Abstract
A-kinase anchoring proteins (AKAPs) streamline signal transduction by localizing signaling enzymes with their substrates. Great strides have been made in elucidating the role of these macromolecular signaling complexes as new binding partners and novel AKAPs are continually being uncovered. The mechanics and dynamics of these multi-enzyme assemblies suggest that AKAP complexes are viable targets for therapeutic intervention. This review will highlight recent advances in AKAP research focusing on local signaling events that are perturbed in disease.
Keywords: AKAP, protein kinase, protein phosphatase, Ca2+, signal transduction, disease, interfering peptide
Introduction
A-kinase anchoring proteins (AKAPs) ensure enzymes are appropriately targeted to optimally facilitate signal transduction. A unifying feature of this group of proteins is their ability to anchor the regulatory (R) subunits of Protein Kinase-A (PKA) in proximity to substrates. However, each anchoring protein associates with a unique subset of signaling effectors comprised of protein kinases, phosphoprotein phosphatases, small GTPases, phosphodiesterases, transmembrane receptors and ion channels [1]. This list is constantly growing as additional AKAP binding partners are continually being discovered. Consequently, local signaling is a burgeoning line of investigation as many research groups are grappling with the intricate spatial relationships that exist within these AKAP complexes. This article highlights examples of the four fundamental tenets of AKAP signaling: specificity, sensitivity, localization and temporal control.
Recently, investigators are shifting their attention from cells in culture toward in vivo models in order to understand how anchored signaling pathways are perturbed during disease. This comes at a time when approximately 50% of marketed pharmaceuticals target G Protein-Coupled Receptors (GPCRs) and increasing numbers of kinase inhibitor drugs are entering the clinic [2] . Thus, our growing knowledge of these anchored enzyme units earmark AKAP complexes as potential targets for therapeutic intervention, especially since the manipulation of local signaling holds a promise of generating therapies with fewer off-target effects.
Signaling specificity through anchoring
Arguably the most extensively characterized anchoring protein, AKAP79/150, organizes a veritable mecca of signaling proteins. These include the beta adrenergic receptor (βAR), adenylyl cyclase (AC), L-type voltage-gated Ca2+ channels (Cav1.2, Cav1.3) protein kinases A and C (PKA, PKC), and protein phosphatases among others [3-5]. The exquisite control AKAP79/150 exerts on its many partner molecules in a variety of cellular contexts makes this anchoring protein a prototypic example of local signaling specificity [6].
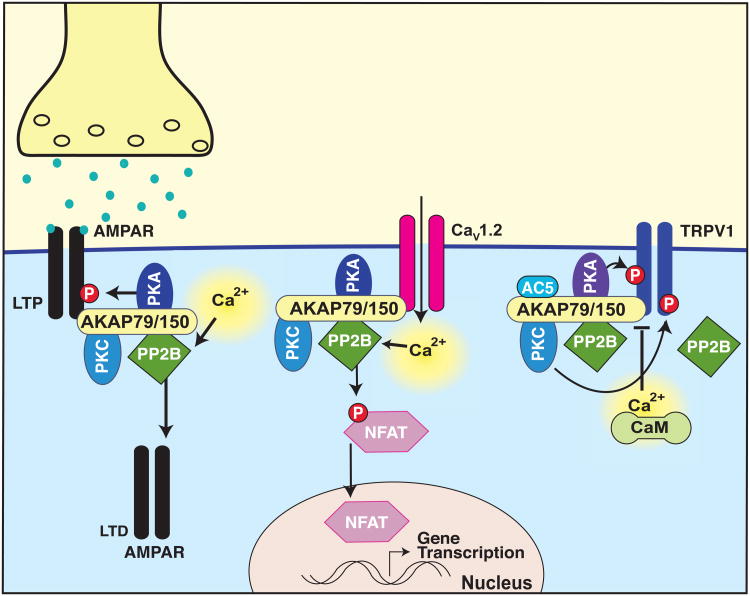
Modulation of signaling molecules through dephosphorylation is a key mechanism of signal transduction [7-9]. A recently defined example is anchoring of the calcium-calmodulin-dependent serine-threonine protein phosphatase 2B/calcineurin (PP2B) to neuronal AKAP79/150 (Figure 1). This enzyme is the target of immunosuppressant drugs cyclosporine A and FK506. A primary downstream effector of PP2B is the transcription factor, Nuclear Factor Activated in T-cells (NFAT). Work with NFAT has identified a PP2B recognition site intrinsic to many PP2B effector proteins, commonly referred to as PIxIxIT sequence [9, 10]. Although the region of AKAP79/150 required for binding to PP2B was originally mapped more than 10 years ago, recent work has identified a specific sequence within this region which bears striking resemblance to the PIxIxIT motif in NFAT [11, 12]. The authors showed that this region was responsible for the AKAP79/150-mediated activation of NFAT by PP2B [11, 12]. In rat hippocampal neurons NFAT signaling is initiated via AKAP79/150-anchored Cav1.2-mediated increases in intracellular Ca2+, PP2B activation and NFAT dephosphorylation, allowing NFAT translocation to the nucleus where it regulates gene transcription. Additionally, AKAP150-dependent nuclear translocation of NFAT regulates gene expression of KCNQ2 and KCNQ3 potassium channels and is abolished in AKAP150 null mice [13].
Figure 1. AKAP79/150 modulates diverse synaptic functions through anchored PP2B.
Diagram depicting PKA-mediated phosphorylation of AMPAR GluA1 subunit primes the receptor for insertion in the postsynaptic membrane during LTP while dephosphorylation by PP2B removes the receptor from the synaptic space during LTD. AKAP79/150-anchored Cav1.2 increases intracellular Ca2+, triggering PP2B activation and NFAT dephosphorylation, allowing NFAT translocation to the nucleus where it regulates gene transcription. AKAP79/150 associated PP2B dephosphorylates the channel in a negative feedback loop. AKAP150-anchored PKA and PKC phosphorylate the TRPV1 channel, increasing channel sensitivity. This process is facilitated by AC5 and blocked by Ca2+/CaM. PP2B dephosphorylates the receptor independently of AKAP anchoring [6, 9-12, 14, 16, 17].
Activation of AKAP-anchored PP2B involves local Ca2+ influx through the L-type voltage-gated Ca2+ channel, Cav1.2. However, negative feedback regulation of the channel also appears to be modulated by PP2B as deletion of the PP2B binding motif on AKAP79/150 (ΔPIX), the use of interfering peptides, or pharmacological PP2B inhibition prevents feedback channel inhibition [14]. Long term potentiation (LTP) and long term depression (LTD) are systemic processes underlying learning and memory. LTP and LTD are Ca2+-dependent processes mediated through AMPA and NMDA-type glutamate receptor ion channels [6, 15]. PKA-mediated phosphorylation of AMPAR GluA1 subunit primes the receptor for insertion in the postsynaptic membrane during LTP while subunit dephosphorylation by PP2B removes the receptor from the synaptic space during LTD. Genetic removal of AKAP79/150 in rat CA1 pyramidal neurons increases AMPA receptor-mediated excitatory postsynaptic current (EPSCs) as well as NMDA-receptor LTD [6]. Interestingly, this is reversed upon re-introduction of wild type AKAP79/150 as well as AKAP mutants lacking the PKA or PKC binding sites, but not with a mutant lacking the PP2B binding domain [16]. The ΔPIX transgenic mice exhibit greater AMPA receptor phosphorylation than wild type animals and display simultaneous decreased LTP and enhanced LTD at hippocampal CA1 synapses [17]. Finally, a novel mechanism of GABAergic LTD through dopamine D2L receptor depends on IP3 receptor activation and AKAP79/150-anchored PP2B activity [18]. These selected examples illustrate the diversity of synaptic functions modulated by AKAP-anchored PP2B. However, this mechanism also plays an important role in other tissues and cellular context.
Modulating glucose homeostasis is another example of the multifaceted role that AKAP150-anchored PP2B can play in pathology [19]. Loss of AKAP150 suppresses insulin secretion in pancreatic β-cells concurrent with decreased Ca2+ currents, and lower cAMP mobilization. Surprisingly, intraperitoneal insulin injection in AKAP150 null animals resulted in significantly reduced blood glucose compared to wild type animals, indicating enhanced insulin action in the peripheral tissues of AKAP150 null animals. Further analysis revealed that knock-in mice lacking the PKA binding site of AKAP150 exhibited very little differences in glucose handling compared to wild type. However, ΔPIX animals, which can no longer anchor PP2B to AKAP150, performed similarly to AKAP150 null animals, indicating that PP2B anchoring is the core molecular basis in AKAP150-mediated coordination of glucose homeostasis [19].
Enzyme anchoring augments signal sensitivity
Damage to nociceptors or peripheral nerves trigger hyperalgesia, an increased sensitivity to pain [20]. At the molecular level these pathological events originate from activation of the nonselective cation channel TRPV1 [20]. Phosphorylation of TRPV1 by anchored PKA or PKC sensitizes this receptor in response to inflammatory stimuli [21, 22]. The interaction domain between TRPV1 and AKAP79/150 is a viable target for the development of novel analgesics that may lack the side effects observed with direct TRPV1 inhibition. Twin studies published earlier this year mapped the interaction sites on both AKAP79/150 and TRPV1 and showed that peptide inhibition of either binding site resulted in abolished PKC-dependent TRPV1 sensitization to capsaicin stimulation (Figure 1). Furthermore, cell permeable peptide analogs mimicking either side of the AKAP-TRPV1 interface suppressed inflammatory thermal and mechanical pain-related behaviors in mice [23, 24]. Additionally, adenylyl cyclase AC5 anchoring to the AKAP79/150-TRPV1 complex is necessary for TRPV1 sensitization to inflammatory stimuli. AC5 anchoring to the complex inhibits TRPV1 desensitization, while interrupting the AKAP-AC association permits channel desensitization [25]. Meanwhile, Ca2+/calmodulin interferes with AKAP79/150 association with TRPV1 potentially decreasing channel activity, while PIP2 at the membrane can interfere with PKA phosphorylation of TRPV1 in an AKAP79/150-dependent manner [26, 27]. However, AKAP79/150 is not required for the PP2B-mediated desensitization of activated TRPV1 channels, as PP2B can effectively dephosphorylate the channel in AKAP150-/- mice [28]. Collectively, these three examples as depicted in Figure 1 highlight how the AKAP79/150 scaffold facilitates enhanced signal sensitivity to distinct physiological events in different cellular contexts.
Kinase localization during cell division and cancer progression
Since phosphorylation is implicated in cell cycle control it is perhaps not surprising that distinct AKAPs coordinate aspects of mitosis and cancer progression. For example, Gravin and the rodent ortholog Src-Suppressed C Kinase Substrate (SSeCKS)/AKAP12 have long been implicated in oncogenesis [29]. The Gravin locus is thought to be associated with susceptibility to malignancies while loss of Gravin in multiple cancers is correlated with poor overall survival and is often attributed in part to Gravin promoter hypermethylation [30-35].
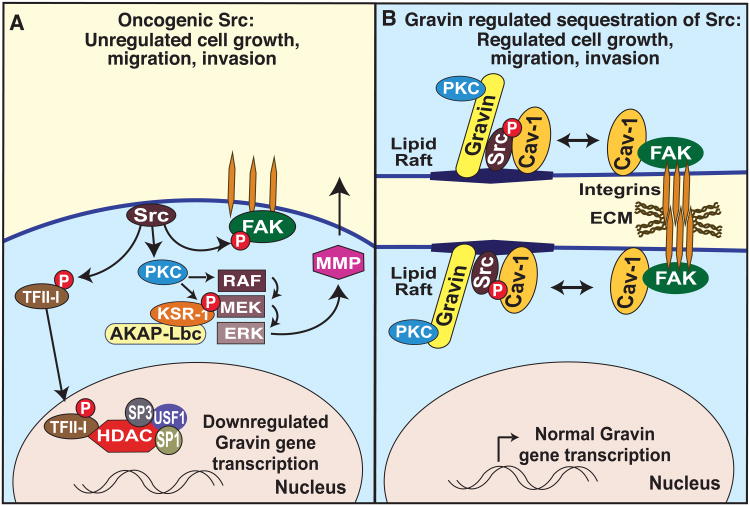
As suggested by the name of the rodent ortholog, Gravin expression is both suppressed in Src-transformed cells and phosphorylated by PKC [29]. Activated Src suppresses Gravin promoter activity through a short proximal sequence that binds upstream transcription factor 1 (USF1), specificity proteins (Sp1 and Sp3), histone deacetylase 1 (HDAC1), as well as the Src-phosphorylated transcription factor TFII-I (Figure 2A) [36]. However, Gravin can in turn inhibit the mitogenic signal transduction mechanisms triggered by these two kinases. Gravin reduces prostate cancer cellular chemotaxis and invasiveness by preventing serum-activated Src from triggering a PKC-Raf-MEK-ERK pathway thus precluding matrix metalloprotease-2 expression and secretion [37]. Activation of this particular MAP kinase cascade may involve another anchored enzyme complex as AKAP-Lbc nucleates a higher order macromolecular assembly that includes a Raf-MEK-ERK module and their scaffolding protein KSR [38]. Furthermore, it has recently been proposed that Gravin does not directly inhibit Src activity, but rather sequesters the active enzyme from its downstream effector molecules. For example, Gravin anchoring to Src occurs via a caveolin-1-like motif on the anchoring protein that drives Src into lipid rafts (Figure 2B). The net effect is to deplete this oncogenic tyrosine kinase from focal adhesions. These events also drive FAK-mediated cell adhesion through increased clustering of integrin β1 [39].
Figure 2. Gravin sequesters Src to reduce mitogenic signaling.
(A) In the absence of Gravin anchoring, activated Src induces PKC to stimulate the Raf-MEK-ERK kinase cascade. Mobilization of this MAP kinase triad triggers MMP-2 expression and secretion. Src also activates FAK, triggering focal adhesion disassembly which can contribute to cell migration and invasion. Recruitment of the MAPK cascade to this location may be mediated in part by another anchoring complex that involves AKAP-Lbc and RAF-MEK-ERK scaffolding protein, KSR. Activated Src suppresses transcription of the Gravin/AKAP12 gene through a short proximal sequence that binds USF1, Sp1, Sp3, HDAC1, as well as the Src-phosphorylated transcription factor TFII-I. Another mechanism of uncontrolled cell growth may be through MAPK scaffolding by AKAP-Lbc [38] (B) Gravin protein association with Src not only inhibits kinase activity, but also sequesters the active enzyme from its downstream effector molecules. The net effect is to drive Src into lipid rafts, and thereby deplete this oncogenic tyrosine kinase from focal adhesions where it can facilitate FAK-mediated cell adhesion and enhanced clustering of integrin β1 [36, 37, 39].
Gravin association with PKC occurs through direct binding of two homologous motifs resulting in kinase inhibition [40, 41]. Cellular confluence increases Gravin-PKC association, while simultaneously decreasing PKC activity. Gravin null murine embryonic fibroblasts (MEFs) exhibit increased PMA-induced PKC activity but not PKC-dependent cytoskeletal rearrangement. PKC activity and prostate cancer cell apoptosis can be restored to normal levels by re-introducing full length Gravin, but not a mutant lacking a PKC binding site [40].
Due to Gravin's antagonistic relationship with various mitogenic factors, it is logical to propose that Gravin plays a key role in limiting cell cycle progression and cell proliferation. A recent in-depth study found an interesting, if unexpected role for Gravin in cell cycle progression [42]. As expected, nude mice developed significantly larger tumors when injected with Gravin shRNA-expressing U251 cells compared to control. Similarly, Gravin mRNA was significantly decreased in human glioblastoma multiform complexes compared to neighboring normal tissue. Yet unexpectedly, shRNA depletion of Gravin actually decreased prostate cancer PC-3 cell proliferation while enhancing mitotic defects. Investigators found that Gravin is phosphorylated at T766 by the cell cycle regulatory kinase CDK1 in a temporally synchronized manner, peaking at mitosis along with other makers of cell cycle progression. Interestingly, CDK1-phosphorylated Gravin is localized to the mitotic spindle and associated with the G2-M transition trigger kinase, Plk1. This concept was validated using a Gravin T766A phosphosite mutant that is unable to bind Plk1 and significantly decreases cell proliferation. Finally, while overall Gravin protein levels were decreased in human glioblastoma multiforme, phosphorylated Gravin was enriched in malignancy compared to control neighboring tissue [42]. This indicates that while many aspects of Gravin signaling are anti-proliferative, there is a specific pool of Gravin which is both spatially and temporally regulated to facilitate cell cycle progression and cell proliferation.
Temporal regulation and the management of cardiac hypertrophy
Cardiovascular disease is the primary cause of morbidity and mortality in the USA. This places severe financial strain on the healthcare system each year. In response to hypertension (increased blood pressure), atherosclerosis (hardening of the blood vessels) or other forms of cardiac stress, the heart enlarges and remodels (cardiac hypertrophy), ultimately leading to heart failure. Several studies have implicated various AKAP-mediated signaling mechanisms in the onset and progression of cardiovascular disease. These include the anchoring proteins m-AKAP, AKAP18 and AKAP-Lbc [43, 1, 44, 45].
Gene silencing of AKAP-Lbc or disrupted anchoring in isolated cardiomyocytes attenuates RhoA activation. The net result is decreased hypertrophic responses induced by GPCRs [43]. However, it is becoming apparent that AKAP-Lbc is also essential for the normal growth and development of the heart. AKAP-Lbc null mice die at E10.5-11 and display deficient sarcomere formation and thin-walled developing hearts [46]. It was speculated that this developmental deficiency may be due to altered anchoring of RhoA or activation of the protein kinase D (PKD) leading to differences in downstream events such as activation the p38- MAP kinase cascade or HDAC5-mediated de-repression of transcriptional remodeling [45]. However, transgenic mice lacking either of these domains in AKAP-Lbc develop normally, implying that the cardiac malformation of AKAP-Lbc null mice is not solely due to the mislocalization of these signaling molecules [47]. Furthermore, a cardioprotective effect of AKAP-Lbc was described which identifies this anchoring protein as underlying the PKA-mediated phosphorylation of Hsp20 on Ser16, thus facilitating the anti-apoptotic actions of the Hsp [48].
A propensity for high blood pressure was recently linked to individuals with a polymorphism in the noncoding region of the AKAP-Lbc gene [49]. Clinical interest in this finding is heightened by evidence that AKAP-Lbc coordinates signaling events underlying pathological cardiac hypertrophy through genetic reprogramming events resulting in aberrant growth of cardiac myocytes. More specifically, AKAP-Lbc synchronizes the actions of GPCR-activated protein kinases and histone remodeling enzymes that impact a variety of transcriptional factors and co-factors. For example, alpha 1 adrenergic receptor (α1AR) stimulation mobilizes a pool of AKAP-Lbc-anchored NFκB inhibitor kinase (IKKβ) to enhance transcription of the NFκB-dependent gene, interleukin-6 [50]. This process results in the recruitment of additional signaling elements to the AKAP-Lbc scaffold as pressure overload via aortic banding results in the addition of RhoA and its effector PKNα along with a p38 mitogen-activated protein kinase (MAPK) cascade. This ultimately triggers cardiomyocyte growth through activation of the mTOR pathway [51, 52]. Targeting this complex may be therapeutically advantageous, particularly since interfering peptides disrupting the AKAP-Lbc/p38 complex impair hypertrophic responses. However, further investigation is necessary to pinpoint which of the growing number of AKAP-Lbc binding partners are the key effectors driving this pathophysiological state.
As described above, pathological cardiac hypertrophy interferes with heart function and ultimately leads to heart failure. However, early compensatory cardiac hypertrophy is initially beneficial in response to increased pressure load or pathological injury, allowing the stressed heart to maintain normal cardiac output and reduce shear stress. While targeted disruption of the AKAP-Lbc/p38 signaling complex impairs hypertrophic responses of transgenic mice, these animals also develop dilated cardiomyopathy and early cardiac dysfunction [51]. These data highlight a pleiotropic role for AKAP-Lbc as anchored signaling is essential for early cardiac development but becomes detrimental during pathological hypertrophy.
The mAKAP-PLCε axis: synchronization of pathophysiological processes during early stage heart disease
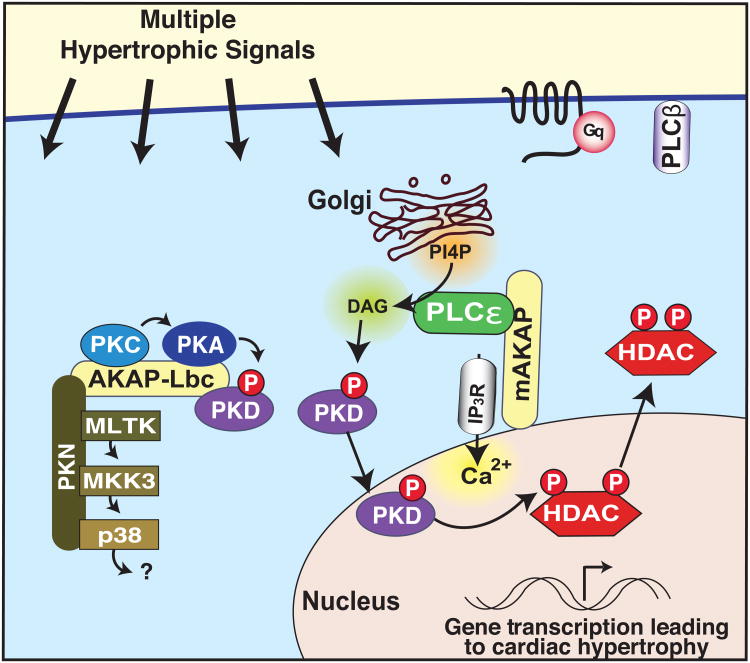
Many contributing factors of cardiovascular disease stimulate GPCRs coupled through Gαq/11 to the activation of phospholipase C (PLC). This results in the formation of diacylglycerol (DAG) and inositol 1,4,5 trisphosphate (IP3) leading to the release of intracellular calcium stores and the activation of PKC. Recently a novel phospholipase isoform PLCε has been implicated in endothelin-1 (ET-1) dependent cardiac hypertrophy in neonatal rat ventricular myocytes (NRVM) (Figure 3) [53-55]. PLCε gene depletion reduces agonist-induced hypertrophy in NRVM. These events occur at the nuclear envelope where PLCε is tethered to mAKAP and peptide mediated mislocalization of PLCε from the anchoring protein attenuates Gq-mediated hypertrophy [55]. This latter report is in contrast to an earlier study indicating that global PLCε gene disruption exacerbates heart failure but is consistent with mouse genetic analysis showing that cardiac-specific conditional knockout of PLCε protects these animals from pressure overload induced hypertrophy [53, 54]. There are several interesting implications arising from this study. First, PLCε is a novel mAKAP binding partner exclusively localized at the nuclear membrane. Second, the anchored enzyme utilizes a previously unrecognized phosphoinositide second messenger P1-4P, the products of which activate protein kinase D [53]. Since PKD is a known AKAP-Lbc binding partner that regulates hypertrophic transcriptional reprogramming it is possible that both anchored enzyme complexes act synergistically to propagate these pathophysiological events [56]. This multifaceted process highlights the utility of AKAPs in the tight temporal control and spatial synchronization of enzymes that catalyze essential cellular processes.
Figure 3. mAKAP spatially synchronizes enzymes to induce cardiac hypertrophy.
Multiple hypertrophic signals work through Gq-coupled GPCRs to activate PLC, resulting in the formation of diacylglycerol (DAG) and inositol 1,4,5 trisphosphate (IP3). The release of intracellular calcium stores act in concert with these lipid signal to activate PKC. In response to ET-1 stimulation, mAKAP anchors PLCε to the nuclear envelope where it hydrolyzes P1-4P from the golgi, producing DAG to activate the lipid responsive enzyme protein kinase D. Activated PKD mediates gene transcription of hypertrophic genes. Peptide-mediated mislocalization of PLCε from the anchoring protein disrupts this signaling pathway to attenuate Gq-mediated hypertrophy [53-55]. Cardiac hypertrophy is also mediated by the AKAP-Lbc signaling platform, which scaffolds the p38 signal transduction cascade, resulting in mTOR activation [51, 52].
AKAPs: towards therapeutic intervention
The previous sections have delineated a role for AKAPs and their associated proteins in disease progression. Thus, selected AKAP complexes may prove to be useful therapeutic targets. Ultimately, this may lead to the design of small molecules that target specific AKAP complexes to correct pathological signaling defects underlying certain diseases.
One translational application of this concept is the use of selected AKAPs as biomarkers for disease. For example, AKAP4 is a cancer testes antigen (CTA) that can also be detected in cervical, ovarian, breast and prostate cancers [57-59]. Since the majority of these patients produce circulating anti-AKAP4 immunoglobulins that can be detected during routine medical screening, it has been proposed that these individuals could be susceptible to AKAP4 immunotherapies [57-59]. Additionally, Gravin promoter hypermethylation is elevated in numerous cancers and is correlated with increased malignancy and metastasis. Therefore, Gravin promoter methylation is a potential biomarker for cancer progression and metastasis that can be detected using methods such as next generation sequencing or methylation-specific PCR [28-33]. This also highlights the utility of personalized medicine as an emerging diagnostic tool.
Chronic and acute pain is a distressing condition that currently affects fifty million Americans. Commonly prescribed analgesic drugs such as non-steroidal anti-inflammatory drugs underperform while opioids are addictive and can cause unpleasant side effects [60]. Consequently, there is a continual need for improved, well-tolerated analgesic agents. As previously discussed, the role TRPV1 plays in noiciception makes it an attractive target for pain management. However, the development of several TRPV1 drugs have stalled in Phase II, largely due to side effects including hyperthermia and impaired noxious heat sensation [60]. Thus disrupting the AKAP-TRPV1 interaction may offer an alternative target for pain management. This notion is supported by recent evidence that cell permeable peptide disruptors of AKAP-TRPV1 interface suppressed inflammatory, thermal and mechanical pain-related behaviors using a mouse foot pad model [23, 24]. While this approach holds some promise, additional studies will be necessary to delineate the role of AKAP79/150 complexes in animal models of chronic pain and whether peptidomimetic or small molecules can be designed to therapeutically manage the AKAP-TRPV1 interface.
Local signaling during cardiac remodeling and hypertrophy is another area of interest. Particularly since protein levels of several AKAPs and associated proteins have been shown to be altered during human heart failure [45, 61]. To date, cultured cells have been used as model systems for most of these studies. Yet it is reassuring that some investigators are moving towards a more translational approach by exploring the in vivo consequences of AKAP mediated signaling in mouse models of cardiac hypertrophy [51]. For example, targeted disruption of the AKAP-Lbc/p38 complex in vivo inhibits pressure-induced cardiac hypertrophy [50]. However, mice expressing the AKAP-Lbc competitor fragment also develop dilated cardiomyopathy and cardiac dysfunction in response to pressure overload [51]. The complexity of these findings highlight a need for further investigations that delineate how uncoupling of AKAP complexes can help treat cardiovascular disease while avoiding undesirable side effects. Another element of this venture will be to establish the precise therapeutic window for these treatments. For example, class 2 HDAC inhibitors currently projected as anti cancer drugs could be repurposed to halt the advance transcriptional reprogramming coordinated by the AKAP-Lbc-PKD-HDAC5 axis that is a hallmark of pathological hypertrophy [45] whereas p38 inhibitors could manage some of the inflammatory responses associated with aberrant AKAP-Lbc signaling in the heart [51].
Concluding remarks: can AKAPs become viable therapeutic targets?
Although the discovery of new AKAPs and their binding partners heightens awareness of local signaling in disease, agents that therapeutically target these macromolecular assemblies remain elusive [62-66]. Currently, interfering peptides that displace anchored enzymes dominate AKAP research [4]. Perhaps the most established mode of this targeted enzyme modulation is to exploit the RII–AKAP interface [67, 68]. The original PKA disruptor peptide Ht31 [69], derived from the anchoring helix of AKAP-Lbc, has been widely used to establish a role for PKA anchoring in a variety of biological systems (reviewed in [4]). Subsequent derivatives have been developed that are more potent than Ht31 [70], and distinguish between anchored type I [71] and type II [67] PKA regulatory subunits. These peptides are useful research tools that probe the cellular ramifications of various anchored signaling events and have been successfully used to reverse pathological phenotypes associated with kinase or phosphatase tethering to AKAPs in animal models [72, 38]. Nevertheless, there are difficulties in targeting peptides to appropriate tissues because of their restricted cellular permeability and the rapid degradation of peptides in vivo. Yet, perhaps the overriding limitation of these anchoring disruptors is their inability to discriminate between the contributions of individual AKAP-PKA complexes. For these reasons, investigators are now pursuing alternate approaches to achieve these goals.
A more promising target for peptidometic interference of local second messenger signaling events is the PIxIxIT motif that displaces phosphatase PP2B from AKAPs and other binding partners. Success in developing small molecule inhibitors targeting AKAPs is promising but has thus far been limited [73]. Current drugs cyclosporin and FK506 are a standard treatment following organ transplant, albeit with significant side effects including elevated blood glucose and hypertension. The seven-amino acid sequence (PIAIIIT) on the anchoring protein AKAP150 controls phosphatase activity and also modulates glucose homeostasis [19]. Hence small molecules that perturb phosphatase tethering to this anchoring protein could boost insulin sensitivity when used in combination with immunosuppressive drugs. The aim of this new therapeutic approach would be to protect organ transplant patients against the onset of diabetes.
An alternative approach to target AKAP-PKA complexes is structure-based phage screening has yielded modified RII subunits of PKA with AKAP-selective binding properties [74]. Still, the utility of these RII selective reagents has yet to be established. Clearly additional insight into the structure and topology of anchored enzyme interfaces will prove invaluable. However, even with high-resolution structural information, AKAP-enzyme interfaces targeting these protein-protein interactions may have its challenges. A glimpse into this complexity comes from evidence that certain well-characterized and potent small molecule ATP analog inhibitors of PKCs are rendered ineffective when the enzyme is associated with AKAP79/150 [75]. This observation is reminiscent of recent reports that Akt and B-Raf can become resistant to the inhibitor drugs A-443654 and PLX4032, respectively [75-78]. Thus, kinase association with endogenous binding partners that confer resistance to ATP analog inhibitors could have important ramifications for drug discovery and research projects predicated on the selectivity of pharmacological protein kinase inhibitors [75]. Certainly this body of work emphasizes that the cellular context of the anchored enzymes must be considered in any viable therapeutic strategy.
Highlights.
AKAPs streamline signal transduction by localizing enzymes with their substrates
AKAPs coordinate aspects of mitosis and cancer progression
AKAP-mediated signaling is implicated in onset & progression of cardiovascular disease
Selected AKAPs may serve as biomarkers for disease
Acknowledgments
The authors wish to thank Lorene Langeberg for help preparing the figures and Melanie Milnes for proofreading and formatting. JDS is supported in part by the Howard Hughes Medical Institute and National Institutes of Health (NIH) grant R01 HL088366. JLE is the recipient of the Heart and Stroke Foundation of Canada Postdoctoral Fellowship.
Abbreviations
- AKAP
A-kinase anchoring protein
- PKC
protein kinase-C
- PP2B
protein phosphatase 2B
- AMPAR
A-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- NFAT
nuclear factor activated in T-cells
- CaV1.2
L-type calcium channel
- LTP
long-term potentiation
- LTD
long-term depression
- FAK
focal adhesion kinase
- MMP
matrix metalloprotease
- ECM
extracellular matrix
- MEK
mitogen activated protein kinase kinase
- ERK
extracellular signal-regulated kinase
- HDAC
histone deacetylase
- TFII-I
transcription factor II-I
- USF
upstream stimulatory factor
- PLC
phospholipase C
- DAG
diacylglycerol
- IP3
inositol 1,4,5 trisphosphate
- RyR2
ryanodine receptor
- PI4P
phosphatidylinositol-4 phosphate
- PKD
protein kinase-D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dodge-Kafka KL, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajagopal S, et al. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanderson JL, Dell'Acqua ML. AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist. 2011;17:321–336. doi: 10.1177/1073858410384740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott JD, et al. Creating order from chaos: cellular regulation by kinase anchoring. Annu Rev Pharmacol Toxicol. 2013;53:187–210. doi: 10.1146/annurev-pharmtox-011112-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klauck TM, et al. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 6.Tunquist BJ, et al. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci U S A. 2008;105:12557–12562. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redden JM, Dodge-Kafka KL. AKAP phosphatase complexes in the heart. J Cardiovasc Pharmacol. 2011;58:354–362. doi: 10.1097/FJC.0b013e31821e5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mochida S, Hunt T. Protein phosphatases and their regulation in the control of mitosis. EMBO Rep. 2012;13:197–203. doi: 10.1038/embor.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, et al. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21:91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dell'Acqua ML, et al. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315-360. J Biol Chem. 2002;277:48796–48802. doi: 10.1074/jbc.M207833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez LL, et al. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J Neurosci. 2002;22:7027–7044. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, et al. Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat Struct Mol Biol. 2012;19:337–345. doi: 10.1038/nsmb.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Shapiro MS. Activity-dependent transcriptional regulation of M-Type (Kv7) K(+) channels by AKAP79/150-mediated NFAT actions. Neuron. 2012;76:1133–1146. doi: 10.1016/j.neuron.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveria SF, et al. Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels. J Neurosci. 2012;32:15328–15337. doi: 10.1523/JNEUROSCI.2302-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 16.Jurado S, et al. A calcineurin/AKAP complex is required for NMDA receptor-dependent long-term depression. Nat Neurosci. 2010;13:1053–1055. doi: 10.1038/nn.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanderson JL, et al. AKAP150-anchored calcineurin regulates synaptic plasticity by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J Neurosci. 2012;32:15036–15052. doi: 10.1523/JNEUROSCI.3326-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dacher M, et al. A-kinase anchoring protein-calcineurin signaling in long-term depression of GABAergic synapses. J Neurosci. 2013;33:2650–2660. doi: 10.1523/JNEUROSCI.2037-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinke SA, et al. Anchored phosphatases modulate glucose homeostasis. EMBO J. 2012;31:3991–4004. doi: 10.1038/emboj.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung MK, et al. Role of TRP channels in pain sensation. Adv Exp Med Biol. 2011;704:615–636. doi: 10.1007/978-94-007-0265-3_33. [DOI] [PubMed] [Google Scholar]
- 21.Jeske NA, et al. A-kinase anchoring protein 150 controls protein kinase C-mediated phosphorylation and sensitization of TRPV1. Pain. 2009;146:301–307. doi: 10.1016/j.pain.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, et al. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59:450–461. doi: 10.1016/j.neuron.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Btesh J, et al. Mapping the binding site of TRPV1 on AKAP79: implications for inflammatory hyperalgesia. J Neurosci. 2013;33:9184–9193. doi: 10.1523/JNEUROSCI.4991-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer MJ, et al. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J Neurosci. 2013;33:7407–7414. doi: 10.1523/JNEUROSCI.3721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efendiev R, et al. Scaffolding by A-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel. J Biol Chem. 2013;288:3929–3937. doi: 10.1074/jbc.M112.428144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhury S, et al. AKAP150-mediated TRPV1 sensitization is disrupted by calcium/calmodulin. Mol Pain. 2011;7:34. doi: 10.1186/1744-8069-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeske NA, et al. A-kinase anchoring protein 150 mediates transient receptor potential family V type 1 sensitivity to phosphatidylinositol-4,5-bisphosphate. J Neurosci. 2011;31:8681–8688. doi: 10.1523/JNEUROSCI.0020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Por ED, et al. PP2B/calcineurin-mediated desensitization of TRPV1 does not require AKAP150. Biochem J. 2010;432:549–556. doi: 10.1042/BJ20100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelman IH. Suppression of tumor and metastasis progression through the scaffolding functions of SSeCKS/Gravin/AKAP12. Cancer Metastasis Rev. 2012;31:493–500. doi: 10.1007/s10555-012-9360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi M, et al. Identification of the A kinase anchor protein 12 (AKAP12) gene as a candidate tumor suppressor of hepatocellular carcinoma. J Surg Oncol. 2012;105:381–386. doi: 10.1002/jso.22135. [DOI] [PubMed] [Google Scholar]
- 31.Jo UH, et al. Methylation of AKAP12{alpha} promoter in lung cancer. Anticancer Res. 2010;30:4595–4600. [PubMed] [Google Scholar]
- 32.Kresse SH, et al. Integrative analysis reveals relationships of genetic and epigenetic alterations in osteosarcoma. PLoS One. 2012;7:e48262. doi: 10.1371/journal.pone.0048262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, et al. Quantitative assessment of AKAP12 promoter methylation in colorectal cancer using methylation-sensitive high resolution melting: Correlation with Duke's stage. Cancer Biol Ther. 2010;9:862–871. doi: 10.4161/cbt.9.11.11633. [DOI] [PubMed] [Google Scholar]
- 34.Wu W, et al. Examination of AKAP12 promoter methylation in skin cancer using methylation-sensitive high-resolution melting analysis. Clin Exp Dermatol. 2011;36:381–385. doi: 10.1111/j.1365-2230.2010.03968.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, et al. A genome-wide association study identifies novel loci associated with susceptibility to chronic myeloid leukemia. Blood. 2011;117:6906–6911. doi: 10.1182/blood-2011-01-329797. [DOI] [PubMed] [Google Scholar]
- 36.Bu Y, et al. Role for transcription factor TFII-I in the suppression of SSeCKS/Gravin/Akap12 transcription by Src. Int J Cancer. 2011;128:1836–1842. doi: 10.1002/ijc.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su B, et al. SSeCKS/Gravin/AKAP12 inhibits cancer cell invasiveness and chemotaxis by suppressing a protein kinase C- Raf/MEK/ERK pathway. J Biol Chem. 2010;285:4578–4586. doi: 10.1074/jbc.M109.073494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith FD, et al. AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat Cell Biol. 2010;12:1242–1249. doi: 10.1038/ncb2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su B, et al. Adhesion-mediated cytoskeletal remodeling is controlled by the direct scaffolding of Src from FAK complexes to lipid rafts by SSeCKS/AKAP12. Oncogene. 2013;32:2016–2026. doi: 10.1038/onc.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo LW, et al. Control of protein kinase C activity, phorbol ester-induced cytoskeletal remodeling, and cell survival signals by the scaffolding protein SSeCKS/GRAVIN/AKAP12. J Biol Chem. 2011;286:38356–38366. doi: 10.1074/jbc.M111.258830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nauert JB, et al. Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr Biol. 1997;7:52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- 42.Canton DA, et al. Gravin is a transitory effector of polo-like kinase 1 during cell division. Mol Cell. 2012;48:547–559. doi: 10.1016/j.molcel.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diviani D, et al. A-kinase anchoring proteins: scaffolding proteins in the heart. Am J Physiol Heart Circ Physiol. 2011;301:H1742–1753. doi: 10.1152/ajpheart.00569.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lygren B, et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carnegie GK, et al. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayers CM, et al. The Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J Biol Chem. 2010;285:12344–12354. doi: 10.1074/jbc.M110.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spindler MJ, et al. AKAP13 Rho-GEF and PKD-binding domain deficient mice develop normally but have an abnormal response to beta-adrenergic-induced cardiac hypertrophy. PLoS One. 2013;8:e62705. doi: 10.1371/journal.pone.0062705. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Edwards HV, et al. The A-kinase-anchoring protein AKAP-Lbc facilitates cardioprotective PKA phosphorylation of Hsp20 on Ser(16) Biochem J. 2012;446:437–443. doi: 10.1042/BJ20120570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hong KW, et al. A regulatory SNP in AKAP13 is associated with blood pressure in Koreans. J Hum Genet. 2011;56:205–210. doi: 10.1038/jhg.2010.167. [DOI] [PubMed] [Google Scholar]
- 50.del Vescovo CD, et al. A-kinase-anchoring protein-Lbc anchors IkappaB kinase beta to support interleukin-6-mediated cardiomyocyte hypertrophy. Mol Cell Biol. 2013;33:14–27. doi: 10.1128/MCB.00887-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez Lopez I, et al. A-Kinase Anchoring Protein Lbc Coordinates a p38 Activating Signaling Complex Controlling Compensatory Cardiac Hypertrophy. Mol Cell Biol. 2013;33:2903–2917. doi: 10.1128/MCB.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cariolato L, et al. A-kinase anchoring protein (AKAP)-Lbc anchors a PKN-based signaling complex involved in alpha1-adrenergic receptor-induced p38 activation. J Biol Chem. 2011;286:7925–7937. doi: 10.1074/jbc.M110.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, et al. Phospholipase Cepsilon hydrolyzes perinuclear phosphatidylinositol 4-phosphate to regulate cardiac hypertrophy. Cell. 2013;153:216–227. doi: 10.1016/j.cell.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, et al. Phospholipase C epsilon modulates beta-adrenergic receptor-dependent cardiac contraction and inhibits cardiac hypertrophy. Circ Res. 2005;97:1305–1313. doi: 10.1161/01.RES.0000196578.15385.bb. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L, et al. Phospholipase C epsilon scaffolds to muscle-specific A kinase anchoring protein (mAKAPbeta) and integrates multiple hypertrophic stimuli in cardiac myocytes. J Biol Chem. 2011;286:23012–23021. doi: 10.1074/jbc.M111.231993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carnegie GK, et al. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol Cell. 2004;15:889–899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal S, et al. Expression and humoral response of a-kinase anchor protein 4 in cervical cancer. Int J Gynecol Cancer. 2013;23:650–658. doi: 10.1097/IGC.0b013e31828a0698. [DOI] [PubMed] [Google Scholar]
- 58.Saini S, et al. A novel cancer testis antigen, A-kinase anchor protein 4 (AKAP4) is a potential biomarker for breast cancer. PLoS One. 2013;8:e57095. doi: 10.1371/journal.pone.0057095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiriva-Internati M, et al. Cancer testis antigens: a novel target in lung cancer. Int Rev Immunol. 2012;31:321–343. doi: 10.3109/08830185.2012.723512. [DOI] [PubMed] [Google Scholar]
- 60.Szallasi A, Sheta M. Targeting TRPV1 for pain relief: limits, losers and laurels. Expert Opin Investig Drugs. 2012;21:1351–1369. doi: 10.1517/13543784.2012.704021. [DOI] [PubMed] [Google Scholar]
- 61.Aye TT, et al. Reorganized PKA-AKAP associations in the failing human heart. J Mol Cell Cardiol. 2012;52:511–518. doi: 10.1016/j.yjmcc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Means CK, et al. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc Natl Acad Sci U S A. 2011;108:E1227–1235. doi: 10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uys GM, et al. Myomegalin is a novel A-kinase anchoring protein involved in the phosphorylation of cardiac myosin binding protein C. BMC Cell Biol. 2011;12:18. doi: 10.1186/1471-2121-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burgers PP, et al. A small novel A-kinase anchoring protein (AKAP) that localizes specifically protein kinase A-regulatory subunit I (PKA-RI) to the plasma membrane. J Biol Chem. 2012;287:43789–43797. doi: 10.1074/jbc.M112.395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shanks MO, et al. Chromodomain helicase binding protein 8 (Chd8) is a novel A-kinase anchoring protein expressed during rat cardiac development. PLoS One. 2012;7:e46316. doi: 10.1371/journal.pone.0046316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim M, et al. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32:1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gold MG, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–395. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Kinderman FS, et al. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol Cell. 2006;24:397–408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenmund C, et al. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 70.Alto NM, et al. Bioinformatic design of A-kinase anchoring protein-in silico: a potent and selective peptide antagonist of type II protein kinase A anchoring. Proc Natl Acad Sci U S A. 2003;100:4445–4450. doi: 10.1073/pnas.0330734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carlson CR, et al. Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J Biol Chem. 2006;281:21535–21545. doi: 10.1074/jbc.M603223200. [DOI] [PubMed] [Google Scholar]
- 72.McConnell BK, et al. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J Biol Chem. 2009;284:1583–1592. doi: 10.1074/jbc.M806321200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christian F, et al. Small molecule AKAP-protein kinase A (PKA) interaction disruptors that activate PKA interfere with compartmentalized cAMP signaling in cardiac myocytes. J Biol Chem. 2011;286:9079–9096. doi: 10.1074/jbc.M110.160614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gold MG, et al. Engineering A-kinase anchoring protein (AKAP)-selective regulatory subunits of protein kinase A (PKA) through structure-based phage selection. J Biol Chem. 2013;288:17111–17121. doi: 10.1074/jbc.M112.447326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoshi N, et al. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol Cell. 2010;37:541–550. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poulikakos PI, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brennan DF, et al. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature. 2011;472:366–369. doi: 10.1038/nature09860. [DOI] [PubMed] [Google Scholar]
- 78.Okuzumi T, et al. Inhibitor hijacking of Akt activation. Nat Chem Biol. 2009;5:484–493. doi: 10.1038/nchembio.183. [DOI] [PMC free article] [PubMed] [Google Scholar]