Abstract
Context
Alzheimer’s disease (AD) is usually only diagnosed many years after pathology begins. Earlier detection would allow emerging interventions to have a greater chance to preserve healthy brain function. A rare form of Alzheimer’s disease, caused by autosomal-dominant mutations, affects carriers with 100% certainty and at a younger age specific to their mutation. Studying families with these mutations allows a unique investigation of the temporal sequence of biomarker changes in Alzheimer’s disease.
Objective
To determine whether the pupil flash response (PFR), previously reported to be altered in sporadic Alzheimer’s disease, is different in pre-symptomatic mutation carriers.
Design
Researchers blinded to participant mutation status collected pupil response data from cognitively normal participants in the Dominantly Inherited Alzheimer's Network (DIAN) Study during 2010–2011.
Setting
The pupil response was examined at the McCusker Alzheimer’s Research Foundation in Perth, Western Australia.
Participants
Participants were from a single family harboring an Amyloid-Beta Precursor Protein genetic mutation (APPGlu693Gln). Six carriers and six non-carriers were available for pupil testing (age 43.0±8.3 years old, 2 males and 10 females, 4 with hypertension).
Main Outcome Measure
Pupil response parameter comparison between mutation carriers and non-carriers.
Results
75% recovery time was longer in mutation carriers (p<0.0003, ROC AUC 1.000, Sensitivity 100%, Specificity 100%) and percentage recovery 3.5 seconds after stimulus was less in mutation carriers (p<0.006, ROC AUC 1.000, Sensitivity 100%, Specificity 100%).
Conclusions
PFR changes occur pre-symptomatically in autosomal dominant AD mutation carriers, supporting further investigation of PFR for early detection of AD.
Keywords: Alzheimer’s, autosomal, amyloid, eye, pupil
Introduction
A rare form of Alzheimer’s disease (AD)"autosomal dominant AD” (ADAD), affects carriers of specific gene mutations with penetration approaching 100% [1]. ADAD represents about 1% of all AD cases and can occur in people as young as 30 years of age. It is a genetic disorder with specific mutations in primarily amyloid precursor protein (AβPP), presenilin 1 and presenilin 2 genes known to cause the disease [1–4]. It is inherited in an autosomal dominant manner. Children of affected individuals have a fifty-percent risk to develop AD dementia, with a relatively predictable age at onset within each family.
Despite the difference in underlying cause and age of onset, ADAD and the more common sporadic AD have similar neuropathologic hallmarks and clinical features [5]. Evidence suggests that AD related pathological changes begin at least 15 years before the symptomatic stage [6], providing a window of time in which to detect the disease early and allow emerging therapies the chance to preserve healthy brain function. Hence, new knowledge about the disease process in ADAD has the potential to translate into better methods for early detection of sporadic AD.
Biomarkers of early AD pathology (amyloid plaques and neurofibrillary tangles) are currently being sought in order to confirm early diagnosis, before irreversible neurological damage occurs. Early changes to vision reported in AD have motivated investigations of biomarkers that might exist in the eye [7–9]. The pupil flash response (PFR), the response of the pupil size to a bright flash of light, is one ocular test that has previously been reported to be altered in sporadic AD [10–15].
Studies of families harboring known ADAD mutations provide a powerful opportunity to investigate the temporal sequence of ocular and other AD biomarker changes during disease progression. Studying pre-symptomatic individuals with ADAD mutations alleviates many problems inherent in studies of pre-symptomatic sporadic AD, including uncertainty about disease progression. In addition, the early age of onset in ADAD means that these studies will be less confounded by age-related co-morbidities such as hypertension and cardio-vascular disease.
Here we investigate ocular biomarkers in ADAD by utilizing the Dominantly Inherited Alzheimer Network (DIAN) study cohort [5]. Results from the DIAN study have already established that AD-related cerebro-spinal fluid (CSF) pathology can be detected in mutation carriers more than two decades before their estimated age at dementia onset [6]. Studying AD biomarkers in ADAD could prove useful for the development and evaluation of disease-modifying prevention measures in both ADAD and sporadic AD populations.
Most DIAN study participants are in the pre-symptomatic stages of the disease and hence provide a unique means by which to investigate the time-course of biomarker changes in the lead-up to clinical expression of the disease. Since the parental age of dementia onset within each family is typically known, the stage of disease progression for pre-symptomatic family members (e.g. 10 years prior to estimated age of dementia onset) can be accurately estimated and used as a temporal reference for biomarker changes. This also enables comparison of biomarker changes across families with different mutations and typical age of onset.
Pupil Responses in Alzheimer’s Disease
The pupil controls retinal illumination and responds dynamically to a bright light flash by rapidly constricting and then re-dilating. The neurotransmitters responsible for these two processes are acetyl-choline (Ach; constriction) and norepinephrine (NE; dilation). As the primary neurotransmitter deficit in AD is acetyl-choline [16, 17], pupil responses have become a research topic for AD diagnosis and monitoring.
During the 1990s a number of studies investigated the influence of AD on the effects of pharmacological drugs delivered for pupil constriction or dilation. A hypersensitive pupil response to a cholinergic agonist (pilocarpine - constriction) or antagonist (tropicamide - dilation) was reported for AD patients [18–27]. However, other reports were confounding [12, 28–37], possibly due to problems with stability of eye-drop solutions and variation in corneal penetration.
As an alternative, PFR was investigated, since it is not reliant on delivery of drugs to the eye. Changes to a number of PFR parameters have been found in AD compared to healthy ageing [10–15], with a single parameter (reduced “maximum constriction acceleration”) facilitating perfect classification in one study [13]. The results from these studies are summarized in Table 1.
Table 1.
Summary of published reports of PFR changes in AD.
| Reference | N (AD, HC) |
Parameters | Change in AD |
p | Participants and Medications |
|---|---|---|---|---|---|
| (Prettyman et al., 1997) [11] | 9,9 | Resting pupil diameter | − | 0.041 | All participants medication-free. |
| Constriction Amplitude | − | <0.002 | |||
| 75% Recovery time | − | 0.034 | |||
| Constriction Latency | NS | ||||
| (Ferrario et al., 1998) [14] | 20,44 | Resting pupil diameter | + | <0.05 | No anticholinesterase drugs. |
| Minimum pupil diameter | NS | ||||
| Constriction percentage | NS | ||||
| Constriction latency | + | <0.05 | |||
| Latency to half contraction | NS | ||||
| Latency to minimum pupil size | NS | ||||
| 63% recovery time | NS | ||||
| Maximum constriction velocity | NS | ||||
| Maximum dilation velocity | NS | ||||
| Maximum constriction acceleration | + | <0.05 | |||
| (Fotiou et al., 2000) [10] | 10,5 | Constriction latency | NS | Medication-free participant results reported. Additional donepezil-treated-AD group exhibited similar but non-significant trends. | |
| Constriction amplitude | NS | ||||
| Latency to minimum pupil size | − | 0.000 | |||
| No. of oscillations | − | 0.000 | |||
| (Tales et al., 2001) [15] | 12,12 | Constriction latency | + | NS | Medication free for 2 months, diabetes excluded. |
| Constriction percentage | − | <0.05 | |||
| (Granholm et al., 2003) [12] | 15,15 | Resting pupil diameter | NS | Trend with donepezil-treated-AD having shorter latency-to-minimum-pupil size than AD without treatment. Constriction amplitude was similar for treated/untreated AD. | |
| Constriction amplitude | − | <0.05 | |||
| Latency to minimum pupil size | NS | ||||
| (Fotiou et al., 2007) [13] | 23,23 | Resting pupil diameter | NS | No cardiac glycosides, anticholinergics, sympathomimetics, beta-blockers. | |
| Minimum pupil diameter | NS | ||||
| % Recovery 3.5sec after stimulus | + | <0.001 | |||
| Constriction latency | + | <0.001 | |||
| Latency to max velocity | + | <0.001 | |||
| Latency to minimum pupil size | + | <0.001 | |||
| Max constriction velocity | − | <0.001 | |||
| Maximum constriction acceleration | − | <0.001 | |||
| Constriction amplitude | − | <0.001 | |||
| Constriction amplitude percentage | + | <0.001 |
The majority of the results indicate a ‘sluggish’ PFR in AD, with reduced velocities, accelerations and constriction amplitude, and increased latencies. Similar results have been observed in a larger cohort by our group. The result from Ferrario et al. on increased maximum constriction acceleration in AD stands in contrast, possibly due to different PFR methodology and participant selection. However, consistent with other reports, constriction latency was larger in AD. Some results indicate a faster recovery after stimulus in AD, despite slower constriction and dilation velocities, most likely attributable to the reduced amplitude [11, 13].
Some studies have found that Parkinson’s disease patients exhibit similar PFR changes [12, 38], hence more research is required to confirm the sensitivity and specificity of each of the PFR parameters (or a combined panel) as a screening tool for AD. Here, we investigate the PFR in ADAD mutation carriers, who exhibit similar neuropathological hallmarks and clinical features as sporadic AD[5]. To the authors’ knowledge, no other study has published results on PFR in ADAD mutation carriers.
Subjects and Methods
Participants
Participants for the PFR study were recruited from the DIAN study (DIAN, NIA U19 AG032438, JC Morris, PI) at the McCusker Foundation for AD Research, Perth, Western Australia (www.dian-info.org, clinicaltrials.gov number NCT00869817). DIAN study procedures are published elsewhere (Morris et. al. 2012, in press) [39] and were approved by the Washington University Human Research Protection Office and also the Hollywood Private Hospital Ethics Committee. All PFR experiments were approved by the Ethics Committee of the University of Western Australia. All participants provided their written informed consent for this study.
Exclusion criteria for the PFR study were past history of ocular operations or ophthalmological disease, asymmetrical pupils, receiving anticholinergics, cardiac glycosides, sympathomimetics or ophthalmic agents affecting PFR.
All participants were white Caucasians from a single family harbouring Dutch cerebral amyloid angiopathy (hereditary cerebral hemorrhage with amyloidosis - Dutch type, HCHWA-D). The APP 693 mutation at position 22 of the amyloid-beta fragment (APPGlu693Gln), resulting in a glutamine for glutamic acid, was identified in the proband and his affected sister who died at age 61 and 66 respectively, from recurrent lobar hemorrhages in the brain. Neuritic amyloid plaques were found but neither dementia nor cognitive decline have been diagnosed in this family, possibly due to earlier onset of vascular pathology.
The mean paternal anticipated age of onset (AAO) is 51. All but 2 of the 12 were younger than the parental AAO at time of PFR, and 8 of the 12 were within 15 years younger than the parental AAO. All participants performed above the mini-mental state examination (MMSE) [40] cut off score for dementia (>24), indicating normal cognitive function. The mean MMSE scores were 29.0±0.9 for MC, 27.5±2.1 for NC. Participants were neuroimaged for the presence of fibrillar brain amyloid using positron emission tomography (PET) with Pittsburgh Compound B (PiB) [41, 42]. Results for neocortical standardized uptake value ratio (SUVR) were calculated.
System of Pupillometry
The PFR was collected using a NeurOptics™ VIP™-200 Pupillometer. This is a commercial, monocular device providing fully automated operation and calculation of response parameters. The device produces a white flash stimulus and then measures the pupil size for 5 seconds using infrared illumination. The video frame rate is 33Hz, the stimulus/pulse intensity is 180µW and the stimulus/pulse duration is 31 milliseconds. The pupillometer produces diffuse light over the whole visual field.
The room was darkened for 2 minutes prior to testing. The test was practiced once before recording, all participants performed the experiment with proper cooperation. Occasionally, an extra trial was needed to achieve a recording without blinks or artefacts. Data was rejected if artefacts were present. The right eye was used for all participants. The pupillometer provided automatic calculation of the following eight parameters; resting pupil diameter (mm), minimum pupil diameter (mm), response latency (msec), mean constriction velocity (mm/sec), maximum constriction velocity (mm/sec), mean dilation velocity (mm/sec), 75% recovery time (sec) and constriction percentage (%) relative to initial pupil size. A record of the pupil's diameter as a function of time was exported from the pupillometer. Four extra parameters (constriction amplitude (mm), maximum constriction acceleration (mm/sec), latency to maximum constriction (sec) and percentage recovery 3.5 seconds after stimulus (%)) were calculated from the exported PFR data. PFR trials with artefacts or excessive blinking were discarded. A computer algorithm was used to remove minor blinks.
Genetic Analysis
Genotyping for familial AD mutations and APOE isoforms was performed by the DIAN Genetics Core. Ambient blood samples were shipped from the DIAN performance sites to both the National Cell Repository for Alzheimer’s Disease (NCRAD) and the DIAN Genetics Core at Washington University. DNA was extracted from blood at both sites using standard procedures. DNA sequencing of AβPP, presenilin 1 and presenilin 2 genes was performed by DIAN Genetics Core personnel, using Sanger sequencing methods on an ABI 3130xl, to determine the presence/absence of a disease-causing mutation [43]. APOE genotyping was performed using an ABI predesigned real time TaqMan assay “rs7412 & rs429358” according to the manufacturer’s protocol (ABI, Foster City, CA). DNA fingerprinting was performed with the Cell ID kit, using short tandem repeat (STR) analysis of 10 specific loci in the human genome, nine STR loci and Amelogenin for gender identification (Promega #G9500, Madison, WI) in order to confirm that DNA samples obtained by NCRAD and the DIAN Genetics Core were from the same individual. DNA sequencing, fingerprinting and genotyping was performed on DNA from NCRAD and the DIAN Genetics Core in parallel for each individual, and the data were compared for quality control purposes. All individuals included in this analysis have 100% concordant data for each DNA sample.
Statistical analysis
Demographic analysis between mutation carrier (MC) and non-carrier (NC) groups was performed using a chi square (χ2) test for categorical variables (gender and APOE ϵ4 carrier status), and analysis of variance (ANOVA) for the continuous variables (age and MMSE). ANOVA was also used to compare SUVR between groups.
PFR scores for the MC and NC groups were compared using analysis of variance (ANCOVA) correcting for age, gender and APOE ϵ4 status. Since 12 PFR parameters were analysed, the possibility of false positive results was minimised using the false discovery rate (FDR) method [44].
Receiver-operating characteristic (ROC) curve analysis was also performed to further illustrate the classification accuracy of the PFR parameters. The area under the curve (AUC) of the ROC curves was estimated, an AUC of 1 indicates perfect classification ability into MC or NC, whereas an AUC near 0.5 indicates poor (random) classification ability. All statistical analyses were conducted in XLstat 2011 (Microsoft Excel).
RESULTS
The demographic characteristics of the MC and NC groups are described in Table 2. Groups were not significantly different in age, gender or APOE ϵ4 carrier status. Neocortical plaque burden (Standardised Uptake Value Ratio, SUVR) was higher in the MC group (1.23±0.08) than the NC group (1.08±0.04) (p=0.004).
Table 2.
Demographic characteristics of the mutation carrier and non-carrier groups.
| Mutation Carriers | Non-Carriers | p | |
|---|---|---|---|
| Number of Participants: [N] | 6 | 6 | |
| Age: Years [mean (SD)] | 46.0 ± 5.7 | 40.0 ± 10.0 | 0.229 |
| Gender; Males: [N (%)] | 5 (83%) | 5 (83%) | 1.000 |
| Hypertension: [N (%)] | 2 (33.3%) | 2 (33.3%) | 1.000 |
| APOE ϵ4 Carrier: [N (%)] | 2 (33.3%) | 1 (16.6%) | 0.512 |
| MMSE: [mean ± SD] | 29.0 ± 0.9 | 27.5 ± 2.1 | 0.135 |
| SUVR: [mean ± SD] | 1.23 ± 0.08 | 1.08 ± 0.04 | 0.004 |
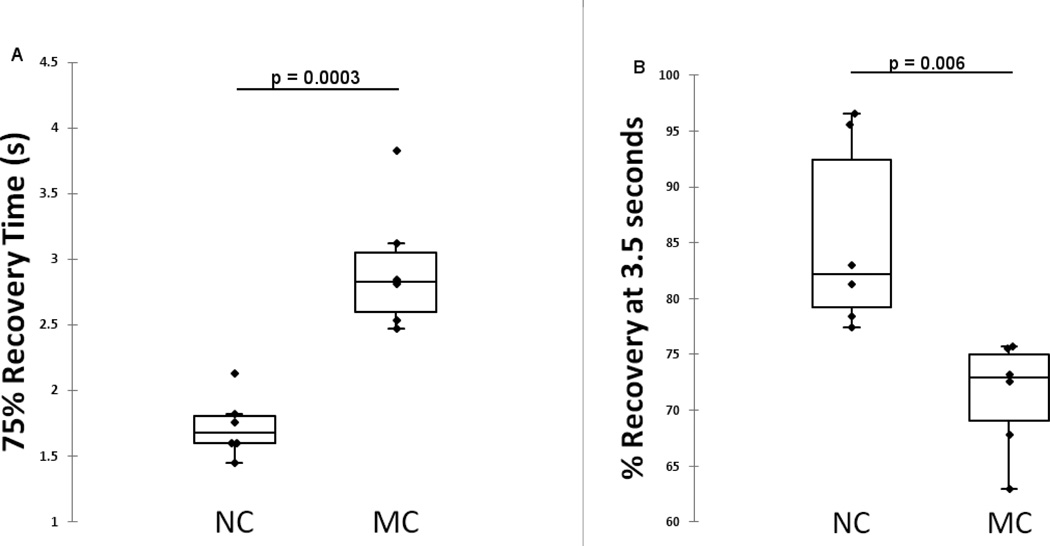
The 75% recovery time (75%RT) was larger in mutation carriers (p=0.0003). Percentage recovery 3.5 seconds after stimulus (PR3.5) was smaller in mutation carriers (p=0.006) (see Figure 1). This slower or ‘sluggish’ PFR trend was also observed in mutation carriers for mean constriction velocity, max constriction velocity, mean dilation velocity and max constriction acceleration, but did not reach statistical significance. Confounders (age, gender and APOE ϵ4 carrier status) were not significant in the ANCOVA models for 75%RT and PR3.5. Confounders were not significant for other PFR parameters with the exception of constriction amplitude which was smaller in APOE ϵ4 carriers (p<0.0001) and in males (p<0.0001).
Figure 1.
Difference in (A) 75% Recovery Time and (B) Percentage recovery 3.5 seconds after stimulus, between 6 mutation carriers and 6 non-carriers from the same family harboring an APP ADAD mutation.
Both the 75%RT and PR3.5 parameters provided perfect classification of mutation carriers vs non-carriers (sensitivity=100%, specificity=100%) in this cohort. Receiver operator characteristic (ROC) area under the curve (AUC) was thus 1.0 for both parameters (75%RT: 95%CI=0.84–1.00, p<0.0001; PR3.5: 95%CI=0.84–1.00, p<0.0001). The effect of estimated years to onset of symptoms (EYO = AAO - age) on 75%RT and PR3.5 was then investigated to explore possible changes in these parameters as part of the temporal sequence of biomarker changes during disease progress. The mean EYO was 5.0±5.9 for MC and for 11.2±9.5 for NC. No evidence of temporal relationships in these PFR parameters was found.
DISCUSSION
We provide evidence from pupil flash response (PFR) testing that carriers of the APPGlu693Gln mutation exhibit slower recovery from pupil flash response, with longer 75% recovery time and smaller percentage recovery 3.5 seconds after stimulus, than non-carriers in the same family. The parameter ‘75% recovery time’ describes the time taken, after constriction to minimum pupil size, to recover 75% of the constriction amplitude through re-dilation. The parameter ‘percentage recovery after 3.5 seconds’ refers to the percentage of the constriction amplitude recovered by re-dilation at time 3.5 seconds after stimulus. Both parameters are measures of the recovery time for the pupil after flash stimulus.
A ‘sluggish’ PFR has previously been reported in sporadic AD [10–13], with reduced resting pupil size, amplitude of constriction and reduced velocity and acceleration of the pupil size. Despite reduced constriction and dilation velocities, some of these studies reported a quicker recovery after PFR stimulus in sporadic AD. This is likely due to the overwhelmingly reduced amplitude of the response. The present study found non-significant trends for reduced mean constriction velocity, maximum constriction velocity, mean dilation velocity and maximum constriction acceleration in mutation carriers. Statistically significant results were found for increased ‘75% recovery time’ and reduced ‘percentage recovery after 3.5 seconds’ in mutation carriers. APPGlu693Gln mutation carriers appear to exhibit the sporadic-AD trends for reduced pupil velocity, but not reduced amplitude, resulting in a significantly slower recovery from stimulus.
The study of families with ADAD mutations provides three major benefits over studies into sporadic AD. Firstly, mutation carriers progress to disease with 100% certainty and at a well defined age-range. Hence, although all participants in the present study were pre-symptomatic, genetic testing enabled identification of those destined to progress to disease. Secondly, a typically younger age of onset (about 51 years for the present analysis), usually results in fewer confounds due to age-related medical co-morbidities. However, in the present study we still observed 2 individuals with hypertension in each group. Thirdly, utilizing within-family comparisons reduces the chance of other genetic influences.
Most families in the DIAN study have genetic mutations that result in AD dementia. Aβ deposition, as measured by positron-emission tomography with Pittsburgh compound B (PET-PiB), has been found to be higher in mutation carriers in the DIAN cohort, up to 15 years before expected symptom onset [6]. However, the ADAD mutation investigated in the present study is a mutation of the Amyloid Precursor Protein (APP) at position 22 of the Aβ fragment (APPGlu693Gln), replacing glutamic acid with glutamine. Mutations in this region of APP are associated with cerebral amyloid angiopathy (CAA) and ultimately cerebral hemorrhage. Indeed both the proband and his sister exhibited CAA and recurrent lobar hemorrhages in the brain, but neuritic amyloid plaques were also found, indicating ADAD pathology may also result from this mutation. PET-PiB imaging results from the participants in the present PFR study demonstrated that the mutation carriers had higher SUVR (1.23±0.08) than non-carriers (1.08±0.04) (p=0.004), although no participants were categorized as high-SUVR (SUVR>1.5). Neither dementia nor cognitive decline has been observed in this family, possibly due to earlier onset of CAA and fatal cerebral hemorrhages.
Despite the difference in underlying cause and age of onset, ADAD and sporadic AD have similar neuropathologic hallmarks and clinical features [5]. The present results indicate that PFR changes may occur in the early pathogenic but pre-symptomatic stages of CAA or ADAD. The lack of correlation between the PFR parameters and estimated years to onset (EYO) may indicate that PFR changes occur early and subsequently stabilize over the EYO range of this cohort, but larger cohorts will be required to investigate this hypothesis. The major limitation of this study is the relatively small number of subjects studied, however the results support further investigation with larger cohorts and other ADAD mutations.
PFR changes in AD are considered to be due to either degeneration in pupil-control relays in the midbrain or cholinergic deficits in the peripheral parasympathetic pathway. Mechanisms by which CAA could impact on PFR could also include damage to pupil-control relays in the midbrain. As changes to pupil response have now been reported in both sporadic AD and APPGlu693Gln mutation carriers, PFR deserves further investigation as a useful adjunct to assist the diagnosis of early AD. PFR may also be useful in monitoring CAA or AD for management of risk factors and treatment.
Footnotes
Disclosure/Conflict of interest
Authors report no competing interests with regards to this manuscript.
Contributor Information
Shaun M. Frost, Commonwealth Scientific and Industrial Research Organisation (CSIRO), Perth, WA, Australia; Preventative Health Flagship, Australian e-Health Research Centre, Perth, WA, Australia; And School of Psychiatry and Clinical Neurosciences, University of Western Australia, Perth, WA, Australia
Yogesan Kanagasingam, Commonwealth Scientific and Industrial Research Organisation (CSIRO), Perth, WA, Australia; and Preventative Health Flagship, Australian e-Health Research Centre, Perth, WA, Australia.
Hamid R. Sohrabi, School of Psychiatry and Clinical Neurosciences, University of Western Australia, Crawley, WA, Australia; Centre of Excellence for Alzheimer's Disease Research and Care, School of Medical Sciences, Edith Cowan University, Perth, WA, Australia; and The McCusker Alzheimer's Research Foundation, Hollywood Medical Centre, Perth, WA, Australia
Kevin Taddei, Centre of Excellence for Alzheimer's Disease Research and Care, School of Medical Sciences, Edith Cowan University, Perth, WA, Australia; The Sir James McCusker Alzheimer's Disease Research Unit, Hollywood Private Hospital, Nedlands, WA, Australia.
Randall Bateman, Department of Neurology, Washington University School of Medicine, St. Louis, MO, USA.
John Morris, Knight Alzheimer's Disease Research Center, Washington University School of Medicine, St. Louis, MO, USA.
Tammie Benzinger, Department of Radiology, Washington University School of Medicine, St. Louis, MO, USA.
Alison Goate, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, USA.
Colin L. Masters, Mental Health Research Institute, The University of Melbourne, Parkville, VIC 3052, Australia
Ralph N. Martins, Centre of Excellence for Alzheimer's Disease Research and Care, School of Medical Sciences, Edith Cowan University, Perth, WA, Australia; The McCusker Alzheimer's Research Foundation, Suite 22, Hollywood Medical Centre, 85 Monash Ave, Nedlands, WA 6009, Australia; and School of Psychiatry and Clinical Neurosciences, University of Western Australia, Crawley, WA, Australia
References
- 1.Sherrington R, Froelich S, Sorbi S, Campion D, Chi H, Rogaeva EA, et al. Alzheimer's disease associated with mutations in presenilin 2 is rare and variably penetrant. Hum Mol Genet. 1996 Jul;5(7):985–988. doi: 10.1093/hmg/5.7.985. [DOI] [PubMed] [Google Scholar]
- 2.Campion D, Flaman JM, Brice A, Hannequin D, Dubois B, Martin C, et al. Mutations of the presenilin I gene in families with early-onset Alzheimer's disease. Hum Mol Genet. 1995 Dec;4(12):2373–2377. doi: 10.1093/hmg/4.12.2373. [DOI] [PubMed] [Google Scholar]
- 3.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 4.Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991 Oct 4;254(5028):97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 5.Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, et al. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res Ther. 2011;3(1):1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer's Disease. N Engl J Med. 2012 Jul 11; doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz B, Rimmer S. Ophthalmologic manifestations of Alzheimer's disease. Surv Ophthalmol. 1989 Jul-Aug;34(1):31–43. doi: 10.1016/0039-6257(89)90127-6. [DOI] [PubMed] [Google Scholar]
- 8.Sadun AA, Borchert M, DeVita E, Hinton DR, Bassi CJ. Assessment of visual impairment in patients with Alzheimer's disease. Am J Ophthalmol. 1987 Aug 15;104(2):113–120. doi: 10.1016/0002-9394(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 9.Frost S, Martins RN, Kanagasingam Y. Ocular biomarkers for early detection of Alzheimer's disease. J Alzheimers Dis. 2010;22(1):1–16. doi: 10.3233/JAD-2010-100819. [DOI] [PubMed] [Google Scholar]
- 10.Fotiou F, Fountoulakis KN, Tsolaki M, Goulas A, Palikaras A. Changes in pupil reaction to light in Alzheimer's disease patients: a preliminary report. Int J Psychophysiol. 2000 Jul;37(1):111–120. doi: 10.1016/s0167-8760(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 11.Prettyman R, Bitsios P, Szabadi E. Altered pupillary size and darkness and light reflexes in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1997 Jun;62(6):665–668. doi: 10.1136/jnnp.62.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granholm E, Morris S, Galasko D, Shults C, Rogers E, Vukov B. Tropicamide effects on pupil size and pupillary light reflexes in Alzheimer's and Parkinson's disease. Int J Psychophysiol. 2003 Feb;47(2):95–115. doi: 10.1016/s0167-8760(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 13.Fotiou DF, Brozou CG, Haidich AB, Tsiptsios D, Nakou M, Kabitsi A, et al. Pupil reaction to light in Alzheimer's disease: evaluation of pupil size changes and mobility. Aging Clin Exp Res. 2007 Oct;19(5):364–371. doi: 10.1007/BF03324716. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario E, Molaschi M, Villa L, Varetto O, Bogetto C, Nuzzi R. Is videopupillography useful in the diagnosis of Alzheimer's disease? Neurology. 1998 Mar;50(3):642–644. doi: 10.1212/wnl.50.3.642. [DOI] [PubMed] [Google Scholar]
- 15.Tales A, Troscianko T, Lush D, Haworth J, Wilcock GK, Butler SR. The pupillary light reflex in aging and Alzheimer's disease. Aging (Milano) 2001 Dec;13(6):473–478. [PubMed] [Google Scholar]
- 16.Herholz K, Weisenbach S, Zundorf G, Lenz O, Schroder H, Bauer B, et al. In vivo study of acetylcholine esterase in basal forebrain, amygdala, and cortex in mild to moderate Alzheimer disease. Neuroimage. 2004 Jan;21(1):136–143. doi: 10.1016/j.neuroimage.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 17.Tohgi H, Abe T, Hashiguchi K, Saheki M, Takahashi S. Remarkable reduction in acetylcholine concentration in the cerebrospinal fluid from patients with Alzheimer type dementia. Neurosci Lett. 1994 Aug 15;177(1–2):139–142. doi: 10.1016/0304-3940(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 18.Scinto LF, Daffner KR, Dressler D, Ransil BI, Rentz D, Weintraub S, et al. A potential noninvasive neurobiological test for Alzheimer's disease. Science. 1994 Nov 11;266(5187):1051–1054. doi: 10.1126/science.7973660. [DOI] [PubMed] [Google Scholar]
- 19.Idiaquez J, Alvarez G, Villagra R, San Martin RA. Cholinergic supersensitivity of the iris in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1994 Dec;57(12):1544–1545. doi: 10.1136/jnnp.57.12.1544-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pomara N, Sitaram N. Detecting Alzheimer's disease. Science. 1995 Mar 17;267(5204):1579–1580. doi: 10.1126/science.7886438. author reply 80-1. [DOI] [PubMed] [Google Scholar]
- 21.Iijima A, Haida M, Ishikawa N, Ueno A, Minamitani H, Shinohara Y. Reevaluation of tropicamide in the pupillary response test for Alzheimer's disease. Neurobiol Aging. 2003 Oct;24(6):789–796. doi: 10.1016/s0197-4580(02)00235-x. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Tortosa E, del Barrio A, Jimenez-Alfaro I. Pupil response to tropicamide in Alzheimer's disease and other neurodegenerative disorders. Acta Neurol Scand. 1996 Aug;94(2):104–109. doi: 10.1111/j.1600-0404.1996.tb07038.x. [DOI] [PubMed] [Google Scholar]
- 23.Grunberger J, Linzmayer L, Walter H, Rainer M, Masching A, Pezawas L, et al. Receptor test (pupillary dilatation after application of 0.01% tropicamide solution) and determination of central nervous activation (Fourier analysis of pupillary oscillations) in patients with Alzheimer's disease. Neuropsychobiology. 1999;40(1):40–46. doi: 10.1159/000026595. [DOI] [PubMed] [Google Scholar]
- 24.Kalman J, Kanka A, Magloczky E, Szoke A, Jardanhazy T, Janka Z. Increased mydriatic response to tropicamide is a sign of cholinergic hypersensitivity but not specific to late-onset sporadic type of Alzheimer's dementia. Biol Psychiatry. 1997 Apr 15;41(8):909–911. doi: 10.1016/S0006-3223(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 25.Robles A, Tourino R, Sesar A, Suarez P, Noya M. [Experience with pupil tropicamide test in Alzheimer's disease] Rev Neurol. 1996 Jan;24(125):65–68. [PubMed] [Google Scholar]
- 26.Kono K, Miyao M, Ishihara S, Takagi A, Ikari H, Suzuki Y, et al. [Hypersensitivity in the pupil dilation response to a cholinergic antagonist in patients with Alzheimer's disease and Down's syndrome] Nippon Ronen Igakkai Zasshi. 1996 Nov;33(11):829–834. doi: 10.3143/geriatrics.33.829. [DOI] [PubMed] [Google Scholar]
- 27.Arai H, Terajima M, Nakagawa T, Higuchi S, Mochizuki H, Sasaki H. Pupil dilatation assay by tropicamide is modulated by apolipoprotein E epsilon 4 allele dosage in Alzheimer's disease. Neuroreport. 1996 Mar 22;7(4):918–920. doi: 10.1097/00001756-199603220-00017. [DOI] [PubMed] [Google Scholar]
- 28.Kardon RH. Drop the Alzheimer's drop test. Neurology. 1998 Mar;50(3):588–591. doi: 10.1212/wnl.50.3.588. [DOI] [PubMed] [Google Scholar]
- 29.Caputo L, Casartelli M, Perrone C, Santori M, Annoni G, Vergani C. The 'eye test' in recognition of late-onset Alzheimer's disease. Arch Gerontol Geriatr. 1998 Sep-Oct;27(2):171–177. doi: 10.1016/s0167-4943(98)00112-5. [DOI] [PubMed] [Google Scholar]
- 30.FitzSimon JS, Waring SC, Kokmen E, McLaren JW, Brubaker RF. Response of the pupil to tropicamide is not a reliable test for Alzheimer disease. Arch Neurol. 1997 Feb;54(2):155–159. doi: 10.1001/archneur.1997.00550140031009. [DOI] [PubMed] [Google Scholar]
- 31.Fridh M, Havelius U, Elofsson G, Hindfelt B. The pupillary response to tropicamide in Alzheimer's disease. Acta Ophthalmol Scand. 1996 Jun;74(3):276–279. doi: 10.1111/j.1600-0420.1996.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 32.Growdon JH, Graefe K, Tennis M, Hayden D, Schoenfeld D, Wray SH. Pupil dilation to tropicamide is not specific for Alzheimer disease. Arch Neurol. 1997 Jul;54(7):841–844. doi: 10.1001/archneur.1997.00550190031011. [DOI] [PubMed] [Google Scholar]
- 33.Kurz A, Marquard R, Fremke S, Leipert KP. Pupil dilation response to tropicamide: a biological test for Alzheimer's disease? Pharmacopsychiatry. 1997 Jan;30(1):12–15. doi: 10.1055/s-2007-979476. [DOI] [PubMed] [Google Scholar]
- 34.Marx JL, Kumar SR, Thach AB, Kiat-Winarko T, Frambach DA. Detecting Alzheimer's disease. Science. 1995 Mar 17;267(5204):1577. doi: 10.1126/science.7741897. author reply 80-1. [DOI] [PubMed] [Google Scholar]
- 35.Reitner A, Baumgartner I, Thuile C, Baradaran Dilmaghani R, Ergun E, Kaminski S, et al. The mydriatic effect of tropicamide and its diagnostic use in Alzheimer's disease. Vision Res. 1997 Jan;37(1):165–168. doi: 10.1016/s0042-6989(96)00119-8. [DOI] [PubMed] [Google Scholar]
- 36.Loupe DN, Newman NJ, Green RC, Lynn MJ, KK WI, Geis TC, et al. Pupillary response to tropicamide in patients with Alzheimer disease. Ophthalmology. 1996 Mar;103(3):495–503. doi: 10.1016/s0161-6420(96)30666-0. [DOI] [PubMed] [Google Scholar]
- 37.Treloar AJ, Assin M, Macdonald AJ. Pupillary response to topical tropicamide as a marker for Alzheimer's disease. Br J Clin Pharmacol. 1996 Mar;41(3):256–257. doi: 10.1111/j.1365-2125.1996.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 38.Fotiou DF, Stergiou V, Tsiptsios D, Lithari C, Nakou M, Karlovasitou A. Cholinergic deficiency in Alzheimer's and Parkinson's disease: Evaluation with pupillometry. International Journal of Psychophysiology. 2009 Aug;73(2):143–149. doi: 10.1016/j.ijpsycho.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Morris Developing an international network for Alzheimer research. Clinical Investigation. doi: 10.4155/cli.12.93. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 41.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004 Mar;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 42.Klunk WE, Lopresti BJ, Ikonomovic MD, Lefterov IM, Koldamova RP, Abrahamson EE, et al. Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-beta in Alzheimer's disease brain but not in transgenic mouse brain. J Neurosci. 2005 Nov 16;25(46):10598–10606. doi: 10.1523/JNEUROSCI.2990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kauwe JS, Jacquart S, Chakraverty S, Wang J, Mayo K, Fagan AM, et al. Extreme cerebrospinal fluid amyloid beta levels identify family with late-onset Alzheimer's disease presenilin 1 mutation. Ann Neurol. 2007 May;61(5):446–453. doi: 10.1002/ana.21099. [DOI] [PubMed] [Google Scholar]
- 44.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]