SUMMARY
Vancomycin-resistant Enterococcus bloodstream infections (VRE-BSI) are a growing problem with few clinical trials to guide therapy. We conducted a retrospective study of management and predictors of mortality for VRE-BSI at a tertiary care centre from January 2005 to August 2008. Univariate and multivariable analyses examined the relationship of patient characteristics and antibiotic therapy with 30-day all-cause mortality. Rates of VRE-BSI increased from 0.06 to 0.17 infections/thousand patient days (p=0.03). Among 235 patients, 30-day mortality was 34.9%. Patients were primarily treated with linezolid (44.2%) or daptomycin (36.5%). Factors associated with mortality were haemodialysis (OR 3.2, 95% CI 1.6-6.3, p=0.007), mechanical ventilation (OR 3.7, 95% CI 1.3-10.4, p=0.01), and malnutrition (OR 2.0, 95% CI 1.0-4.0, p=0.046). Use of linezolid, but not daptomycin (p=0.052) showed a trend toward an association with survival. In conclusion, VRE-BSI is a growing problem, associated with significant 30-day mortality. Multiple factors were associated with poor outcomes at our hospital.
INTRODUCTION
Enterococci are the second most common cause of nosocomial bloodstream infections in the United States [1] and vancomycin-resistance occurs in nearly one third of isolates. Bloodstream infections due to vancomycin-resistant Enterococcus sp. (VRE-BSI) are associated with increased morbidity, mortality, and healthcare expenditures for hospitalized patients [2-12]. Fortunately, novel antimicrobials with activity against VRE (daptomycin, linezolid, quinupristin-dalfopristin, and tigecycline) provide a therapeutic option for physicians.
The impact of newer antimicrobial agents on predictors of mortality from VRE-BSI needs further investigation [5, 13-16]. While previous research has identified factors associated with poor outcomes from VRE-BSI (Charlson Co-morbidity Index, the number of positive blood cultures, parenteral nutrition, severity of illness, renal function, liver disease, malignancy, and prior VRE infection) [4, 5, 7, 16-18], most of these studies were conducted prior to widespread use of novel antimicrobials with activity against VRE. Herein, we explore outcomes of patients with VRE-BSI at a tertiary care medical center, focusing on antimicrobial therapy and factors associated with mortality for VRE-BSI.
PATIENTS AND METHODS
Clinical Data
We conducted a retrospective study of patients with nosocomial VRE-BSI at University Hospital in Birmingham, Alabama between January 1, 2005 and August 1, 2008. All patients who met CDC criteria for nosocomial BSIs with vancomycin-resistant Enterococcus spp were included in the study[19]. All blood cultures were analyzed with an automated culture system (BacT/ALERT, bioMerieux Industries, Hazelwood, Missouri) and automated susceptibility testing (Microscan Walkaway 96 SI, Siemens Healthcare Diagnostics, Deerfield, IL). Rates of VRE-BSI were calculated as number of patients with bacteremia per 1,000 patient days of care.
Medical records were reviewed for demographic information, presence of co-morbidities, laboratory data, antimicrobial treatment, and outcomes. The following definitions were used: cancer – any active malignancy; liver disease – cirrhosis due to any cause, chronic viral hepatitis, or transaminase levels at least four times the upper limit of normal; recent surgery – surgery within the prior sixty days; transplant – receipt of a hematopoietic stem cell or solid organ transplant; leukocytosis – white blood cell (WBC) count >20,000 cells/mm3; neutropenia – absolute neutrophil count <500 neutrophils/mm3; malnutrition – serum albumin < 2.0 mg/dL; malnutrition – serum albumin < 2.0 mg/dL; concurrent bloodstream infection - positive blood culture for bacteria or fungi with associated findings of infection within 14 days before or after the first positive VRE culture. Renal insufficiency was defined as creatinine > 2 g/dl or hemodialysis at time of VRE culture. Immunosuppression was defined as receipt of any immunosuppressive medication, excluding antineoplastic agents for the treatment of cancer, during the index hospitalization. Corticosteroid doses equivalent to less than 10 mg of prednisone were not included as immunosuppression. An aggregate measure of underlying illness was measured using the Charlson co-morbidity index (CCI) [20]. The CCI is predictive of mortality for a patient who may have a range of co-morbid conditions such as heart disease, HIV/AIDS, or cancer (a total of 22 conditions). Each condition is assigned a score from 1 to 6 depending on the risk of dying associated with the condition. The CCI is the sum of these scores and a higher score is associated with a higher likelihood of death.
Primary antimicrobial therapy for VRE was defined as receipt of daptomycin, linezolid, quinupristin-dalfopristin, or tigecycline for at least three of the initial four days of VRE treatment. Time to initiation of antibiotics was determined from collection of the first blood culture that subsequently grew VRE to the start date of antibiotics, measured in days. Microbiologic failure was defined as persistently positive blood cultures while on appropriate therapy for at least 24 h or recurrence of positive blood cultures within one week of completing therapy. Antimicrobial stewardship guidelines were in place throughout the hospital for the entire course of the study. Daptomycin and linezolid were unrestricted for the treatment of VRE, but quinupristin-dalfopristin and tigecycline required verbal approval from an infectious disease specialist. Infectious disease consultation was recommended, but not required for all VRE infections. The primary outcome of interest was all-cause mortality at 30 days following the first positive culture for VRE.
Statistical analyses
Frequencies of categorical variables and means, medians and standard deviations of continuous variables were calculated for the overall population and for patients who received appropriate treatment. For analysis of the relationships of variables to survivors and non-survivors, univariate analyses were performed using Chi-square or Fisher’s exact methods for categorical variables and student’s t-test or the Wilcoxon rank sum test for continuous variables. The primary model for factors associated with mortality was created using stepwise multiple logistic regression analysis. Models using all-cause mortality as the dependent variable were determined for the overall population, and then for patients who received appropriate treatment. All variables significant at α=0.20 in univariate analyses were considered as possible predictor variables for the multivariable analyses. Age was entered into the final model as a continuous variable. The criterion for entry into the models was significance at α=0.20, while the criterion for remaining in the model was significance at α=0.05. Odds ratios and corresponding 95% confidence intervals were calculated. Model fit was assessed using the Hosmer-Lemeshow goodness-of-fit statistic, and it was determined that all models fit the data well. A potential interaction between ICU stay and ventilator use was evaluated by incorporating an interaction term into the final model. In addition to the primary models, where co-morbidities were entered individually, models were constructed using the CCI as a continuous variable.
All statistical tests were two-tailed and were performed using a 0.05 significance level. Statistical analyses were conducted using SAS (version 9.1; SAS Institute, Inc., Cary, NC). This study was approved by the University of Alabama at Birmingham Institutional Review Board.
RESULTS
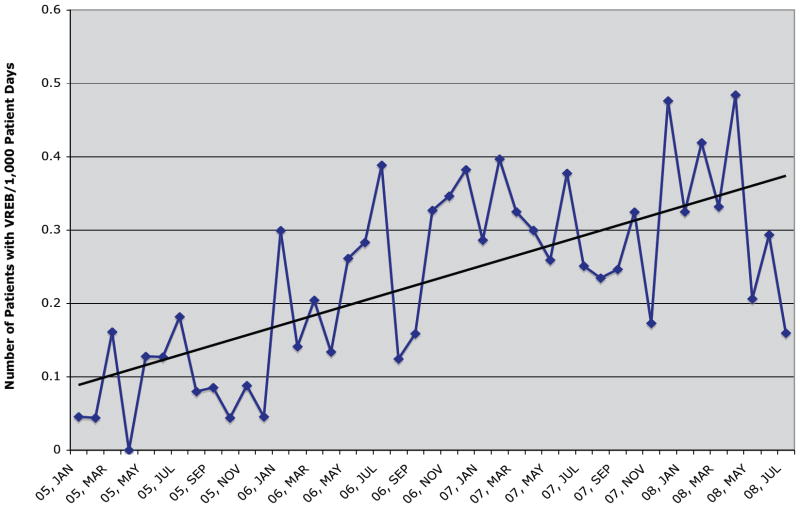
Of two hundred thirty seven patient episodes of VRE-BSI that occurred during the study period, 235 had outcome data available and were included in analyses. Over the study period, rates of VRE-BSI increased significantly from a baseline of 0.06 infections/thousand patient days to 0.17 infections/thousand patient days (p=0.03, Figure 1). Mean age of patients with VRE-BSI was 53.7 years and 42.3% were male. E. faecium caused 227 (96.6%) VRE-BSI, with the remaining due to E. faecalis. Patient characteristics among survivors and non-survivors are shown in Table 1. Frequent underlying illnesses present at the time of the VRE-BSI included malnutrition (60.3%), concurrent bloodstream infection (51%), renal insufficiency (48.5%), mechanical ventilation (29.3%), and cancer (31.6%). More than one third of patients (39.2%) were located in an intensive care unit when the index blood cultures were drawn. Medical co-morbidities among patients were common; with a CCI score ≥6 in 149 (63.4%) patients.
Figure 1.
The number of patients with VRE bloodstream infections per 1,000 patient days of care plotted per month at the University Hospital in Birmingham, Alabama. Linear trend added.
Table 1.
Characteristics of 235 patients with VRE bloodstream infections with comparison of Survivors versus Non-Survivors using T-test or Chi-Square analysis.
| Characteristic | Total N(%) | Survivors | Non-survivors | P value |
|---|---|---|---|---|
| N=235 | (N=153) | (N=82) | ||
| Age, mean ± SDa | 53.7±14.3 | 52.6±14.4 | 55.9±13.9 | 0.09 |
| Male sex | 99(42) | 68(44) | 31(38) | 0.31 |
| Black race | 115(49) | 72(47) | 43(52) | 0.43 |
| Cancer | 71(30) | 44(29) | 27(33) | 0.31 |
| Recent surgery | 87(37) | 54(35) | 33(40) | 0.43 |
| Leukocytosis | 52(22) | 30(20) | 22(27) | 0.20 |
| Mechanical ventilation | 69(29) | 31(20) | 38(46) | <0.001 |
| ICUb | 92(39) | 49(32) | 43(52) | 0.002 |
| Renal Insufficiency | 114(49) | 60(39) | 54(66) | <0.001 |
| Liver disease | 53(23) | 30(20) | 23(28) | 0.27 |
| Malnutrition | 117(50) | 64(42) | 53(64) | 0.01 |
| Diabetes mellitus | 82/228(36) | 53/149(36) | 29/79(37) | 0.86 |
| Immunosuppression | 52(22) | 35(23) | 17(21) | 0.68 |
| Neutropenia | 36(15) | 24(16) | 12(15) | 0.83 |
| Transplant recipient | 37(16) | 28(18) | 9(11) | 0.14 |
| Concurrent BSI | 121(51) | 80(52) | 41(50) | 0.73 |
| HIV/AIDSc | 7(3) | 6(4) | 1(1) | 0.24 |
| ID consultationd | 34(15) | 27(18) | 7(9) | 0.06 |
| No Antibiotic Therapy | 45(19) | 23(15) | 22(27) | 0.03 |
| Daptomycin Therapy | 86/190(45) | 54/130(42) | 32/60(53) | 0.14 |
| Linezolid Therapy | 104/190(55) | 76/130(58) | 28/60(47) | 0.10 |
| Tigecycline Therapy | 2/190(1) | 2/130(2) | 0/60(0) | 0.56 |
| Enterococcus faecium | 227(97) | 147(96) | 80(98) | 0.55 |
| Timing of antibiotics (mean ± SD) | 2.65 ± 1.6 | 2.78 ± 1.7 | 2.36 ± 1.4 | 0.17 |
| CCIe (mean ± SD) | 5.5 ± 2.9 | 5.15 ± 2.9 | 6.14 ± 2.7 | 0.03 |
SD = standard deviation;
ICU = intensive care unit;
HIV/AIDS = human immunodeficiency virus/acquired immunodeficiency syndrome;
ID = infectious diseases;
CCI = Charlson comorbidity index
All cause mortality at 30 days was 34.9% (82/235). Significant differences between survivors and non-survivors in univariate analysis included presence of mechanical ventilation (20.3% vs. 46.3%, p < 0.001); location in ICU (32.0% vs. 52.4%, p=0.002); renal insufficiency (39.2% vs. 65.9%, p < 0.001); malnutrition (53.0% vs. 71.6%, p=0.01); receipt of antibiotics active against the VRE isolate (85.0% vs. 73.2%, p=0.03); and mean CCI (5.15 vs. 6.14, p=0.03).
Independent predictors of mortality
Predictors of mortality in the overall population were explored in a multivariable logistic regression model (Table 2). Factors independently associated with 30-day mortality included mechanical ventilation (OR 3.32, 95% CI 1.7-10.4, p=0.007), renal insufficiency (OR 3.2, 95% CI 1.6-6.3, p=0.007) and malnutrition (OR 2.0, 95% CI 1.0-4.0, p=0.046). There was an association with receipt of VRE-active antibiotics and increased survival (OR 0.53, 95% CI 0.2-1.2, p=0.13), but this was not statistically significant. In a model with age, sex, race, timing of antibiotics, transplant and CCI (instead of individual co-morbidities), CCI was an independent predictor of mortality (OR 1.13, 95% CI 1.03-1.25, p=0.01).
Table 2.
Multivariable logistic regression analysisa of factors related to 30-day mortality in the overall population (n=235).
| Variable | Univariate | Multivariableb | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| Age | 1.0 (0.9-1.04) | 0.09 | ||
| Male Sex | 0.8 (0.4-1.3) | 0.31 | ||
| Black race | 1.2 (0.7-2.1) | 0.43 | ||
| Ventilator | 3.4 (1.9-6.1) | <0.001 | 3.32 (1.7-6.6) | 0.007 |
| ICU location | 2.3 (1.4-4.1) | 0.003 | ||
| Renal Insufficiency | 3.0 (1.7-5.2) | <0.001 | 3.2 (1.6-6.3) | 0.007 |
| Malnutrition | 2.2 (1.2-4.1) | 0.01 | 2.0 (1.0-4.0) | 0.046 |
| Antibiotic Treatment (vs. No Antibiotic Treatment) | 0.5 (0.25-0.93) | 0.03 | 0.53 (0.2-1.2) | 0.13 |
| ID Consultation | 0.4 (0.2-1.05) | 0.06 | 0.4 (0.2-1.2) | 0.06 |
| Transplant | 0.5 (0.2-1.2) | 0.14 | ||
Logistic regression analysis. Variables with p<0.20 on univariate analysis were included in a multivariable stepwise regression model in addition to variables for race and sex. Results are listed for variables with p<0.20. p-values obtained are two-tailed.
Each of the multivariable odds ratios are adjusted for all of the other variables remaining in the final model.
OR = odds ratio; CI = confidence interval.
VRE Antimicrobial Therapy
Linezolid was the primary therapy for VRE-BSI in 104 (44%) patients, daptomycin in 86 (37%) patients and tigecycline in 2 (1%) patients. No patient received quinupristin-dalfopristin. The remaining 43 (18%) did not receive VRE-specific therapy. Among those who did not receive specific antimicrobial therapy, 22 (51%) of 43 died by day 30. Eighteen (42%) of 43 died before blood culture results were identified VRE.
Microbiologic failure occurred in 18 patients treated with linezolid (17.5%) and 25 patients treated with daptomycin (29%). No failure occurred in 2 patients treated with tigecycline. There were 28 (27%) deaths in patients treated with linezolid, 32 (37.2%) deaths in patients treated with daptomycin and no deaths in patients treated with tigecycline. Concurrent BSI was noted in 50/86 patients in the daptomycin group (58%) and 54/104 patients in the linezolid group (52%).
A multivariable logistic regression analysis was conducted to identify independent predictors of mortality for those patients who received VRE-active therapy (Table 3). Patients treated with tigecycline were excluded from the analysis because of limited sample size. Among this population of 190 patients, mechanical ventilation (OR 3.4, 95% CI 1.5-7.7, p=0.003) and renal insufficiency (OR 4.1, 95% CI 1.9-9.1, p <0.001) were predictors of increased mortality. Receipt of a solid organ or stem cell transplant was associated with improved survival (OR 0.2, 95% CI 0.06-0.8, p=0.027). Delayed administration of antibiotics was associated with decreased mortality (OR 0.8, 95% CI 0.55-1.00, p=0.051), but this was not significant. In addition, there was an association of daptomycin therapy and increased mortality (OR 2.1, 95% CI 0.99-4.7, p=0.052), but this did not reach statistical significance. In a model with age, sex, race, timing of antibiotics, transplant and CCI (instead of individual co-morbidities), only CCI was an independent predictor of mortality (OR 1.13, 95% CI 1.00-1.29, p=0.03).
Table 3.
Multivariable logistic regression analysis of factors related to 30-day mortality in patients who received VRE-active therapy (n=190)ab.
| Variable | Univariate | Multivariablec | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| Age | 1.01 (0.99-1.03) | 0.40 | ||
| Male Sex | 0.9 (0.5-1.6) | 0.67 | ||
| Black race | 1.4 (0.7-2.5) | 0.32 | ||
| Ventilator | 3.1 (1.6-6.0) | <0.001 | 3.4 (1.5-7.7) | 0.003 |
| ICU | 2.2 (1.2-4.1) | 0.01 | ||
| Renal Insufficiency | 3.2 (1.7-6.0) | <0.001 | 4.1 (1.9-9.1) | 0.004 |
| Malnutrition | 1.8 (0.9-3.7) | 0.01 | ||
| Daptomycin (vs. Linezolid) | 1.6 (0.87-3.0) | 0.13 | 2.1 (0.99-4.7) | 0.052 |
| Liver Disease | 1.7 (0.8-3.6) | 0.16 | ||
| Timing of Antibiotics | 0.8 (0.7-1.0) | 0.09 | 0.8 (0.55-1.0) | 0.051 |
| Transplant | 0.4 (0.15-1.1) | 0.07 | 0.2 (0.06-0.8) | 0.027 |
Logistic regression analysis. Variables with p<0.20 on univariate analysis were included in a multivariable stepwise regression model in addition to variables for age, sex and race. Results are listed for variables with p<0.10. p-values obtained are two-tailed.
Due to limited sample size two patients treated with Tigecycline were not included in this analysis.
Each of the multivariable odds ratios are adjusted for all of the other variables remaining in the final model.
OR = odds ratio; CI = confidence interval.
Characteristics of patients receiving either linezolid or daptomycin were compared in a sub-analysis (Table 4). Neutropenia was more common among patients who received daptomycin (29% vs. 7.7%, p < 0.001); and ID consultation was more common in patients who received linezolid (24.0% vs. 8.1%, p=0.003). Overall length-of-therapy was a median of 11 days (range, 3-47 days), and was not significantly different between the two groups.
Table 4.
Characteristics of patients treated with linezolid (n=104) or daptomycin (n=86).
| Characteristic | Linezolid | Daptomycin | P value |
|---|---|---|---|
| N=104 (%) | N=86 (%) | ||
| Age, mean ± SD | 54.5±14.3 | 50.1±13.5 | 0.52 |
| Male sex | 42(40) | 41(48) | 0.28 |
| Black race | 47(45) | 41(48) | 0.73 |
| Cancer | 28(27) | 32(37) | 0.11 |
| Recent surgery | 47(45) | 28(33) | 0.09 |
| Leukocytosis | 28(27) | 15(17) | 0.12 |
| Ventilator | 30(29) | 24(28) | 0.90 |
| ICU | 43(41) | 33(38) | 0.67 |
| Renal Insuffieciency | 48(46) | 42(49) | 0.71 |
| Liver disease | 25/84(30) | 14/76(18) | 0.10 |
| Malnutrition | 51/82(62) | 41/74(55) | 0.39 |
| Diabetes | 38(37) | 32(37) | 0.80 |
| Immunosuppressives | 25(24) | 19(22) | 0.80 |
| Neutropenia | 8(8) | 25(29) | <0.001 |
| Transplant | 15(15) | 14(16) | 0.70 |
| Concurrent BSI | 54(52) | 50(58) | 0.43 |
| ID consultation | 25(24) | 7(8) | 0.003 |
| HIV/AIDS | 2(2) | 3(3) | 0.50 |
| Enterococcus faecium | 101(99) | 85(99) | 0.41 |
| Timing of Antibiotics, mean ±SD | 2.38 ± 1.7 | 2.25 ± 1.5 | 0.58 |
| CCI, mean ±SD | 5.0 ± 2.8 | 4.6 ± 2.8 | 0.4 |
DISCUSSION
This retrospective cohort study of 235 patients represents one of the largest published investigations of nosocomial VRE-BSI. Our findings provide important insights into the epidemiology and management of this growing health problem. For example, we observed nearly a three-fold increase in VRE-BSI incidence during the 2.5-year study period. We also report a 30-day all-cause mortality of 35%, consistent with previous observations [2-12]. These data provide “real world” evidence that VRE-BSI is a growing problem for hospitalized patients in the United States [2, 3, 21, 22].
A crucial component of the epidemiology of VRE-BSI is the identification of factors that relate to patient outcomes. Previous studies found the CCI, number of positive blood cultures, parenteral nutrition, severity of illness, decreased renal function, liver disease, malignancy, and prior VRE infection to be associated with poor outcomes among cases of VRE infection [4, 5, 7, 16-18]. Most of these studies were conducted when there were relatively limited treatment options for VRE. Our study confirmed several previous findings. In both the overall population and those receiving antibiotics, renal insufficiency and mechanical ventilation were independent predictors of increased mortality [5, 7, 17, 23]. Similar to the recent study from Camins and colleagues, we also observed that CCI was a significant predictor of mortality [17]. Previous studies have not addressed specifically lack of antibiotics and mortality. In our cohort, treatment with VRE active antimicrobial was associated with improved survival in univariate analysis (p=0.03). Although these findings did not reach statistical significance in multivariable analysis (p=0.13), the authors favor treatment for patients with VRE-BSI.
Providers at our institution prescribed daptomycin and linezolid frequently; prescribed tigecycline infrequently; and did not use quinupristin-dalfopristin. Antimicrobial stewardship guidelines during the study period required infectious disease consultation or specific approval prior to use of quinupristin-dalfopristin and tigecycline, which probably led to decreased use of these agents. However, the lack of consensus on treatment strategy is also likely related to lack of published data to guide therapy. To date, there are a few retrospective observational investigations comparing treatment options for VRE–BSI[5,16]. Furthermore, most data supporting the use of newer agents with in vitro activity against VRE; daptomycin, linezolid, quinupristin-dalfopristin, and tigecycline; are based on case series and case reports [5, 13-16, 24-31]. Additional research, preferably an adequately powered randomized controlled trial, is needed to determine optimal selection of antimicrobial therapy in this context.
When limiting our analysis to patients who received VRE-active antimicrobial therapy, use of daptomycin showed a trend toward an association with mortality, but was not significant in multivariable analysis (p=0.052). Our observations that daptomycin therapy may be associated with poorer outcomes when compared with linezolid were unexpected; however, ours is the second investigation to suggest a potential difference. In a retrospective study of 98 patients with VRE bacteremia, Mave and colleagues demonstrated a trend towards higher mortality with daptomycin when compared to linezolid [16]. Our observations, while similar, should not be considered conclusive, as they are likely influenced by several important factors. First, when we forced the CCI into the multivariable model (data not shown), the association of daptomycin and mortality was less pronounced (OR 1.7), suggesting that “sicker” people received daptomycin therapy. Second, there were important differences in patients who received daptomycin or linezolid therapy, including neutropenia and number of ID consultations. Differences in ID consultation may be relevant, as published reports demonstrate decreased mortality in patients with candidemia and staphylococcal bacteremia in patients who received ID consultation [32, 33]. Lastly, our finding that transplant patients had a lower mortality is likely spurious and suggests confounding in this investigation. Although our data are limited by being observational in nature, further research into the comparative effectiveness of these two agents is warranted.
Our study has several other limitations. A high number of concurrent BSI (n=120) was observed. The high frequency of BSI (51%) is likely the result of our generous timeframe for concurrent infection (+/- 14 days). Although the majority of patients in our cohort received effective therapy for concurrent BSI (>90%), the bloodstream isolates were heterogeneous in grade of bacteremia (number of positive cultures) and causative organism, e.g. Klebsiella, Candida, Staphylococcus, and other spp. Future studies should consider a different time interval (+7/-2 days) and make specific adjustments for type and grade of BSI. Although this represents a large cohort of VRE-BSI patients, some analyses were limited by small variable frequencies. Our study did not evaluate treatment of concurrent pneumonias. As daptomycin has limited efficacy in lung parenchyma, there may have been increased mortality due to pulmonary infections not captured. We were unable to determine appropriate dosing or levels of daptomycin in patients on hemodialysis. As this was a retrospective study, we were unable to collect robust severity-of-illness data such as APACHE scores.
In conclusion, this investigation confirms that VRE bloodstream infections continue to be a challenging problem and are associated with increased mortality. Our observations on “real-world” management of VRE-BSI indicate that limited clinical trial data has lead to treatment equipoise. Given the growing burden of disease and the serious consequences of infection, there is a pressing need to understand optimal management of VRE-BSI, especially the impact of newer antimicrobial therapy. Identifying predictors of mortality in VRE-BSI patients treated with newer antimicrobial agents may aid in future study design.
Acknowledgments
We thank Darlene Green, Aaron Jones, Steve Duncan, and Marga Jones for assistance in data collection and organization. We thank Dr. Arnold Bayer and Dr. Loren Miller for their review of the manuscript. We recognize the logistic support of the M01 RR 00425 grant to the GCRC at Harbor-UCLA Medical Center.
JWB has received research support from Pfizer.
Footnotes
Declaration of Interest:
There was no financial support for this project.
JM: None
MP is a member of the Speaker’s Bureaus for Cubist Pharmaceuticals and Astra-Zeneca Pharmaceuticals
RS: None
DFK: None
SAM: None
References
- 1.Hidron AI, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infection Control and Hospital Epidemiology. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande LM, et al. Antimicrobial resistance and molecular epidemiology of vancomycin-resistant enterococci from North America and Europe: a report from the SENTRY antimicrobial surveillance program. Diagnostic Microbiology and Infectious Diseases. 2007;58:163–170. doi: 10.1016/j.diagmicrobio.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 3.System NNIS. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. American Journal of Infection Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 4.Vergis EN, et al. Determinants of vancomycin resistance and mortality rates in enterococcal bacteremia. a prospective multicenter study. Annals of Internal Medicine. 2001;135:484–492. doi: 10.7326/0003-4819-135-7-200110020-00007. [DOI] [PubMed] [Google Scholar]
- 5.Erlandson KM, et al. Impact of the more-potent antibiotics quinupristin-dalfopristin and linezolid on outcome measure of patients with vancomycin-resistant Enterococcus bacteremia. Clinical Infectious Diseases. 2008;46:30–36. doi: 10.1086/523588. [DOI] [PubMed] [Google Scholar]
- 6.DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. Journal of Infectious Diseases. 2005;191:588–595. doi: 10.1086/427512. [DOI] [PubMed] [Google Scholar]
- 7.Gearhart M, et al. Consequences of vancomycin-resistant Enterococcus in liver transplant recipients: a matched control study. Clinical Transplantation. 2005;19:711–716. doi: 10.1111/j.1399-0012.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 8.Garbutt JM, et al. Association between resistance to vancomycin and death in cases of Enterococcus faecium bacteremia. Clinical Infectious Diseases. 2000;30:466–472. doi: 10.1086/313694. [DOI] [PubMed] [Google Scholar]
- 9.Song JH, et al. Clinical implications of vancomycin-resistant Enterococcus faecium (VRE) with VanD phenotype and vanA genotype. Journal of Antimicrobial Chemotherapy. 2008;61:838–844. doi: 10.1093/jac/dkn025. [DOI] [PubMed] [Google Scholar]
- 10.Song X, et al. Effect of nosocomial vancomycin-resistant enterococcal bacteremia on mortality, length of stay, and costs. Infection Control and Hospital Epidemiology. 2003;24:251–256. doi: 10.1086/502196. [DOI] [PubMed] [Google Scholar]
- 11.Papanicolaou GA, et al. Nosocomial infections with vancomycin-resistant Enterococcus faecium in liver transplant recipients: risk factors for acquisition and mortality. Clinical Infectious Diseases. 1996;23:760–766. doi: 10.1093/clinids/23.4.760. [DOI] [PubMed] [Google Scholar]
- 12.DiazGranados CA, et al. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clinical Infectious Diseases. 2005;41:327–333. doi: 10.1086/430909. [DOI] [PubMed] [Google Scholar]
- 13.Warren RE. Daptomycin in endocarditis and bacteraemia: a British perspective. Journal of Antimicrobial Chemotherapy. 2008;62(Suppl 3):iii25–33. doi: 10.1093/jac/dkn370. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz BS, Ngo PD, Guglielmo BJ. Daptomycin treatment failure for vancomycin-resistant Enterococcus faecium infective endocarditis: impact of protein binding? Annals of Pharmacotherapy. 2008;42:289–290. doi: 10.1345/aph.1k548. [DOI] [PubMed] [Google Scholar]
- 15.Mohr JF, et al. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE) International Journal of Antimicrobial Agents. 2009;33:543–548. doi: 10.1016/j.ijantimicag.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Mave V, et al. Vancomycin-resistant enterococcal bacteraemia: is daptomycin as effective as linezolid? Journal of Antimicrobial Chemotherapy. 2009;64:175–180. doi: 10.1093/jac/dkp154. [DOI] [PubMed] [Google Scholar]
- 17.Camins BC, et al. A population-based investigation of invasive vancomycin-resistant Enterococcus infection in metropolitan Atlanta, Georgia, and predictors of mortality. Infection Control and Hospital Epidemiology. 2007;28:983–991. doi: 10.1086/518971. [DOI] [PubMed] [Google Scholar]
- 18.Bhavnani SM, et al. A nationwide, multicenter, case-control study comparing risk factors, treatment, and outcome for vancomycin-resistant and -susceptible enterococcal bacteremia. Diagnostic Microbiology and Infectious Diseases. 2000;36:145–158. doi: 10.1016/s0732-8893(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 19.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. American Journal of Infection Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Boucher HW, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clinical Infectious Diseases. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 22.Reik R, et al. The burden of vancomycin-resistant enterococcal infections in US hospitals, 2003 to 2004. Diagnostic Microbiology and Infectious Diseases. 2008;62:81–85. doi: 10.1016/j.diagmicrobio.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Ghanem G, et al. Outcomes for and risk factors associated with vancomycin-resistant Enterococcus faecalis and vancomycin-resistant Enterococcus faecium bacteremia in cancer patients. Infection Control and Hospital Epidemiology. 2007;28:1054–1059. doi: 10.1086/519932. [DOI] [PubMed] [Google Scholar]
- 24.Poutsiaka DD, et al. Daptomycin in the treatment of vancomycin-resistant Enterococcus faecium bacteremia in neutropenic patients. Journal of Infection. 2007;54:567–571. doi: 10.1016/j.jinf.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segreti JA, Crank CW, Finney MS. Daptomycin for the treatment of gram-positive bacteremia and infective endocarditis: a retrospective case series of 31 patients. Pharmacotherapy. 2006;26:347–332. doi: 10.1592/phco.26.3.347. [DOI] [PubMed] [Google Scholar]
- 26.Raad II, et al. Vancomycin-resistant Enterococcus faecium: catheter colonization, esp gene, and decreased susceptibility to antibiotics in biofilm. Antimicrobial Agents and Chemotherapy. 2005;49:5046–5050. doi: 10.1128/AAC.49.12.5046-5050.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moellering RC, et al. The efficacy and safety of quinupristin/dalfopristin for the treatment of infections caused by vancomycin-resistant Enterococcus faecium. Synercid Emergency-Use Study Group. Journal of Antimicrobial Chemotherapy. 1999;44:251–261. doi: 10.1093/jac/44.2.251. [DOI] [PubMed] [Google Scholar]
- 28.Kvirikadze N, et al. Daptomycin for the treatment of vancomycin resistant Enterococcus faecium bacteremia. Scandinavian Journal of Infectious Diseases. 2006;38:290–292. doi: 10.1080/00365540500434687. [DOI] [PubMed] [Google Scholar]
- 29.Cunha BA, Mickail N, Eisenstein L. E. faecalis vancomycin-sensitive enterococcal bacteremia unresponsive to a vancomycin tolerant strain successfully treated with high-dose daptomycin. Heart and Lung. 2007;36:456–461. doi: 10.1016/j.hrtlng.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Levine DP. Clinical experience with daptomycin: bacteraemia and endocarditis. J Antimicrobial Chemotherapy. 2008;62(Suppl 3):iii 35–39. doi: 10.1093/jac/dkn369. [DOI] [PubMed] [Google Scholar]
- 31.Chien JW, Kucia ML, Salata RA. Use of linezolid, an oxazolidinone, in the treatment of multidrug-resistant gram-positive bacterial infections. Clinical Infectious Diseases. 2000;30:146–151. doi: 10.1086/313597. [DOI] [PubMed] [Google Scholar]
- 32.Fowler VG, Jr, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clinical Infectious Diseases. 1998;27:478–486. doi: 10.1086/514686. [DOI] [PubMed] [Google Scholar]
- 33.Patel M, et al. Initial management of candidemia at an academic medical center: evaluation of the IDSA guidelines. Diagnostic Microbiology and Infectious Diseases. 2005;52:29–34. doi: 10.1016/j.diagmicrobio.2004.12.010. [DOI] [PubMed] [Google Scholar]