Abstract
E2F is a heterodimeric transcription factor that regulates the expression of genes at the G1/S boundary and is composed of two related but distinct families of proteins, E2F and DP. E2F/DP heterodimers form complexes with the retinoblastoma (Rb) protein, the Rb-related proteins p107 and p130, and cyclins/cdks in a cell cycle-dependent fashion in vivo. E2F is encoded by at least five closely related genes, E2F-1 through -5. Here we report studies of DP-2, the second member of the DP family of genes. Our results indicate that (i) DP-2 encodes at least five distinct mRNAs, (ii) a site of alternative splicing occurs within the 5' untranslated region of DP-2 mRNA, (iii) at least three DP-2-related proteins (of 55, 48, and 43 kDa) are expressed in vivo, (iv) each of these proteins is phosphorylated, and (v) one DP-2 protein (43 kDa) carries a truncated amino terminus. Our data also strongly suggest that the 55-kDa DP-2-related protein is a novel DP-2 isoform that results from alternative splicing. Thus, we conclude that DP-2 encodes a set of structurally, and perhaps functionally, distinct proteins in vivo.
Full text
PDF

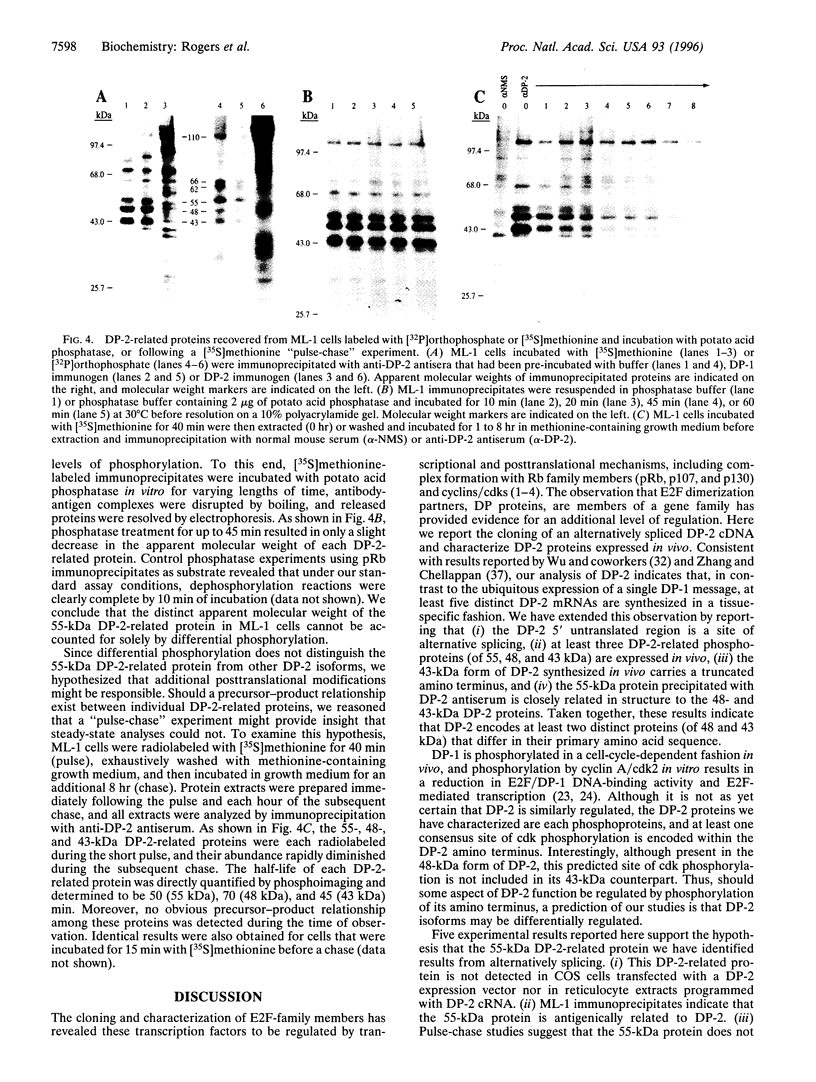

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi S., Weinmann R., Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991 Jun 14;65(6):1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., Adamczewski J. P., Hunt T., La Thangue N. B. Cyclin A and the retinoblastoma gene product complex with a common transcription factor. Nature. 1991 Jul 18;352(6332):249–251. doi: 10.1038/352249a0. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., Buck V. M., Zamanian M., Johnston L. H., La Thangue N. B. Functional synergy between DP-1 and E2F-1 in the cell cycle-regulating transcription factor DRTF1/E2F. EMBO J. 1993 Nov;12(11):4317–4324. doi: 10.1002/j.1460-2075.1993.tb06116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara L. R., Lam E. W., Sørensen T. S., Zamanian M., Girling R., La Thangue N. B. DP-1: a cell cycle-regulated and phosphorylated component of transcription factor DRTF1/E2F which is functionally important for recognition by pRb and the adenovirus E4 orf 6/7 protein. EMBO J. 1994 Jul 1;13(13):3104–3114. doi: 10.1002/j.1460-2075.1994.tb06609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijersbergen R. L., Kerkhoven R. M., Zhu L., Carlée L., Voorhoeve P. M., Bernards R. E2F-4, a new member of the E2F gene family, has oncogenic activity and associates with p107 in vivo. Genes Dev. 1994 Nov 15;8(22):2680–2690. doi: 10.1101/gad.8.22.2680. [DOI] [PubMed] [Google Scholar]
- Cao L., Faha B., Dembski M., Tsai L. H., Harlow E., Dyson N. Independent binding of the retinoblastoma protein and p107 to the transcription factor E2F. Nature. 1992 Jan 9;355(6356):176–179. doi: 10.1038/355176a0. [DOI] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., Kaelin W. G., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. doi: 10.1016/0092-8674(91)90559-h. [DOI] [PubMed] [Google Scholar]
- Cobrinik D., Whyte P., Peeper D. S., Jacks T., Weinberg R. A. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993 Dec;7(12A):2392–2404. doi: 10.1101/gad.7.12a.2392. [DOI] [PubMed] [Google Scholar]
- DeGregori J., Kowalik T., Nevins J. R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995 Aug;15(8):4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto S. H., Mudryj M., Pines J., Hunter T., Nevins J. R. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell. 1992 Jan 10;68(1):167–176. doi: 10.1016/0092-8674(92)90215-x. [DOI] [PubMed] [Google Scholar]
- Dynlacht B. D., Flores O., Lees J. A., Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994 Aug 1;8(15):1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Slansky J. E., Kollmar R. The role of E2F in the mammalian cell cycle. Biochim Biophys Acta. 1993 Aug 23;1155(2):125–131. doi: 10.1016/0304-419x(93)90001-s. [DOI] [PubMed] [Google Scholar]
- Flemington E. K., Speck S. H., Kaelin W. G., Jr E2F-1-mediated transactivation is inhibited by complex formation with the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6914–6918. doi: 10.1073/pnas.90.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girling R., Partridge J. F., Bandara L. R., Burden N., Totty N. F., Hsuan J. J., La Thangue N. B. A new component of the transcription factor DRTF1/E2F. Nature. 1993 Mar 4;362(6415):83–87. doi: 10.1038/362083a0. [DOI] [PubMed] [Google Scholar]
- Helin K., Harlow E., Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993 Oct;13(10):6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helin K., Wu C. L., Fattaey A. R., Lees J. A., Dynlacht B. D., Ngwu C., Harlow E. Heterodimerization of the transcription factors E2F-1 and DP-1 leads to cooperative trans-activation. Genes Dev. 1993 Oct;7(10):1850–1861. doi: 10.1101/gad.7.10.1850. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W. Regions of the retinoblastoma gene product required for its interaction with the E2F transcription factor are necessary for E2 promoter repression and pRb-mediated growth suppression. Mol Cell Biol. 1993 Jun;13(6):3384–3391. doi: 10.1128/mcb.13.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans E. M., Voorhoeve P. M., Beijersbergen R. L., van 't Veer L. J., Bernards R. E2F-5, a new E2F family member that interacts with p130 in vivo. Mol Cell Biol. 1995 Jun;15(6):3082–3089. doi: 10.1128/mcb.15.6.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Krek W., Sellers W. R., DeCaprio J. A., Ajchenbaum F., Fuchs C. S., Chittenden T., Li Y., Farnham P. J., Blanar M. A. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992 Jul 24;70(2):351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Kovesdi I., Reichel R., Nevins J. R. Identification of a cellular transcription factor involved in E1A trans-activation. Cell. 1986 Apr 25;45(2):219–228. doi: 10.1016/0092-8674(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Krek W., Ewen M. E., Shirodkar S., Arany Z., Kaelin W. G., Jr, Livingston D. M. Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell. 1994 Jul 15;78(1):161–172. doi: 10.1016/0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Krek W., Livingston D. M., Shirodkar S. Binding to DNA and the retinoblastoma gene product promoted by complex formation of different E2F family members. Science. 1993 Dec 3;262(5139):1557–1560. doi: 10.1126/science.8248803. [DOI] [PubMed] [Google Scholar]
- Lam E. W., La Thangue N. B. DP and E2F proteins: coordinating transcription with cell cycle progression. Curr Opin Cell Biol. 1994 Dec;6(6):859–866. doi: 10.1016/0955-0674(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Lees E., Faha B., Dulic V., Reed S. I., Harlow E. Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes Dev. 1992 Oct;6(10):1874–1885. doi: 10.1101/gad.6.10.1874. [DOI] [PubMed] [Google Scholar]
- Li Y., Graham C., Lacy S., Duncan A. M., Whyte P. The adenovirus E1A-associated 130-kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1993 Dec;7(12A):2366–2377. doi: 10.1101/gad.7.12a.2366. [DOI] [PubMed] [Google Scholar]
- Martin K., Trouche D., Hagemeier C., Sørensen T. S., La Thangue N. B., Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995 Jun 22;375(6533):691–694. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- Mudryj M., Devoto S. H., Hiebert S. W., Hunter T., Pines J., Nevins J. R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991 Jun 28;65(7):1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Ormondroyd E., de la Luna S., La Thangue N. B. A new member of the DP family, DP-3, with distinct protein products suggests a regulatory role for alternative splicing in the cell cycle transcription factor DRTF1/E2F. Oncogene. 1995 Oct 19;11(8):1437–1446. [PubMed] [Google Scholar]
- Pagano M., Draetta G., Jansen-Dürr P. Association of cdk2 kinase with the transcription factor E2F during S phase. Science. 1992 Feb 28;255(5048):1144–1147. doi: 10.1126/science.1312258. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardet C., Vidal M., Cobrinik D., Geng Y., Onufryk C., Chen A., Weinberg R. A. E2F-4 and E2F-5, two members of the E2F family, are expressed in the early phases of the cell cycle. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2403–2407. doi: 10.1073/pnas.92.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodkar S., Ewen M., DeCaprio J. A., Morgan J., Livingston D. M., Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992 Jan 10;68(1):157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Sterner J. M., Murata Y., Kim H. G., Kennett S. B., Templeton D. J., Horowitz J. M. Detection of a novel cell cycle-regulated kinase activity that associates with the amino terminus of the retinoblastoma protein in G2/M phases. J Biol Chem. 1995 Apr 21;270(16):9281–9288. doi: 10.1074/jbc.270.16.9281. [DOI] [PubMed] [Google Scholar]
- Udvadia A. J., Rogers K. T., Higgins P. D., Murata Y., Martin K. H., Humphrey P. A., Horowitz J. M. Sp-1 binds promoter elements regulated by the RB protein and Sp-1-mediated transcription is stimulated by RB coexpression. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3265–3269. doi: 10.1073/pnas.90.8.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vairo G., Livingston D. M., Ginsberg D. Functional interaction between E2F-4 and p130: evidence for distinct mechanisms underlying growth suppression by different retinoblastoma protein family members. Genes Dev. 1995 Apr 1;9(7):869–881. doi: 10.1101/gad.9.7.869. [DOI] [PubMed] [Google Scholar]
- Wu C. L., Zukerberg L. R., Ngwu C., Harlow E., Lees J. A. In vivo association of E2F and DP family proteins. Mol Cell Biol. 1995 May;15(5):2536–2546. doi: 10.1128/mcb.15.5.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chellappan S. P. Cloning and characterization of human DP2, a novel dimerization partner of E2F. Oncogene. 1995 Jun 1;10(11):2085–2093. [PubMed] [Google Scholar]
- Zhu L., van den Heuvel S., Helin K., Fattaey A., Ewen M., Livingston D., Dyson N., Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993 Jul;7(7A):1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]