Abstract
Background
The aortic valve interstitial cell (AVIC) has been implicated in the pathogenesis of calcific aortic stenosis. When appropriately stimulated, AVICs undergo a phenotypic change from that of a myofibroblast to that of a bone-forming-like cell. Elevated blood levels of LDL-cholesterol are a clinical risk factor for aortic stenosis, and oxidized LDL-cholesterol (ox-LDL) is consistently found in calcified aortic valve leaflets. But whether it plays a role in the pathogenesis of aortic stenosis is unknown. The process of aortic valve leaflet calcification is associated with deposition of calcium- phosphate, mediated in part by the phosphate inorganic transporter 1 (PiT-1), a sodium-phosphate ion co-transporter. Therefore, we hypothesized that ox-LDL induces an osteogenic change in human AVICs marked by the induction of PiT-1. Using isolated human AVICs, the purpose of this study was to examine the effect of ox-LDL on the expression of PiT-1 and the osteogenic factor bone morphogenetic protein 2 (BMP-2), which is a protein necessary for bone formation.
Methods
Human AVICs were isolated from non-stenotic aortic valves obtained from explanted hearts of patients undergoing cardiac transplantation (n=4) and grown in culture. The cells were treated with serum free media, serum free media with DMSO (vehicle control), 40 μg/ml of ox-LDL or 40 μg/ml of Ox-LDL + 2.5 mM phosphonoformic acid. Phosphonoformic acid (PFA) is a competitive inhibitor of PiT-1 by mimicking inorganic phosphate. Cell lysis was performed at 24 hours following treatment. Cell lysates were analyzed via immunoblot and densitometry for PiT-1 and BMP-2. Statistics were by ANOVA. P < 0.05 was significant.
Results
ox-LDL stimulation of AVICs induced an increase in PiT-1 and BMP-2. Ox-LDL induced increased production of the phosphate transporter, PiT-1, and the osteogenic factor, BMP-2. Inhibition of PiT-1 with PFA prevented ox-LDL-induced BMP-2 expression.
Conclusions
These data offer mechanistic insight into the pathogenesis of calcific aortic stenosis.
Keywords: aortic stenosis, aortic valve interstitial cell, oxLDL, PiT-1
Introduction
Despite the prevalence of calcific aortic stenosis, the cellular mechanisms by which aortic valve leaflets become calcified have not been elucidated (1). Theories as to the pathogenesis of calcific aortic stenosis have been derived from the examination of explanted valve leaflets. Examination of such leaflets has demonstrated histological evidence of inflammation and markers of osteogenesis. These histological findings are very similar to those found with atherosclerosis and imply that the cellular mechanisms responsible for aortic stenosis and atherosclerosis are similar (2-3).
The principal cell type found in the aortic valve leaflet is the aortic valve interstitial cell (AVIC). The human AVIC has phenotypic features of a myoblast and fibroblast, and is therefore considered a myofibroblast (4). The human AVIC has been implicated in the pathogenesis of aortic stenosis (5, 6). When stimulated by mechanisms of inflammation, its phenotype changes from that of a myofibroblast to that of an osteoblast-like cell (4, 7, 8). Such an osteogenic phenotype is characterized by the production of bone-forming proteins such as bone morphogenetic protein-2 (BMP-2) (8).
The clinical risk factors for calcific aortic stenosis are virtually the same as those for vascular atherosclerosis, including hypercholesterolemia (9). LDL-cholesterol has a critical role in the pathogenesis of atherosclerosis. Retained within the arterial wall, LDL is modified by oxidation (ox-LDL); it incites an inflammatory-atherosclerotic process (10). The vascular smooth muscle cells within the vessel wall have been shown to be important in the pathogenesis of atherosclerosis. Following ox-LDL inflammatory stimulation, vascular smooth muscle cells undergo an osteogenic phenotypic change (11, 12). This is in part driven by increased phosphate uptake leading to the deposition of calcium phosphate. PiT-1 is a sodium-phosphate co-transporter that has been implicated in this process (13).
It is therefore significant that ox-LDL is found in calcified aortic valve leaflets and co-localized with histological evidence of inflammation and calcium deposits in calcified aortic valve leaflets (12). Further, an association has been demonstrated between circulating ox-LDL and aortic valve remodeling in aortic stenosis (11). While such circumstantial evidence is provocative, the role of ox-LDL in aortic valve calcification and stenosis has not been determined. Therefore, we hypothesized that ox-LDL induces an osteogenic change in human AVICs marked by the induction of PiT-1.
The purpose of this study was to determine the effects of ox-LDL on human AVICs. The results of this study demonstrate that ox-LDL induces an osteogenic phenotype that includes an increased expression of PiT-1. The results further demonstrate that PiT-1 may play a role in ox-LDL-induced pro-osteogenic signaling.
Methods
This study was approved by the Colorado Multiple Institutional Review Board of the University of Colorado School of Medicine. All patients provided written informed consent.
Chemicals and Reagents
Medium 199 was purchased from Lonza (Walkersville, MD). The PiT-1 inhibitor sodium phosphonofomate hexahydrate (PFA) was purchased from Alfa Aesar (Ward Hill, MA). Rabbit polyclonal antibody against human PiT-1 (H-130) and BMP-2 (N-14) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Human oxidized LDL cholesterol (OxLDL) was purchased from Biomedical Technologies Inc. (Stoughton, MA). Protein assay reagents and chemiluminescent substrate (ECL) were purchased from Thermo Scientific (Rockford, IL). 4-20% gradient polyacrylamide Ready gels, nitrocellulose membranes, and 2× Laemmli sample buffer were purchased from Bio-Rad (Hercules, CA). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO).
Cell Isolation and Culture
Non-stenotic aortic valve leaflets were obtained from the explanted hearts of patients undergoing cardiac transplantation at the University of Colorado Hospital (n=4) for idiopathic dilated cardiomyopathy (males, ages 36-47 years). Grossly, all leaflets were thin, pliable and grossly normal without overt calcification. Isolation was by collagenase digestion as previously described and AVICs were cultured and maintained as independent cultures in medium 199 with penicillin G, streptomycin, amphotericin B, and 10% fetal bovine serum in an incubator supplied with 5% carbon dioxide (4). Briefly, aortic valves were treated under sterile conditions in the operating room and placed immediately into 4°C in sterile saline. After 3 vigorous washes with sterile saline, the valves were sectioned and segments were either placed into 4% formaldehyde in PBS, flash frozen, or placed in OCT for frozen sections. The remaining sections were washed five times with Earl's Balanced Salt Solution (EBSS) placed in 2.5 mg/mL collagenase in full medium 199 for 30 minutes and incubated at 37°C. The supernatant was disposed and valve sections were washed once with EBSS in order to remove endothelial cells. Aortic valve segments underwent further digestion for three hours in 0.8 mg/mL collagenase in full medium 199 and cells were pelleted by centrifugation, resuspended in full medium 199 and grown in culture (Passage zero). Cells from passages 3-6 were used for all experiments grown to 70-90% confluence and subcultured to 24-well plates for immunoblotting experiments.
AVIC PiT-1 Inhibitor Treatments
AVICs that were treated with PiT-1 inhibition were first pre-treated with 5 mM PFA (dissolved in dimethyl sulfoxide (DMSO)) for thirty minutes in serum-free medium, serum-free medium with DMSO as a vehicle control, and serum-free medium alone (control). Media were aspirated and 40 μg/mL of human OxLDL was added to the collected media then returned to their respective wells. (In a preliminary experiment, the optimal concentration of OxLDL was determined to be 40 μg/mL; data not presented). Cells were washed twice with cold phosphate buffered saline (PBS) and were lysed using 1× Laemmli sample buffer with 1:40 β-mercaptoethanol and cell-scraping.
Immunoblotting
Immunoblotting was used to analyze PiT-1 and BMP-2 production in cell lysates. AVICs in culture were lysed using 1× Laemmli sample buffer with β-mercaptoethanol. Lysates were loaded into 15-well 4-20% gradient Ready gels (Bio-Rad) and run at 200 V for 30 minutes. Transfer was to nitrocellulose membranes at 100 V for 70 minutes, cross-linked using a UV Stratalinker (Stratagene, La Jolla, CA) twice, and then blocked using 5% dry milk in 0.1% Tween in PBS (T-PBS). After three washes with 0.1% T-PBS, the blocked membranes were incubated overnight at 4°C with primary antibodies which were diluted (1:300 to 1:10,000) in 5% BSA in 0.1% T-PBS. Again, after three washes in 0.1% T-PBS, membranes were incubated in appropriate horseradish peroxidase-conjugated secondary antibodies diluted to 1:5000 in 5% dry milk in 0.1% T-PBS for one hour at room temperature. After three washes in 0.1% T-PBS, membranes were incubated in ECL for 5 minutes at room temperature and exposed on X-ray film. Images were scanned using a flatbed scanner (Epson, Long Beach, CA) and images were analyzed using the NIH densitometry software, Image J.
Statistical Analysis
Data are presented as means ± standard error and statistical analysis was performed using ANOVA (StatView 5.0, SAS Intstitute, Cary NC) with significance defined as p<0.05.
Results
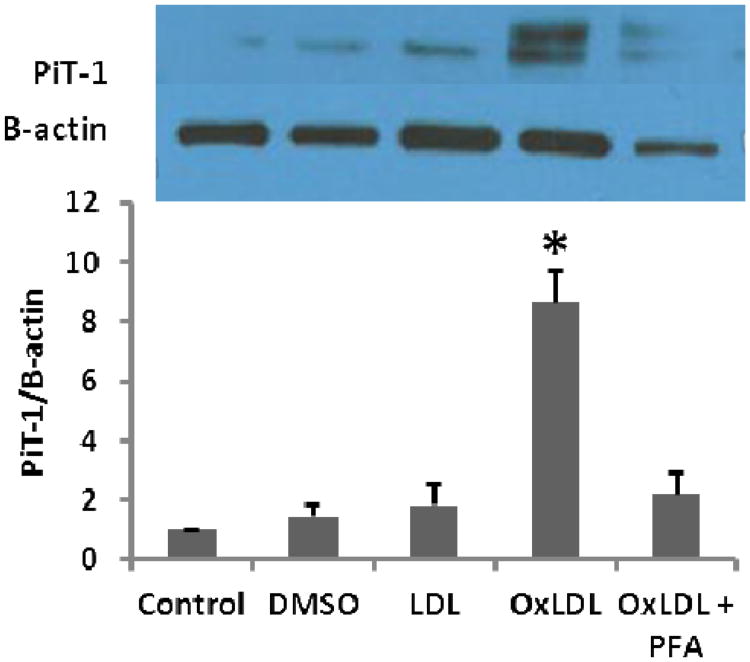
Ox-LDL stimulation of human AVICs induced an increase in PiT-1 (Figure 1)
Figure 1.
OxLDL induced an osteogenic response in isolated human AVICs. Following stimulation with OxLDL, the expression of the sodium-phosphate co-transporter, PiT-1, was significantly increased (* p < 0.05). DMSO is a vehicle control and LDL is used as cholesterol control. Addition of the PiT-1 inhibitor, PFA, prevented the increase in PiT-1 expression.
OxLDL induced an 8-fold increase in PiT-1 expression in comparison to base line (p<0.05). Treatment with the PiT-1 inhibitor, PFA, effectively prevented ox-LDL-induced expression of Pit-1.
OxLDL stimulation of human AVICs induced an increase in BMP-2, which was prevented by PiT-1 inhibition (Figure 2)
Figure 2.
OxLDL induced an osteogenic phenotype in human AVICs, which was prevented by addition of the PiT-1 inhibitor, PFA. BMP-2 expression was significantly increased after stimulation with OxLDL(*p<0.05). DMSO is a vehicle control and LDL is used as a cholesterol control. Inhibition of PiT-1 with PFA prevented the osteogenic response, suggesting that PiT-1 plays a role in ox-LDL-induced pro-osteogenic signaling in human AVICs.
Ox-LDL stimulation induced a greater than 2.5-fold expression in BMP-2 (p<0.05). This ox-LDL-induced expression of BMP-2 was prevented by inhibition of PiT-1 inhibitor (PFA).
Discussion
The results of the present study demonstrate an important mechanism by which ox-LDL can induce osteogenesis in isolated human AVICs. Stimulation by ox-LDL induced the production of the important bone-forming protein, BMP-2, and the sodium-phosphate co-transporter, Pit-1. When expression of PiT-1was blocked, ox-LDL-induced expression of BMP-2 was inhibited. In addition to its function as a sodium-phosphate co-transporter, these data suggest that PiT-1 may be involved in ox-LDL pro-osteogenic signaling
The limitations of the present study must be acknowledged. In the present study, isolated AVICs were studied in vitro. As with any study of isolated cells, a limitation of the present study is that the behavior of the cells in vitro may differ from the behavior of those in vivo. However, we have previously demonstrated that isolated human AVICs that have been grown through multiple passages in cell culture have functions comparable to those of freshly isolated cells (8). A second limitation of any study of isolated cells is that it is not possible to understand how cell-cell interactions in vivo may affect the responses seen in vitro. Nonetheless, despite these limitations, the findings of the present study have important implications.
The AVIC has been implicated in the pathogenesis of aortic stenosis. When stimulated by mechanisms of inflammation, these cells assume an osteogenic phenotype (4, 7, 8). In its role in the pathogenesis of atherosclerosis, the pro-inflammatory actions of ox-LDL are well recognized (10-12). Hence, the present study focused on the effects of ox-LDL on human AVICs. The results of the present study suggest that ox-LDL may have actions in the aortic valve leaflet that are similar to its actions in the arterial wall. Therefore, mechanistic parallels may exist between the pathogenesis of aortic stenosis and that of vascular atherosclerosis.
The role of hypercholesterolemia in the pathogenesis of atherosclerosis is well known. Given that the clinical risk factors for aortic stenosis, including hypercholesterolemia, are virtually the same as for atherosclerosis, clinical trials have been conducted in which the effect of cholesterol-lowering medications (statins) on aortic stenosis have been examined (14). The results of these trials have been disappointing: statin therapy has not been demonstrated to slow the progression of aortic stenosis (15, 16). However, the patients in these clinical trials had been diagnosed (echocardiography) with some degree of aortic stenosis. Hence, an important limitation of all of these clinical trials is that the statin therapy was initiated after the disease was already underway. In other words, the therapy may have been initiated too late to alter the course of the disease.
The results of the present study suggest that stimulation of normal human AVICs by ox-LDL may initiate the pathogenic mechanisms of aortic stenosis. Stimulation of isolated human AVICs from normal aortic valve leaflets by ox-LDL induced an osteogenic phenotype (BMP-2 expression). This ox-LDL-induced BMP-2 expression was prevented by inhibition of Pit-1. While the results of the present study were obtained through the study of isolated AVICs, it is tempting to speculate that the actions of ox-LDL may play a role in the genesis of aortic stenosis in vivo.
In summary, the results of the present study demonstrate that ox-LDL induces an osteogenic phenotype in isolated human AVICs. These data offer mechanistic insight into the pathogenesis of aortic stenosis.
Acknowledgments
Funded by grants from the American Heart Association (AHA: 11GRNT7900016) and the National Institutes of Health (NIH RO1 HL106582-01).
Footnotes
Presented at the 8th Annual Meeting of the Academic Surgical Congress, New Orleans, Louisiana, February 5-7, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hakuno D, Kimura N, Yoshoika M, et al. Molecular mechanisms underlying the onset of degenerative valve disease. J Mol Med. 2009;87:17–24. doi: 10.1007/s00109-008-0400-9. [DOI] [PubMed] [Google Scholar]
- 2.Olsson M, Dalsgaard CJ, Haegerstrand A, et al. Accumulationo of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J Am Coll Cardiol. 1994;23:1162–1170. doi: 10.1016/0735-1097(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 3.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19:1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 4.Meng X, Ao L, Song Y, et al. Expression of functional toll-like receptors 2 and 4 in human aortic valve interstitial cells: potential roles in aortic valve inflammation and stenosis. Am J Physiol Cell Physiol. 2008;294:C29–C35. doi: 10.1152/ajpcell.00137.2007. [DOI] [PubMed] [Google Scholar]
- 5.Mohler ER, Chawla MK, Chang AW, et al. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–260. [PubMed] [Google Scholar]
- 6.Mohler ER, Gannon F, Reynolds C, et al. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 7.Kaden JJ, Dempfle CE, Grobholz R, et al. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170:205–211. doi: 10.1016/s0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 8.Babu AN, Meng X, Zou N, et al. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg. 2008;86:71–6. doi: 10.1016/j.athoracsur.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Iung B, Baron G, Butchart EG, et al. A prospective study of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg D, Witztum JL. Oxidized Low-Density Lipoprotein and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 11.Côté C, Pibarot P, Després JP, et al. Association between circulating oxidized low-density lipoprotein and fibrocalcific remodeling of the aortic valve and aortic stenosis. Heart. 2008;94:1175–1180. doi: 10.1136/hrt.2007.125740. [DOI] [PubMed] [Google Scholar]
- 12.Mohty D, Pibarot P, Després JP, et al. Association between plasma LDL particle size, valvular accumulation of oxidized LDL and inflammation in patients with aortic stenosis. Arterioscler Thromb Vasc Biol. 2007;28:187–193. doi: 10.1161/ATVBAHA.107.154989. [DOI] [PubMed] [Google Scholar]
- 13.Villa-Bellacosta R, Sorribas V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler Thromb Vasc Biol. 2009;29:761–766. doi: 10.1161/ATVBAHA.108.183384. [DOI] [PubMed] [Google Scholar]
- 14.Novo G, Fazio G, Visconti C, et al. Atherosclerosis, Aortic Stenosis and Statins. Current Drug Targets. 2011;12:115–121. doi: 10.2174/138945011793591545. [DOI] [PubMed] [Google Scholar]
- 15.Chan KL, Teo K, Dumesnil JG, et al. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) Trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 16.Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with Simvastatin and Ezetimibe in Aortic Stenosis. N Engl J Med. 2008;359:1343–56. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]