice by sonication and centrifuged at 14,000xg at 4°C. The resulting supernatants were analyzed for total protein levels by BCA assay and analyzed by SDS-PAGE and immunoblotting using the antibodies described above.
2.9 Calcineurin activity
Calcineurin activity was measured using a calcineurin cellular activity assay (Enzo Life Sciences) according to manufacturer’s protocols. Extracted brain and cell lysates were placed in a desalting column to remove excess phosphates and nucleotides. Total phosphatase activity was assessed in the samples by incubating with a phosphopeptide substrate. Calcium-independent phosphatase activity was assessed in the samples by incubating samples with EGTA and a phosphopeptide substrate. The resulting total phosphatase activity was subtracted from calcium-independent phosphatase activity to obtain calcineurin activity levels.
2.10 Statistical Analyses
PPP3R1 and PPP3CA protein levels were log transformed to achieve a normal distribution. Stepwise discriminant analyses were used to determine covariates that significantly contribute to the model in SAS. The association of PPP3R1 and PPP3CA protein levels with clinical dementia rating (CDR), disease status, and Braak tangle/plaque staging was conducted by an analysis of covariance (ANCOVA), with ApoE genotype included as a covariate. CDR is a clinical measure of dementia, which incorporates six domains of cognitive and functional abilities: memory, orientation, problem solving, community involvement, home, and personal care (Morris, 1993). Power for the analyses was calculated using proc power in SAS.
3. Results
3.1 Calcineurin and AD brain pathology
We have previously demonstrated that the minor allele of a SNP in the regulatory subunit of calcineurin (PPP3R1- rs1868402) is associated with elevated levels of CSF ptau181 and accelerated disease progression (Cruchaga, et al., 2010). Furthermore, individuals carrying the minor allele at rs1868402 have significantly less PPP3R1 mRNA in the brain and significantly higher Braak tangle stage (Cruchaga, et al., 2010).
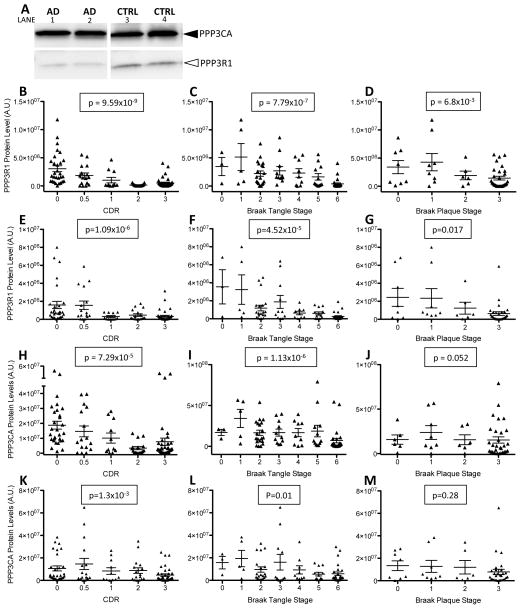
To understand the mechanism by which the regulatory subunit of calcineurin contributes to AD pathology, calcineurin protein and activity levels were measured in AD and cognitively normal brains. Brain tissue from the parietal lobe was extracted in non-ionic detergent to isolate a total cell lysate and immunoblotted with antibodies specific to the regulatory and catalytic subunits of calcineurin. Protein levels were quantified, adjusted for total protein levels to correct for overall cell loss that occurs in AD brains, and analyzed for association with clinical and pathological measures of dementia (Fig. 1A). PPP3R1 protein levels were inversely correlated with CDR (p=9.59×10−9) (Fig. 1B), where the lowest PPP3R1 protein levels are detected in individuals with the highest CDR. Calcineurin is a phosphatase that dephosphorylates tau at several phospho-epitopes, and calcineurin knockout mice exhibit hyperphosphorylated tau in the brain (Kayyali, et al., 1997). Thus, we tested whether PPP3R1 protein levels correlate with the abundance of tau pathology in the brain. PPP3R1 protein levels were significantly correlated with Braak tangle stage (Fig. 1C), where lower PPP3R1 protein levels are associated with increased Braak tangle stage (p=7.79×10−7). PPP3R1 protein levels were also associated with Braak plaque stage (p=0.0068) (Fig. 1D). The association of PPP3R1 protein levels with tangle pathology is much stronger than the association with plaque pathology, suggesting that PPP3R1 protein has a greater influence on tau pathology in the brain.
Figure 1.
Calcineurin protein levels are altered in AD brains. Human brain samples from AD patients (N=73) and non-demented controls (N=39) were extracted in non-ionic detergent. A. Total cell lysate extracted from brains (100 ug) were analyzed by SDS-PAGE and immunoblots were probed with antibodies to the regulatory (PPP3R1) and catalytic (PPP3CA) subunits of calcineurin. B–M. Quantification of immunoblots. B–D and H–J. To correct for total protein, PPP3R1 and PPP3CA protein levels are expressed relative to β-tubulin protein levels. E–G and K–M. To correct for neuron number, PPP3R1 and PPP3CA protein levels are expressed relative to Tuj1 protein levels. B–G. Calcineurin regulatory subunit (PPP3R1) protein levels are associated with clinical and pathologic measures of AD. B,E. PPP3R1 protein levels are plotted with CDR. C,F. PPP3R1 protein levels plotted with Braak tangle staging. D,G. PPP3R1 protein levels are plotted with Braak plaque stage. H–M. Protein levels of the catalytic subunit of calcineurin (PPP3CA) are associated with clinical and pathologic measures of AD. H,K. PPP3CA protein levels are plotted with CDR. I,L. PPP3CA protein levels are plotted with Braak tangle staging. J,M. PPP3CA protein levels are plotted with Braak plaque staging.
Calcineurin is a heterodimeric protein composed of a regulatory subunit (PPP3R1) and a catalytic subunit (PPP3CA). To measure the contribution of PPP3CA protein levels to AD pathology, we analyzed the association of PPP3CA protein levels with clinical and pathological measures of dementia. PPP3CA protein levels were also inversely correlated with CDR (p=7.29×10−5), Braak tangle stage (p=1.13×10−6), and Braak plaque stage (p=0.052) (Fig 1H–J). PPP3R1 and PPP3CA protein levels are highly correlated in this brain cohort (r2=0.63; Fig. 2A), and the correlation between PPP3R1 and PPP3CA protein levels was strongest in AD cases compared with cognitively normal controls (r2=0.75 and r2=0.68, respectively; Fig. 2B–C).
Figure 2.
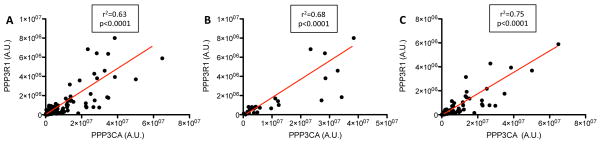
PPP3R1 and PPP3CA protein levels are highly correlated in brain tissue. PPP3R1 and PPP3CA protein levels were expressed relative to Tuj1 protein levels. A. Complete brain series. B. Control brains. C. AD brains.
Calcineurin expression is enriched in neuronal cells, and extensive neuronal cell death is a hallmark pathological feature in AD brains. To determine if the observed association of CDR and Braak staging is strictly due to neuronal loss, we immunoblotted brain fractions with Tuj1, which is specifically expressed in neurons. Tuj1 protein levels were quantified, and PPP3R1 and PPP3CA protein levels were adjusted based on the abundance of Tuj1 reactivity. After adjusting for neuron loss, PPP3R1 (Fig. 1E–F) and PPP3CA (Fig. 1 K–L) protein levels were inversely correlated with CDR (p=1.09×10−6 and p=1.3×10−3, respectively) and Braak tangle stage (p=4.52×10−5 and p=0.01, respectively). PPP3R1 protein levels, but not PPP3CA protein levels, were significantly associated with Braak plaque stage (Fig.1G, p=0.017 and Fig. 1M; p=0.28, respectively). These results demonstrate that our initial observations that calcineurin protein levels are associated with Braak plaque stage were largely driven by neuronal cell loss. Additionally, the association of PPP3R1 with AD pathology was stronger than the association of PPP3CA with AD pathology, suggesting that the levels of PPP3R1 protein have a greater impact on AD pathology than PPP3CA protein levels. Taken together, these data suggest that calcineurin modifies tau pathology in AD brains independent of neuronal cell loss.
Calcineurin is activated when calcium and calmodulin form a complex with the PPP3R1 and PPP3CA subunits. Based on the reduced PPP3R1 and PPP3CA protein levels observed in AD brains, we predicted that calcineurin activity levels would also be affected. Calcineurin activity was measured indirectly in a highly sensitive colorimetric assay by subtracting calcium-independent phosphatase activity from total phosphatase activity. Calcineurin activity was then corrected for total protein to account for cell loss in AD brains. Calcineurin activity was significantly reduced in AD cases compared with cognitively normal controls (Fig. 3A). Furthermore, PPP3R1, but not PPP3CA, protein levels are significantly associated with calcineurin activity (p=0.0357 and 0.51, respectively). Because neuron loss is a hallmark pathology in AD brains and calcineurin is expressed in neurons, calcineurin activity was adjusted based on Tuj1 immunoreactivity. After controlling for neuron number, calcineurin activity was significantly elevated in AD brains compared with controls (Fig. 3B). Calcineurin activity adjusted for neuron number was associated with PPP3R1 and PPP3CA protein levels (p=1.153×10−15 and 5.175×10−19, respectively). These conflicting results suggest that calcineurin activity is globally reduced in AD brains and that calcineurin activity is up-regulated in the surviving neurons in AD brains. Together, this evidence demonstrates that calcineurin protein levels and activity are altered in AD brains.
Figure 3.
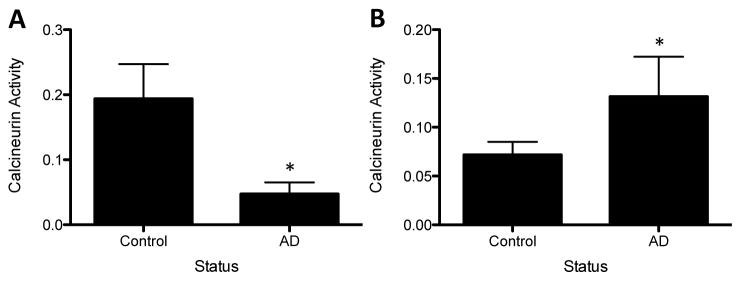
Calcineurin activity levels are altered in AD brains. Total cell lysate extracted from brains and lysates were used in a cellular activity assay. Calcineurin activity was assessed by subtracting the calcium-independent phosphatase activity from total phosphatase activity A. Corrected for β-tubulin levels (* p=0.0001). B. Corrected for Tuj1 levels (*p=0.0006). Values are expressed as arbitrary units, which arise from the correction of calcineurin activity (nmol/PO4) for β-tubulin or Tuj1 protein levels (band intensity).
3.2 Modeling extracellular tau in cultured cells
We have previously observed an association between a SNP in PPP3R1 and CSF ptau181, and here, we demonstrate an association between PPP3R1 protein levels and AD brain pathology. Thus, we were interested in studying the effects of calcineurin expression and function in a cell model of tau metabolism. The effects of calcineurin on tau phosphorylation and the subsequent effects on microtubule binding have been described previously in cell and mouse models (Garver, et al., 1999, Luo, et al., 2008, Yu, et al., 2006b). We were interested in using a cell culture system to model changes in CSF ptau181 (Cruchaga, et al., 2010). Tau is an intracellular protein. However, the presence of tau in the CSF in cognitively normal controls and in the interstitial fluid (ISF) of transgenic mice (Yamada, et al., 2011) suggests that cells release tau during normal tau metabolism. Furthermore, Kim and colleagues have demonstrated that extracellular tau is detectable in neuroblastoma cells overexpressing human wild-type tau (Kim, et al., 2010a, Kim, et al., 2010b).
To determine if extracellular tau is detectable in cells expressing endogenous tau, we measured total tau in culture media by ELISA in undifferentiated human neuroblastoma cells (SH-SY5Y). Extracellular total tau is present in the media of SH-SY5Y cells after 24 hours in culture (Fig. 4A). We also found that a proportion of extracellular tau is phosphorylated at several epitopes: Thr181 and Ser396 (Fig. 4A). Extracellular total tau represents approximately 1% of the total tau detected within the cell (Fig. 4B). The proportion of phospho-tau to total tau was higher inside (0.27) compared with outside (0.02) the cell (Fig. 4A and 4B). We were also able to detect intracellular (Fig. 4D) and extracellular (Fig. 4C) total and phospho-tau in non-neuronal cells (HEK293-T) transiently overexpressing human wild-type tau protein (2N4R). Thus, extracellular tau is detectable in neuronal (SH-SY5Y) and non-neuronal (HEK293-T) cell lines.
Figure 4.
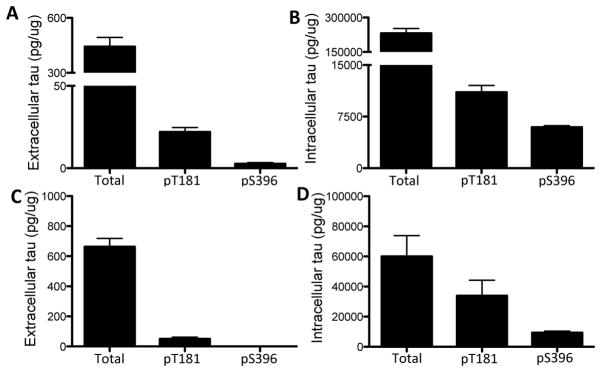
Measuring extracellular tau in cultured cells. A–B. Human neuronal SH-SY5Y cells express endogenous human tau. A. Extracellular tau was measured with ELISAs specific to total, pT181, and pS396 tau. B. Intracellular tau was measured with ELISAs specific to total, pT181, and pS396 tau. C–D. HEK293-T cells, which do not express tau, were transiently transfected with 1 ug human tau (longest isoform: 2N4R) for 24 hrs. C. Extracellular tau was measured with ELISAs specific to total, pT181, and pS396 tau. D. Intracellular tau was measured with ELISAs specific to total, pT181, pS396 tau. Values are expressed relative to total intracellular protein, as measured by a BCA assay. Image shown is representative of six replicate experiments.
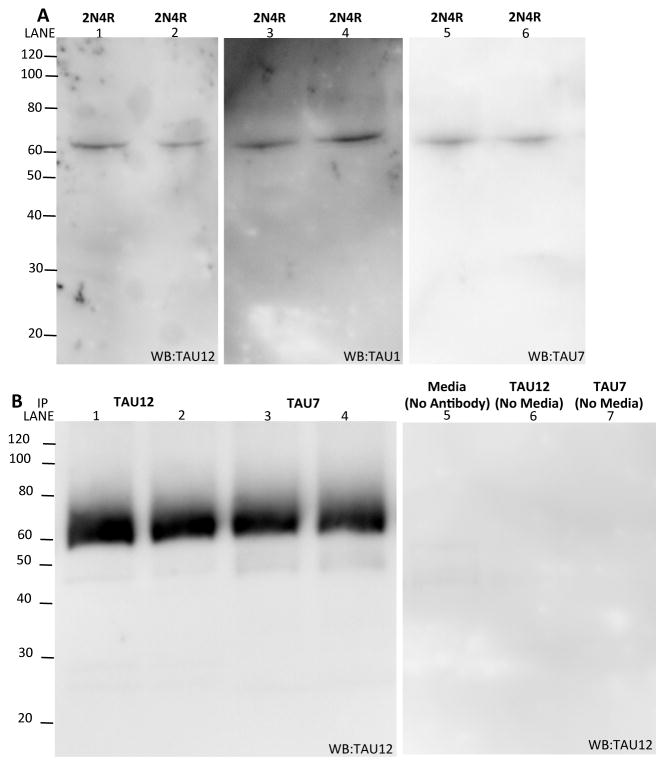
Truncated forms of intracellular tau are detectable in cell models (Wang, et al., 2007) and in tangles from autopsy confirmed AD brains (Gamblin, et al., 2003, Horowitz, et al., 2004). We sought to determine if truncated tau is present outside the cell. Media from cells expressing 2N4R tau for 24 hours was collected and analyzed by immunoblotting. We exclusively detected full-length tau in the media when immunoblotting with antibodies to the N-terminus (Tau12), central domain (Tau1), and C-terminus (Tau7) (Fig. 5A). To determine if truncated tau represents a much smaller fraction of the total extracellular tau that is not captured by direct immunoblotting, tau in the media was immunoprecipitated with antibodies directed to the N-terminus (Tau12) and C-terminus (Tau7) of the tau protein. Immunoblotting with an antibody directed to the N-terminus (Tau12) reveals that extracellular tau is full-length (Fig. 5B). Thus, we demonstrate that extracellular tau is predominantly full-length in cultured cells.
Figure 5.
Extracellular tau is predominantly full-length. A. Media from HEK293-T cells transiently expressing human tau (2N4R) was isolated and analyzed by SDS-PAGE. Immunoblots were probed with antibodies specific to the N-terminus (Tau12), central domain (Tau1), and C-terminus (Tau7) of tau. B. Media from HEK293-T cells expressing human tau (2N4R) was immunoprecipitated with antibodies specific to the N-terminus (Tau12) and C-terminus (Tau7) of tau. Immunoblots were probed with an antibody specific to the N-terminus (Tau12) of tau. Images shown are representative of four replicate experiments.
3.3 Calcineurin influences extracellular tau
Using this cell model, we sought to determine how calcineurin activity and protein levels affect extracellular tau. To address the contribution of calcineurin activity to extracellular total and ptau181 levels, SH-SY5Y cells, expressing endogenous human tau, were treated with compounds that reduce calcineurin activity: cyclosporin A (CsA) and FK506. CsA and FK506 reduce calcineurin activity (Fig. 6A) via alternate mechanisms without altering calcineurin protein levels (Luo, et al., 2008, Yu, et al., 2006b). CsA binds to cyclophylin and the resulting complex blocks calcineurin activity, while FK506 interacts with the FK506 binding protein to inhibit calcineurin activity. Treatment of SH-SY5Y cells with calcineurin inhibitors, CsA and FK506, for 6 hours produced a significant increase in intracellular ptau181 (Fig. 6B). The inhibition of calcineurin by CsA and FK506 resulted in a significant increase in extracellular total tau and ptau181 (Fig. 6C and 6D). Tau may be released into the media when cells undergo cell death; however, we did not observe cytotoxicity in cells treated with calcineurin inhibitors nor in cells treated with DMSO (Fig. 6E), indicating that it is unlikely that the observed effects are due to the passive release of tau by cell death.
Figure 6.
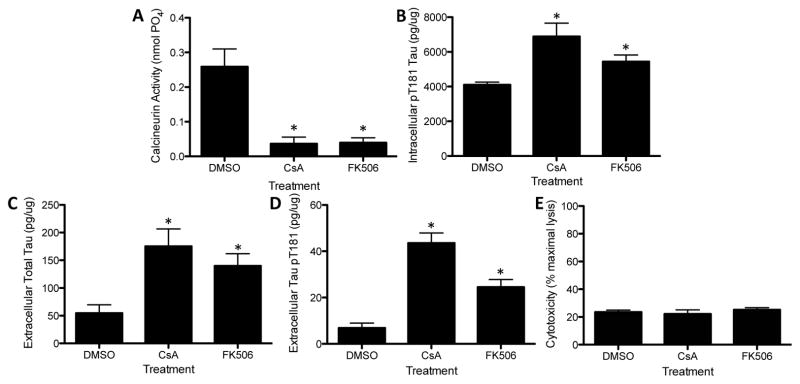
Inhibition of calcineurin activity in SH-SY5Y increases extracellular tau levels. Human neuronal SH-SY5Y cells were treated with CsA (10 uM), FK506 (10 uM), or a control compound (DMSO) for 6 hours. Media and cell lysates were analyzed for total tau and phospho-tau levels and for calcineurin activity. A. Calcineurin activity in cell lysates. B. Intracellular pT181 was measured in cell lysates with an ELISA specific to human pT181. C. Extracellular total tau was measured in cell media with an ELISA specific to human total tau. D. Extracellular pT181 was measured in cell media with an ELISA specific to human pT181. E. Calcineurin inhibitors do not induce cytotoxicity in cultured cells. Cytotoxicity was measured by LDH assay. * p<0.01. Image shown is representative of six replicate experiments.
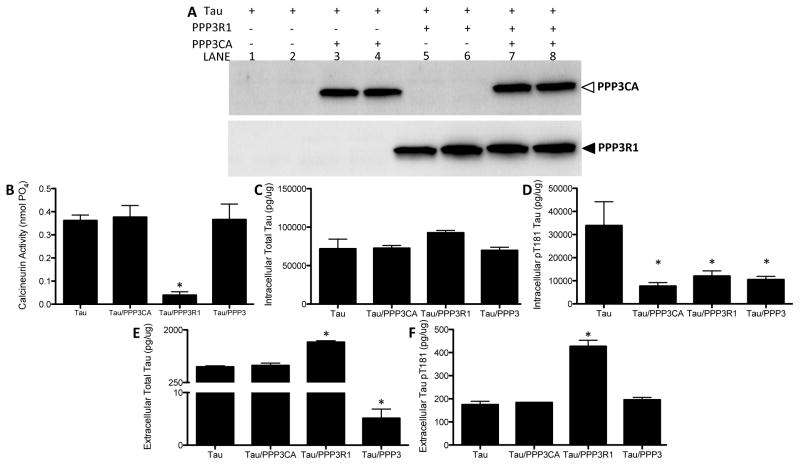
To determine how calcineurin protein levels contribute to changes in extracellular tau in our cell model, we overexpressed calcineurin in HEK293-T cells and measured intracellular and extracellular tau by ELISA. HEK293-T cells do not express tau; so, we transiently overexpressed a vector containing human 2N4R tau with vectors containing each calcineurin subunit (PPP3R1 and/or PPP3CA). To control for non-specific effects of the vector, 2N4R tau was co-transfected with a vector containing GFP. Transient co-expression of vectors containing calcineurin subunits or the control vector, GFP, produced similar levels of intracellular total tau protein levels (Fig. 7C). Overexpression of tau with the regulatory subunit of calcineurin (PPP3R1) resulted in a significant decrease in calcineurin activity (Fig. 7B). Calcineurin activity remained unchanged in cells overexpressing the catalytic subunit of calcineurin (PPP3CA) or both subunits of calcineurin (PPP3R1 and PPP3CA) (Fig. 7B). These results indicate that increasing PPP3R1 protein levels, which functions to inhibit the catalytic subunit, is sufficient to reduce calcineurin activity in the cell. Overexpression of the calcineurin subunits resulted in a significant decrease in intracellular ptau181 (Fig. 7D), without affecting intracellular total tau levels (Fig. 7C). Extracellular total and ptau181 were significantly elevated in cells expressing tau with PPP3R1 (Fig. 7E and 7F); however, in cells expressing both calcineurin subunits, extracellular total (Fig. 7E) but not ptau181 (Fig. 7F) was significantly reduced. Together, these findings demonstrate that altering calcineurin protein levels, with or without an accompanying change in calcineurin activity, can influence extracellular tau levels. Furthermore, our findings suggest that calcineurin may influence tau metabolism via a mechanism that is independent of the enzymatic activity of calcineurin.
Figure 7.
Overexpression of calcineurin subunits reduces extracellular tau levels. HEK293-T cells were transiently transfected with vectors containing human tau (2N4R) and GFP, PPP3R1, and/or PPP3CA cDNA. Media and cell lysates were collected and analyzed for total tau and phospho-tau levels. A. Cell lysates were analyzed by SDS-PAGE and immunoblots were probed with antibodies directed to calcineurin subunits. B. Calcineurin activity in cell lysates. C. Intracellular total tau was measured in cell lysates with an ELISA specific to human total tau. D. Intracellular pT181 was measured in cell lysates with an ELISA specific to human pT181. E. Extracellular total tau was measured in cell media with an ELISA specific to human total tau. F. Extracellular pT181 was measured in cell media with an ELISA specific to human ptau181. *p<0.01. Image shown is representative of four replicate experiments.
The regulatory and catalytic subunits of calcineurin are reported to directly interact with tau (Yu, et al., 2008). To determine if calcineurin subunits interact with tau in our cell system, cell lysates were immunoprecipitated with antibodies to tau (Tau12) or calcineurin (specific to both subunits). Immunoblots were then probed with antibodies to tau (Tau12) or calcineurin (specific to both subunits) (Fig. 8A–B). In this cell model, calcineurin interacts with tau. Thus, when calcineurin is expressed and active, it may be able to sequester tau within the cell.
Figure 8.
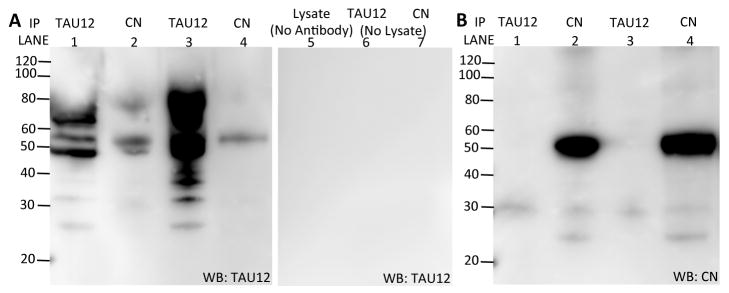
Calcineurin subunits directly interact with human tau. Cell lysates were immunoprecipitated with antibodies to tau (Tau12) and calcineurin. Immunoblots were probed with an antibody specific to tau (left panel) and calcineurin (right panel). Image shown is representative of three replicate experiments.
4. Discussion
We have previously demonstrated that the minor allele of a SNP in the regulatory subunit of calcineurin (PPP3R1- rs1868402) is associated with elevated levels of CSF ptau181, with decreased PPP3R1 mRNA expression in the brain, and with increased Braak tangle stage (Cruchaga, et al., 2010). In this study, we demonstrate that PPP3R1 and PPP3CA protein levels are inversely correlated with CDR and Braak tangle stage in human brain tissue. Furthermore, calcineurin activity is significantly reduced in AD brains compared with cognitively normal controls after correcting for total cell loss. Reducing calcineurin activity in immortalized neuroblastoma cells results in increased levels of total and phospho-tau in the media from cultured cells. These findings are consistent with our observations in AD patients, who have elevated CSF tau and significantly lower calcineurin activity in brain extracts.
The minor allele of rs1868402 in PPP3R1 is associated with decreased PPP3R1 mRNA in the brain and with increased Braak tangle stage (Cruchaga, et al., 2010). We failed to detect a significant association between rs1868402 and PPP3R1 protein levels or calcineurin activity; however, our study may be underpowered (0.054 and 0.056 power, respectively), masking more subtle effects of the SNP on protein levels and protein function. Additionally, because the rs18648402 polymorphism lies in an intronic region of PPP3R1, it is unlikely that this SNP represents the true functional polymorphism. Identification of the functional polymorphism will be essential to further elucidate the mechanism by which calcineurin contributes to AD pathology.
Calcineurin is an interesting target in AD because of its critical role in learning and memory (reviewed in (Rusnak and Mertz, 2000)). Overexpression of calcineurin in mice results in altered synaptic function and deficits in memory retention (Kayyali, et al., 1997). However, the role of calcineurin in the brain may be very complicated. Knockdown of calcineurin activity facilitates PKA dependent long-term potentiation and increased learning and memory (Malleret, et al., 2001). Yet, completely knocking-out calcineurin in Purkinje cells or in the anterior cortex impairs postsynaptic long-term potentiation and worsens cognition (Schonewille, et al., 2010, Zeng, et al., 2001). Additionally, in transplant patients, long-term treatment with CsA, a calcineurin inhibitor and immunosuppressant, results in reversible dementia (Bertoli, et al., 1988). Together, these studies demonstrate that tight regulation of calcineurin protein levels and activity in the brain is required to maintain normal learning and memory.
Calcineurin activity is reportedly altered in AD; however, studies differ as to whether calcineurin activity is upregulated or downregulated in AD brains. Lian and colleagues described reduced calcineurin activity in the frontal cortex of AD brains, which was inversely correlated with neurofibrillary tangles after correcting for total protein (Lian, et al., 2001). More recently, however, Lui et al. described increased calcineurin activity in the hippocampus of AD brains in response to the addition of Ca2+/calmodulin (Liu, et al., 2005). Using a highly sensitive method to measure total phosphatase and calcium-dependent phosphatase activity by the conversion of a phosphopeptide substrate in parietal lobe tissue from 73 individuals with AD, we observed a significant decrease in calcineurin activity after correcting for total brain protein levels. However, correcting for neuron number resulted in the opposite effect: calcineurin activity levels were significantly elevated in AD brains. These findings may provide insight into the inconsistencies in the literature regarding calcineurin activity in the brain. Correcting calcineurin activity for total protein produced a value that is reflective of the global reduction of calcineurin activity in AD brains. Growing evidence suggests that protein changes in the CSF are reflective of global changes in the brain, rather than regional or cell specific changes (Bero, et al., 2011, Yamada, et al., 2011). Thus, we interpret a global reduction of calcineurin activity in AD brains to be correlated with elevated CSF total/ptau181. Our findings that calcineurin activity is elevated in AD brains after correcting for neuronal number is surprising given that PPP3R1 and PPP3CA protein levels are significantly lower in AD brains after correcting for total or neuron number. However, the reported increase in calcineurin activity in AD brains in response to the addition of calcineurin activators suggests that calcineurin proteins are more responsive in diseased brains (Liu, et al., 2005). Thus, we hypothesize that elevated calcineurin activity in the surviving neurons of AD brains reflects an aberrant gain of toxic function that results in constitutively active calcineurin.
Calcineurin is expressed in astrocytes. The regulation of calcineurin activity in astrocytes is reportedly altered in AD (Norris, 2005). Our observations that calcineurin activity differs in AD brains after correcting for total or neuronal cell populations suggests that calcineurin activity in cell types other than neurons contributes to pathological and clinical aspects of AD. Previous observations in astrocytes (Norris, 2005) taken with the neuronal specific effects on calcineurin activity that we observed in this study leads us to conclude that the cell-type specific effects of regulation and function of calcineurin is altered in AD brains.
In this study, calcineurin protein levels, both the catalytic and regulatory protein subunits, were inversely correlated with clinical (CDR) and pathological (Braak tangle stage) measures. Exposure of primary neurons to Aβ42 results in reduced calcineurin expression levels (Celsi, et al., 2007), which fits with our findings in autopsy brain tissue. Abdul and colleagues demonstrate that the catalytic subunit of calcineurin is translocated to the nuclear fraction in a small number of mild cognitive impairment (MCI) and AD brains without a change in the total calcineurin levels (Abdul, et al., 2009). However, we did not examine subcellular changes in calcineurin protein levels in this study.
CSF total tau/ptau181 levels increase in patients with AD (Galasko, et al., 1998, Vigo-Pelfrey, et al., 1995). CSF total tau is increased in other neurodegenerative diseases including stroke, frontotemporal dementia, corticobasal degeneration, and Creutzfeldt-Jacob disease (Itoh, et al., 2001); however, increases in CSF ptau181 and other phospho-epitopes is specific to AD (Buerger, et al., 2002, Hampel and Teipel, 2004, Itoh, et al., 2001, Kohnken, et al., 2000). Thus, cells in the brain aberrantly release total and phospho-tau in AD either by a passive mechanism, such as cell death, or by an unknown active mechanism. The presence of total and phospho-tau in the CSF of healthy individuals suggests that tau (both total and phospho-tau) is actively released by the cell. We hypothesize that tau is actively released by the cell and that genetic factors enriched in AD patients alter the release of tau from cells in the brain.
To model our observations in this study, we sought to use a cell system in which extracellular tau can be measured and quantified. Tau is an intracellular, microtubule-binding protein; however, we can detect tau in the CSF of healthy individuals and mouse ISF (Yamada, et al., 2011), and more recently, Kim and colleagues have described extracellular tau in human neuroblastoma cells measured by immunoblotting concentrated media (Kim, et al., 2010a). We were able to detect extracellular tau, total and phospho-tau, in human neuroblastoma cells (SH-SY5Y) and in non-neuronal cells (HEK293-T) transiently expressing human wild-type tau. These cell models can provide us with powerful tools to model the impact of genetic variants, identified in human studies, on extracellular tau.
Mouse and cell models of calcineurin inhibition produce changes in phospho-tau. Calcineurin knockout mice exhibit increased hyperphosphorylated tau in the hippocampus (Kayyali, et al., 1997). In non-transgenic mice, reduction of calcineurin protein and activity by anti-sense oligonucleotides also results in increased phosphorylation at Thr181 and Thr231 in tau (Garver, et al., 1999). Inhibition of calcineurin with CsA or FK506 treatment in Tg2576 mice results in the reduction of calcineurin activity and enhanced tau phosphorylation at Ser262, Ser198, Ser199, Ser202, Ser396, and Ser404 (Luo, et al., 2008, Yu, et al., 2006a). In our cell model, upon treatment of cells with calcineurin inhibitors, we detected increases in intracellular ptau181 and extracellular total and ptau181. Our observations in this cell model mimicked our observations in human studies, where reduced calcineurin activity in the brain is associated with hyperphosphorylated tau pathology in the brain and increased levels of total tau and ptau181 in the CSF.
Calcineurin may sequester tau within the cell in two ways: (1) by facilitating microtubule binding by dephosphorylating tau and (2) by binding to tau. Tau interacts with microtubules when it is dephosphorylated. We hypothesize that when tau is bound to and stabilizing microtubules, it is unable to be released by the cell. Our observation that the inhibition of calcineurin produces an increase in extracellular tau compared with controls fit this hypothesis. Inhibition of calcineurin results in an increase in phospho-tau in the cell without changing total tau protein levels. This increase in phospho-tau, mediated by the inhibition of calcineurin, could result in a smaller pool of microtubule-bound tau and a larger pool of unbound, soluble tau in the cell. Calcineurin may also sequester tau within the cell by direct or indirect interaction. In this study and in others (Yu, et al., 2008), we demonstrate that calcineurin can interact with tau within the cell. Furthermore, we found that overexpressing calcineurin in HEK293-T cells increased calcineurin protein levels without affecting overall activity. When both calcineurin subunits were overexpressed, we observed a significant decrease in extracellular total tau. Thus, increasing calcineurin protein levels is sufficient to sequester tau within the cell.
Recent evidence in cell and mouse models suggests that tau aggregates propagate between cells (Clavaguera, et al., 2009, Frost, et al., 2009). Additionally, Gomez-Ramos and colleagues have demonstrated that treatment of cells with exogenous, recombinant wild-type tau induces increases in intracellular calcium and cytotoxicity via the M1 and M3 muscarinic receptors (Gómez-Ramos, et al., 2008). Together, this evidence suggests that once outside the cell, tau has the ability to induce cellular abnormalities. Thus, the ability of the cell to maintain a balance between the levels of tau protein inside and outside the cell, whether by calcineurin or other genetic factors, may be critical to normal cell function.
Acknowledgments
We thank the Neuropathology and Clinical Cores of the Charles F. and Joanne Knight Alzheimer’s Disease Research Center for generously sharing brain samples, clinical data, and pathological data. We also thank Dr. Virginia Lee and Dr. Lester Binder for providing reagents and Dr. Carlos Cruchaga for thoughtful advice. This work was supported by AstraZeneca and National Institutes of Health [P01AG003991 and P50AG005681 to John C. Morris].
Footnotes
Disclosure Statement
This work was supported by a research grant from AstraZeneca.
References
- Abdul HM, Sama MA, Furman JL, Mathis DM, Beckett TL, Weidner AM, Patel ES, Baig I, Murphy MP, LeVine H, Kraner SD, Norris CM. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J Neurosci. 2009:12957–69. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H. Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain pathology. 2008;18(4):484–96. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C, Lee VM-Y, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007:663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nature neuroscience. 2011;14(6):750–6. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoli M, Romagnoli GF, Margreiter R. Irreversible dementia following cyclosporin therapy in a renal transplant patient. Nephron. 1988;49(4):333–4. doi: 10.1159/000185087. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta neuropathologica. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttilä T, Schapiro MB, Rapoport SI, Möller H-J, Davies P, Hampel H. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002:1267–72. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- Celsi F, Svedberg M, Unger C, Cotman CW, Carrì MT, Ottersen OP, Nordberg A, Torp R. Beta-amyloid causes downregulation of calcineurin in neurons through induction of oxidative stress. Neurobiology of Disease. 2007:342–52. doi: 10.1016/j.nbd.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, Fraser G, Stalder AK, Beibel M, Staufenbiel M, Jucker M, Goedert M, Tolnay M. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer’s disease. Neurobiology of Disease. 2009:128–40. doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruchaga C, Kauwe JSK, Mayo K, Spiegel N, Bertelsen S, Nowotny P, Shah AR, Abraham R, Hollingworth P, Harold D, Owen MM, Williams J, Lovestone S, Peskind ER, Li G, Leverenz JB, Galasko D, Morris JC, Fagan AM, Holtzman DM, Goate AM Initiative AsDN. SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer’s disease. PLoS Genet. 2010 doi: 10.1371/journal.pgen.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Steiner JP, Lyons WE, Fotuhi M, Blue M, Snyder SH. The immunophilins, FK506 binding protein and cyclophilin, are discretely localized in the brain: relationship to calcineurin. Neuroscience. 1994:569–80. doi: 10.1016/0306-4522(94)90389-1. [DOI] [PubMed] [Google Scholar]
- Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009:12845–52. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D, Chang L, Motter R, Clark CM, Kaye J, Knopman D, Thomas R, Kholodenko D, Schenk D, Lieberburg I, Miller B, Green R, Basherad R, Kertiles L, Boss MA, Seubert P. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998:937–45. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003:10032–7. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver TD, Kincaid RL, Conn RA, Billingsley ML. Reduction of calcineurin activity in brain by antisense oligonucleotides leads to persistent phosphorylation of tau protein at Thr181 and Thr231. Molecular Pharmacology. 1999:632–41. [PubMed] [Google Scholar]
- Gómez-Ramos A, Díaz-Hernández M, Rubio A, Miras-Portugal MT, Avila J. Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci. 2008:673–81. doi: 10.1016/j.mcn.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Goto S, Matsukado Y, Mihara Y, Inoue N, Miyamoto E. Calcineurin in human brain and its relation to extrapyramidal system. Immunohistochemical study on postmortem human brains. Acta Neuropathol. 1986:150–6. doi: 10.1007/BF00685977. [DOI] [PubMed] [Google Scholar]
- Hampel H, Teipel SJ. Total and phosphorylated tau proteins: evaluation as core biomarker candidates in frontotemporal dementia. Dement Geriatr Cogn Disord. 2004:350–4. doi: 10.1159/000077170. [DOI] [PubMed] [Google Scholar]
- Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E, Blennow K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neuroscience letters. 2001;297(3):187–90. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci. 2004:7895–902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Arai H, Urakami K, Ishiguro K, Ohno H, Hampel H, Buerger K, Wiltfang J, Otto M, Kretzschmar H, Moeller HJ, Imagawa M, Kohno H, Nakashima K, Kuzuhara S, Sasaki H, Imahori K. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer’s disease. Ann Neurol. 2001:150–6. doi: 10.1002/ana.1054. [DOI] [PubMed] [Google Scholar]
- Kauwe JSK, Cruchaga C, Mayo K, Fenoglio C, Bertelsen S, Nowotny P, Galimberti D, Scarpini E, Morris JC, Fagan AM, Holtzman DM, Goate AM. Variation in MAPT is associated with cerebrospinal fluid tau levels in the presence of amyloid-beta deposition. Proceedings of the National Academy of Sciences. 2008:8050–4. doi: 10.1073/pnas.0801227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayyali US, Zhang W, Yee AG, Seidman JG, Potter H. Cytoskeletal changes in the brains of mice lacking calcineurin A alpha. Journal of Neurochemistry. 1997:1668–78. doi: 10.1046/j.1471-4159.1997.68041668.x. [DOI] [PubMed] [Google Scholar]
- Kim W, Lee S, Hall GF. Secretion of human tau fragments resembling CSF-tau in Alzheimer’s disease is modulated by the presence of the exon 2 insert. FEBS Lett. 2010a:3085–8. doi: 10.1016/j.febslet.2010.05.042. [DOI] [PubMed] [Google Scholar]
- Kim W, Lee S, Jung C, Ahmed A, Lee G, Hall GF. Interneuronal transfer of human tau between Lamprey central neurons in situ. J Alzheimers Dis. 2010b:647–64. doi: 10.3233/JAD-2010-1273. [DOI] [PubMed] [Google Scholar]
- Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, DeBernardis J, Shen J, Möller HJ, Davies P, Hampel H. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett. 2000:187–90. doi: 10.1016/s0304-3940(00)01178-2. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lian Q, Ladner CJ, Magnuson D, Lee JM. Selective changes of calcineurin (protein phosphatase 2B) activity in Alzheimer’s disease cerebral cortex. Experimental Neurology. 2001:158–65. doi: 10.1006/exnr.2000.7534. [DOI] [PubMed] [Google Scholar]
- Liu F, Grundke-Iqbal I, Iqbal K, Oda Y, Tomizawa K, Gong C-X. Truncation and activation of calcineurin A by calpain I in Alzheimer disease brain. J Biol Chem. 2005:37755–62. doi: 10.1074/jbc.M507475200. [DOI] [PubMed] [Google Scholar]
- Luo J, Ma J, Yu D-y, Bu F, Zhang W, Tu L-H, Wei Q. Infusion of FK506, a specific inhibitor of calcineurin, induces potent tau hyperphosphorylation in mouse brain. Brain Res Bull. 2008:464–8. doi: 10.1016/j.brainresbull.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Malleret G, Haditsch U, Genoux D, Jones MW, Bliss TV, Vanhoose AM, Weitlauf C, Kandel ER, Winder DG, Mansuy IM. Inducible and reversible enhancement of learning, memory, and long-term potentiation by genetic inhibition of calcineurin. Cell. 2001:675–86. doi: 10.1016/s0092-8674(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Tau in Alzheimer’s disease. Trends Cell Biol. 1998:425–7. doi: 10.1016/s0962-8924(98)01368-3. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Norris CM. Calcineurin Triggers Reactive/Inflammatory Processes in Astrocytes and Is Upregulated in Aging and Alzheimer’s Models. Journal of Neuroscience. 2005:4649–58. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost M, Nylen K, Csajbok L, Ohrfelt AO, Tullberg M, Wikkelso C, Nellgard P, Rosengren L, Blennow K, Nellgard B. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67(9):1600–4. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- Polli JW, Billingsley ML, Kincaid RL. Expression of the calmodulin-dependent protein phosphatase, calcineurin, in rat brain: developmental patterns and the role of nigrostriatal innervation. Brain Res Dev Brain Res. 1991:105–19. doi: 10.1016/0165-3806(91)90071-p. [DOI] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000:1483–521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, Hosy E, Hoebeek FE, Elgersma Y, Hansel C, De Zeeuw CI. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010:618–28. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Kay K, Matsouka R, Rozkalne A, Betensky RA, Hyman BT. Calcineurin inhibition with systemic FK506 treatment increases dendritic branching and dendritic spine density in healthy adult mouse brain. Neurosci Lett. 2011:260–3. doi: 10.1016/j.neulet.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigo-Pelfrey C, Seubert P, Barbour R, Blomquist C, Lee M, Lee D, Coria F, Chang L, Miller B, Lieberburg I. Elevation of microtubule-associated protein tau in the cerebrospinal fluid of patients with Alzheimer’s disease. Neurology. 1995:788–93. doi: 10.1212/wnl.45.4.788. [DOI] [PubMed] [Google Scholar]
- Wang YP, Biernat J, Pickhardt M, Mandelkow E, Mandelkow E-M. Stepwise proteolysis liberates tau fragments that nucleate the Alzheimer-like aggregation of full-length tau in a neuronal cell model. Proc Natl Acad Sci USA. 2007:10252–7. doi: 10.1073/pnas.0703676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, Binder LI, Mandelkow EM, Diamond MI, Lee VM, Holtzman DM. In Vivo Microdialysis Reveals Age-Dependent Decrease of Brain Interstitial Fluid Tau Levels in P301S Human Tau Transgenic Mice. J Neurosci. 2011;31(37):13110–7. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D-y, Luo J, Bu F, Song G-J, Zhang L-q, Wei Q. Inhibition of calcineurin by infusion of CsA causes hyperphosphorylation of tau and is accompanied by abnormal behavior in mice. Biological Chemistry. 2006a:977–83. doi: 10.1515/BC.2006.121. [DOI] [PubMed] [Google Scholar]
- Yu D-y, Luo J, Bu F, Zhang W, Wei Q. Effects of cyclosporin A, FK506 and rapamycin on calcineurin phosphatase activity in mouse brain. IUBMB Life. 2006b:429–33. doi: 10.1080/15216540600791555. [DOI] [PubMed] [Google Scholar]
- Yu D-y, Tong L, Song G-J, Lin W-l, Zhang L-q, Bai W, Gong H, Yin Y-x, Wei Q. Tau binds both subunits of calcineurin, and binding is impaired by calmodulin. Biochim Biophys Acta. 2008:2255–61. doi: 10.1016/j.bbamcr.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001:617–29. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]