Abstract
As an alternative to antihelminthic drugs, we are exploiting vaccination to control infections with the abomasal nematode Ostertagia ostertagi in cattle. Our focus for vaccine targets is excretory-secretory (ES) products of this parasite. One of the most abundant antigens in larval and adult Ostertagia ES products is a protein homologous to nematode polyprotein allergens. We found that the Ostertagia polyprotein allergen (OPA) is encoded by a single-copy gene. OPA comprises three or more repeated units, and only the 15-kDa subunits are found in ES products. The native antigen is localized in the intestinal cells of third-stage larvae and in the hypodermis and cuticle of fourth-stage larvae and adult parasites. Vaccination of cattle with native OPA (nOPA) in combination with QuilA resulted in protection against Ostertagia challenge infections. The geometric mean cumulative fecal egg counts in the nOPA-vaccinated animals were reduced by 60% compared to the counts in the control group during the 2-month course of the experiment. Both male and female adult worms in nOPA-vaccinated animals were significantly shorter than the worms in the control animals. In the abomasal mucus of vaccinated animals the nOPA-specific immunoglobulin G1 (IgG1) and IgG2 levels were significantly elevated compared to the levels in the control animals. Reductions in the Ostertagia egg output and the length of the adult parasites were significantly correlated with IgG1 levels. IgG2 titers were only negatively associated with adult worm length. Protected animals showed no accumulation of effector cells (mast cells, globular leukocytes, and eosinophils) in the mucosa. In contrast to the native antigen, recombinant OPA expressed in Escherichia coli did not stimulate any protection.
At present, the control of Ostertagia ostertagi infections in cattle largely depends on the use of chemical antihelminthic drugs. Residues of introduced chemicals in foodstuffs and the environment have become a serious consumer concern. Reports of resistance to antiparasitic drugs for a closely related parasite, Cooperia species, in cattle (5, 31) make the development of alternative control systems even more urgent.
Early attempts to protect cattle against the abomasal nematode O. ostertagi with irradiated larval vaccines (2) or with crude somatic (12) and excretory-secretory (ES) products (13) were not successful. Moderate levels of protection were obtained in calves immunized with gut membrane glycoproteins of O. ostertagi (24). Recently, it was shown that vaccination of cattle with ES products of adult Ostertagia worms enriched for cysteine proteinases by thiol-Sepharose chromatography reduced the fecal egg counts by 60% compared to the counts in the control group (11).
Generally, ES products are considered to be essential for the development and survival of the parasite within the host and are targets for vaccine development (17). Immunoscreening of cDNA libraries of both larvae (third-stage larvae [L3] and L4) and adults of Ostertagia with polyclonal rabbit serum raised against ES products led to identification of 15 genuinely secreted proteins with potential protective capacities (28).
One of these ES antigens showed strong sequence homology to nematode polyprotein allergens (NPAs) of Ascaris suum (25), Dictyocaulus viviparus (1), and Toxocara canis (33). NPAs are synthesized as tandemly repetitive polypeptides composed of 10 or more NPA units and are posttranslationally cleaved at consensus sites into 14-kDa subunits (reviewed in references 15 and 16). NPA units bind fatty acids and retinoids and may play a role in lipid transport in the nematode. NPAs appear to be secreted by parasitic nematodes and may be involved in modification of the local inflammatory and immunological environment of the host tissues which they inhabit (15, 16).
Although NPAs (especially ABA-1 from Ascaris) have been characterized in detail biochemically and molecularly (1, 3, 6, 21, 22, 32, 33), to our knowledge they have never been tested as vaccines in protection trials. Interestingly, allergens (by definition) induce an immunoglobulin E (IgE) antibody response that leads to a type I hypersensitivity reaction similar to the reaction which appears to be associated with protective immune responses to helminth parasites (20). Also, because NPAs are specific to nematodes and have no structural homologues in mammals, they are suitable vaccine candidates.
The objectives of this study were first to molecularly characterize the Ostertagia polyprotein allergen (OPA) (e.g., to determine the genomic organization, expression pattern, and immunolocalization) and then to investigate the protective capacities of both purified native OPA (nOPA) and recombinant OPA (rOPA) in cattle challenged with O. ostertagi.
MATERIALS AND METHODS
Southern blot hybridization and probe preparation.
Genomic DNA (5 μg) (29) was digested overnight at 37°C with XbaI and EcoRI (Amersham Pharmacia Biotech), separated on a 1% agarose gel, and transferred to a nylon membrane (Hybond-N; Amersham Pharmacia Biotech). Hybridization was done overnight at 65°C with a probe generated from OPA (EMBL accession no. Z46800) (Gene Images random prime labeling module; Amersham Life Science), and this was followed by nonradioactive detection with a Gene Images CDP-Star detection module (Amersham Life Science). The sequence of the opa probe contains one restriction site for EcoRI and no restriction site for XbaI. After hybridization the blot was exposed to scientific X-OMAT imaging film for 2 h (Kodak, Rochester, N.Y.).
Levels of expression of opa determined by real-time PCR.
Levels of opa mRNA in L3, L4, and adult Ostertagia were determined by real-time PCR with the Lightcycler system by using an LC-Fast Start reaction mixture with SYBR Green I (Roche Diagnostics). Three micrograms of total RNA, prepared by using the TRIZOL reagent (GibcoBRL, Life Technologies), was converted into first-strand cDNA with oligo(dT) primers (SuperScript choice system for cDNA synthesis; GibcoBRL, Life Technologies). The reaction mixture (total volume, 20 μl) consisted of a master mixture containing Taq DNA polymerase, a deoxynucleoside triphosphate mixture, and SYBR Green, 3 mM MgCl2, 5 pmol of primer 5′-AGATCGTATCGCAGTCGAG-3′, 5 pmol of primer 5′-CCCAAGCTTGTAACCCTCTATGTGGAA-3′), and 2 μl of template cDNA (1/10 dilution). The subsequent steps were initial denaturation for 10 min at 95°C and then 40 cycles of denaturation for 18 s at 95°C, annealing for 25 s at 58°C, and extension for 14 s at 72°C. All real-time PCRs were performed in quadruplicate. The specificity of the PCR products was confirmed by melting curve analysis and subsequent agarose gel electrophoresis. To correct for variations in efficiency of the reverse transcription step and for differences in both RNA quality and RNA quantity between samples, data were normalized with the actin housekeeping gene. The relative amount of opa expression was plotted as a ratio ([number of copies of the target opa gene/number of copies of the housekeeping gene] × 106). For quantification, opa was cloned into plasmids and included in each PCR.
Preparation of parasite ES products.
ES products from exsheathed L3, L4, and adult parasites were prepared as described previously (10). Protein samples were dialyzed against phosphate-buffered saline (PBS) (150 mM, pH 7.4) before use.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed by using standard techniques, essentially as described previously (28). Western blots were probed with monospecific antibodies that were affinity purified from anti-adult ES product rabbit serum (28) or with bovine serum (diluted 1:400 in 2% horse serum [HS] in PBS-Tween [PBST]). The blots were developed with goat anti-rabbit or rabbit anti-bovine horseradish peroxidase conjugate (Sigma-Aldrich, St. Louis, Mo.) that was diluted 1:8,000 in 2% HS in PBST, and recognized antigens were visualized by adding 0.05% 3,3-diaminobenzidine tetrachloride in PBS containing 0.02% (vol/vol) H2O2.
Immunolocalization of OPA.
L3, L4, and adult male and female O. ostertagi parasites were embedded in Tissue-Tek (Sakura Finetek Europe B.V.) and frozen in liquid nitrogen. Twelve-micrometer-thick cryosections (Jung CM3000 cryotome; Leica Instruments GmbH) were mounted on 3-aminopropyltriethoxysilane (Sigma-Aldrich)-coated glass slides. The tissues were fixed in acetone for 10 min at −20°C and air dried. The endogenous peroxidase activity was blocked by incubation of the slides in methanol containing 1.7% hydrogen peroxide for 10 min in the dark. Nonspecific binding was blocked for 30 min with 10% HS in PBS. The slides were incubated with monospecific antibodies against OPA (28) for 4 h at 37°C. To wash away unbound antibodies, the slides were incubated in PBS with gentle shaking (three times for 10 min). Detection was done with Alexa Fluor 594 goat anti-rabbit IgG(H+L) (Molecular Probes) at a final concentration of 0.5 μg/ml for 1 h at room temperature. Red fluorescence was detected by absorption of green light with a Leitz DMRB light microscope (Leica Instruments GmbH). Four negative controls were included; monospecific OPA antibodies were replaced with 2% HS (conjugate control), monospecific OPA antibodies were replaced with negative (preimmune) rabbit serum (at a 1/2,000 dilution in 2% HS), monospecific OPA antibodies were replaced with antiscabies rabbit serum (at a 1/2,000 dilution in 2% HS), and the conjugate was replaced with 2% HS.
Gel filtration chromatography.
Thirty-milligram portions of L3 ES products (650 μg/ml; 3-ml samples) were loaded on a Sephadex G-50 Superfine gel filtration column (70 by 1.6 cm; Pharmacia Biotech, Uppsala, Sweden) at a concentration of 0.3 ml/min. The samples were eluted at 4°C with 10 mM Tris-150 mM NaCl (pH 7.4) at a rate of 0.5 ml/min. Spectrophotometric detection was done at 280 nm. The collected fractions were evaluated by Western blot analysis by using monospecific OPA antibodies (28). The OPA fractions were pooled and concentrated with a Millipore Ultrafree-15 centrifuge filter unit (Sigma-Aldrich). The purity of nOPA was verified by SDS-PAGE followed by Coomassie blue staining. The yield of the purified nOPA was determined by the bicinchoninic acid method (Pierce Chemical Co., Rockford, Ill.). Aliquots (100 μg) of nOPA were stored at −70°C until they were used.
Cloning and expression of rOPA.
A 1,212-bp cDNA fragment (EMBL accession no. Z46800) coding for the C-terminal part of OPA and isolated as described above (28) was initially cloned in the pGEMT-easy vector (Promega Corporation, Madison, Wis.). BamHI and XhoI restriction sites were introduced by PCR (35 cycles of 1 min at 95°C, 1 min at 46°C, and 1.5 min at 72°C with final extension for 10 min at 72°C) onto plasmid DNA (Qiagen plasmid Midi kit; Westburg) by using primers OPAforward (5′-GGATCCCATTCACTTGAAGACGCA-3′) and OPAreverse (5′-CTCGAGCTATGTGGAACGCGT-3′). Digestion of the expression vector pGEX-6P-1 (Amersham Pharmacia Biotech) with BamH1 and XhoI permitted unidirectional cloning of opa. The constructs were transformed into competent Escherichia coli BL21, and transformants were selected. Selected constructs were verified by restriction digestion and sequence analysis (PE Biosystems). Expression of OPA was induced by addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h at 37°C. The glutathione S-transferase fusion protein was purified by affinity chromatography by using a glutathione Sepharose 4B column (Amersham Pharmacia Biotech). The glutathione S-transferase affinity tail was removed by PreScission protease cleavage. The concentration of rOPA was determined by the bicinchoninic acid method (Pierce Chemical Co.). The purity of rOPA was verified by SDS-PAGE followed by Coomassie blue staining. Aliquots (100 μg) of rOPA were stored at −70°C until they were used.
MALDI-TOF mass spectrometry analysis.
The purified nOPA and rOPA protein bands were excised from a one-dimensional SDS-PAGE gel and digested in the gel with trypsin. The sets of peptides generated were mixed with matrix molecules, spotted on an AnchorChip target, and analyzed by matrix-assisted laser desorption ionization (MALDI) coupled to a time of flight (TOF) tube. The identity of OPA was determined by comparing its peptide mass fingerprint with the theoretical molecular weights of peptides that were produced by in silico digestion of each of the protein sequences in the database (EMBL accession no. Z46800).
Vaccination trial.
A vaccination trial was designed essentially as described previously (11). Three groups of seven male MontBéliard calves that were 8 months old were randomly composed. All animals were vaccinated three times by intramuscular injection at 3-week intervals either with 100 μg of nOPA mixed with 750 μg of QuilA, with 100 μg of rOPA mixed with 750 μg of QuilA, or with 750 μg of QuilA as an adjuvant control. Immunogens were randomly assigned to the treatment groups, and the animals were examined daily for adverse reactions to the immunizations. A trickle infection with 1,000 O. ostertagi L3 started on the day of the last immunization and was administered for 25 days (5 days/week). Blood was collected at weekly intervals. Fecal egg counts were determined three times a week (McMaster; sensitivity, 25 eggs per g) (26) starting 21 days postinfection and lasting until the animals were euthanized 36 days later. Parasitological parameters, such as cumulative fecal egg counts, number and size of adult worms, and number of eggs per adult female, were determined by using standard techniques as described previously (11). All parasitological techniques were performed blindly.
Ostertagia antibody detection by microplate ELISA.
Local IgG1, IgG2, IgA, IgE, and IgM levels against Ostertagia L3 ES products or nOPA were determined by an enzyme-linked immunosorbent assay (ELISA). Ostertagia L3 ES products (4 μg/ml) or purified nOPA (0.5 μg/ml) was coated overnight in carbonate buffer (0.025 M, pH 9.6) and incubated in duplicate with an abomasal mucus extract (100 μg in PBS) from all animals. Mucus homogenates were prepared as described previously (11).
Monoclonal antibodies against bovine immunoglobulins were obtained from the ILRI Institute (Nairobi, Kenya) and diluted 1:2,500 (IgG1 and IgM), 1:5,000 (IgA), and 1:25,000 (IgG2) in PBS. Goat anti-mouse IgG coupled to horseradish peroxidase (Sigma) was used as a conjugate (diluted 1:4,000 [IgG1, IgG2, and IgM] or 1:8,750 [IgA] in PBS). O-Phenylenediamine (0.1% in citrate buffer) was used as the substrate. IgE levels were determined as described previously (19). Optical density was measured at 492 nm.
Histochemistry.
Two random samples of mucosal tissue for histological examination were taken from the abomasal fundus at necropsy. The tissue sample used for eosinophil and globule leukocyte staining was fixed with 4% paraformaldehyde in PBS at pH 7.4 for 6 h. The tissue sample used for mast cell counting was fixed in Carnoy's fluid for 4 h. After fixation, the tissues were dehydrated and embedded in paraffin. Five sections that were 8 μm thick were collected from each sample by systematic randomization (14). The sections for mast cell counting were stained with 0.25% toluidine blue. To obtain eosinophil and globular leukocyte counts, the sections were stained by the carbol chromotrope technique. In the first section, three digital photographs of the musocal layer were randomly taken, and two digital photographs of the submucosal layer were taken. In the following four sections, the number of photographs alternated (two and three photographs, three and two photographs, two and three photographs, and three and two photographs in the mucosa and submucosa, respectively), which resulted in 13 pictures of the mucosa and 12 pictures of the submucosa for each animal. The numbers of mast cells were determined in the mucosa and submucosa of each animal by examining areas of approximately 1,014,000 and 936,000 μm2, respectively. The numbers of globule leukocytes and eosinophils were counted in the mucosa and submucosa by examining areas of approximately 451,000 and 413,000 μm2, respectively. The numbers of cells in the mucosa and submucosa were expressed as numbers of cells per 100,000 μm2 of microscopic field.
Statistical analysis.
The significance of differences in parasitological parameters (cumulative fecal egg counts, adult worm counts, adult worm lengths, numbers of eggs per female worm) and immunological parameters (mucosal antibody levels and mast cell, globular leukocyte, and eosinophil counts) between groups was investigated by performing Kruskal-Wallis one-way analysis of variance, followed by a one-tailed Mann-Whitney U test for pairwise comparison of each vaccinated group with the adjuvant control group. The correlation between the different parasitological and immunological parameters was examined by using Spearman's correlation test. A P value of <0.05 was considered statistically significant.
RESULTS
Molecular characterization of nOPA: genomic organization, size, expression and recognition patterns, and immunolocalization.
Southern blot hybridization analysis of XbaI-digested and EcoRI-digested Ostertagia genomic DNA with the OPA probe (EMBL accession no. Z46800) resulted in detection of one major band at approximately 12 kb and two prominent bands at 3.8 and 1.8 kb, respectively (data not shown).
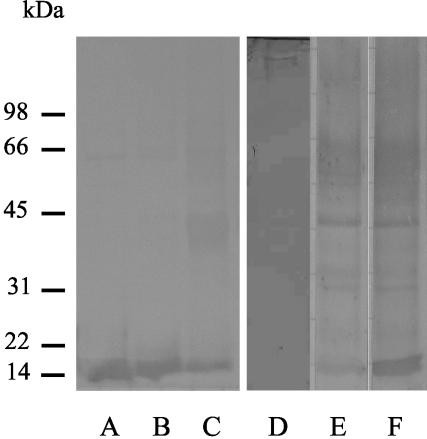
Western blots of somatic extracts from L3, L4, and adult O. ostertagi developed with monospecific antibodies against OPA revealed a ladder-like pattern of reactive bands, and each band was approximately 14 kDa larger than the previous band (14, 28, and 42 kDa) (reference 7 and data not shown). On Western blots of ES products from the three parasitic Ostertagia life stages probed with the same monospecific OPA antibodies, specific recognition of the 14-kDa protein band was detected in all life stages (Fig. 1, lanes A, B, and C). This developmental expression pattern was confirmed by real-time PCR. The highest levels of expression were detected in L3, and these levels were approximately two- and fourfold higher than the levels in the L4 and adult life stages, respectively (Table 1). A Western blot of L3 ES products demonstrated that OPA was not recognized by serum antibodies of preimmune animals (Fig. 1, lane D), was hardly recognized by serum of primary infected animals (helminth-free calves which received a natural challenge infection for 3 weeks) (Fig. 1, lane E), and was very strongly recognized by serum antibodies of immune animals (Fig. 1, lane F) (animals that were naturally infected for two grazing seasons, each of which was approximately 6 months long).
FIG. 1.
Recognition pattern of OPA: Western blot of L3 ES products (lane A), L4 ES products (lane B), and adult ES products (lane C) developed with monospecific antibodies against OPA and Western blot of L3 ES products developed with sera of preimmune animals (lane D), primary infected animals (lane E), and naturally immune animals (lane F).
TABLE 1.
Real-time PCR results: Numbers of copies of opa and the actin gene in the different life stages (L3, L4, and adult) of Ostertagiaa
| Stage | No. of copies of opa | No. of copies of actin gene | No. of copies of opa/no. of copies of actin gene (106) |
|---|---|---|---|
| L3 | 3.41 × 102 | 1.51 × 105 | 2,258 |
| L4 | 4.66 × 101 | 3.38 × 103 | 1,378 |
| Adult | 6.6 × 103 | 1.09 × 107 | 605 |
The relative amount of opa expression was determined as a ratio ([number of copies of target opa/number of copies of the housekeeping gene] × 106).
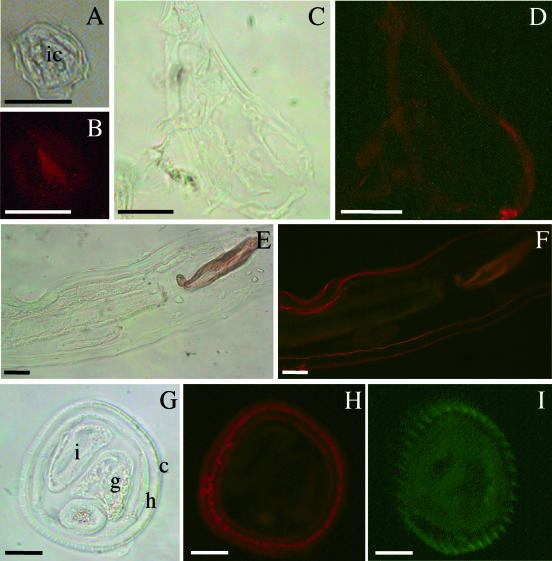
On sections of Ostertagia parasites clear red fluorescence was observed in the intestinal cells of L3 animals (Fig. 2B), in the cuticle of L4 animals (Fig. 2D), and in the cuticle and hypodermis of both male and female adult worms (Fig. 2F and H).
FIG. 2.
Immunolocalization of OPA. Sections of L3 (A and B), L4 (C and D), and male (E and F) and female (G and H) adult O. ostertagi parasites were incubated with monospecific antibodies to OPA, and antibody binding was detected by using Alexa Fluor-conjugated anti-rabbit immunoglobulin. Female adult O. ostertagi parasites were also incubated with negative (preimmune) rabbit serum (at a 1/2,000 dilution in 2% HS) (I). Panels A, C, E, and G are phase-contrast micrographs, and panels B, D, F, H, and I are fluorescence microscopy micrographs (signal indicated by red fluorescence except in panel I). ic, intestinal cells; i, intestine; g, gonads; h, hypodermis; c, cuticle. Bars = 25 μm.
No fluorescence was detected in sections of Ostertagia developed with preimmune rabbit serum (Fig. 2I) or with the irrelevant rabbit serum (antiscabies serum) or in the conjugate controls (data not shown).
Protein profiles of nOPA and rOPA and amino acid sequences.
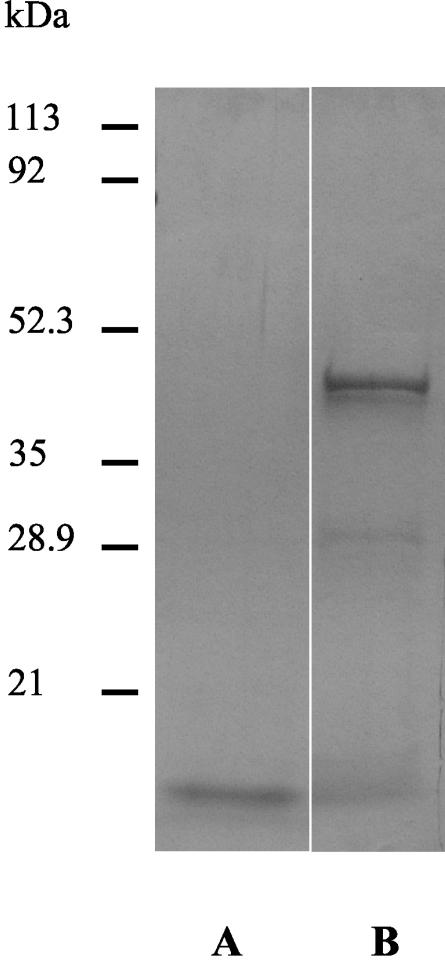
The yield of purified nOPA was around 10% of the total L3 ES products. The yield of rOPA was 1.44 mg/liter. The protein profiles of both nOPA and rOPA obtained by reducing SDS-PAGE and Coomassie blue staining are shown in Fig. 3.
FIG. 3.
Protein profile of OPA. After reducing SDS-PAGE nOPA (lane A) and rOPA (lane B) were visualized by Coomassie blue staining.
The nOPA fraction produced one main band at 14 kDa (Fig. 3, lane A). Although a strong 45-kDa protein band was produced by the rOPA fraction, two additional bands (at approximately 30 and 14 kDa) were visible on the gel (Fig. 3, lane B).
The amino acid sequences of all visible bands, as determined by MALDI-TOF mass spectrometry analysis, showed that all of the protein bands represented nOPA or rOPA. The nOPA band, however, comprised traces of a protein homologous to a 17-kDa L3 ES antigen of Ostertagia (EMBL accession no. AJ318472).
Vaccination trial.
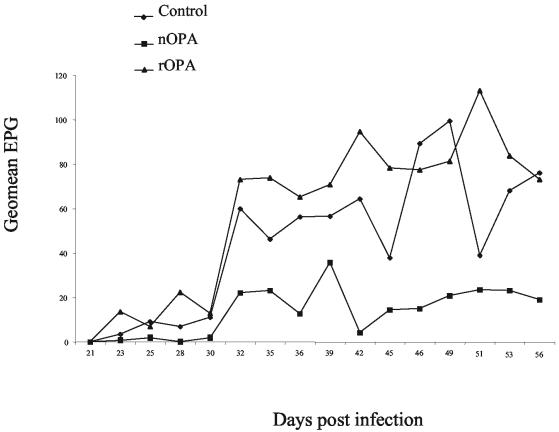
No adverse reactions to the immunizations and no clinical signs of ostertagiosis were observed during the vaccination trial. The fecal egg counts during the course of the trial are expressed as geometric means in Fig. 4. During the whole vaccination trial the geometric mean egg counts for the nOPA-vaccinated animals were below the geometric mean number of eggs per gram for the control QuilA group, which resulted in a significant (60%) reduction (P < 0.001) in the cumulative number of eggs per gram (Table 2). In contrast, there was a trend toward higher geometric values for the number of eggs per gram for the rOPA-vaccinated animals compared to the values for the control QuilA group, although the difference was not statistically significant. The results of the other parasitological tests are summarized in Table 2. There was not a significant difference in the number of adult worms among the three groups. Both female and male adult worms were significantly shorter in the nOPA-vaccinated animals (P < 0.001). This effect was not observed in the rOPA group. The percentages of inhibited L4 and the numbers of eggs per female were similar for all three groups.
FIG. 4.
Geometric mean number of eggs per gram (Geomean EPG). Fecal egg output was determined during the trial for nOPA vaccinated-, rOPA vaccinated-, and control animals.
TABLE 2.
Parasitological parameters
| Group | n | Geometric mean cumulative no. of eggs per g (range) | Geometric mean no. of worms (range) | Geometric mean worm length (mm) (range)
|
% of L4 (range) | Geometric mean no. of eggs per female (range) | |
|---|---|---|---|---|---|---|---|
| Females | Males | ||||||
| Control | 7 | 2,235 (1,300-3,338) | 8,300 (3,100-14,000) | 9.51 (9.26-9.67) | 8.04 (7.91-8.07) | 1 (0-1.38) | 20 (15-29) |
| nOPA | 7 | 914 (350-1,275)a | 8,129 (6,150-11,250) | 9.05 (8.59-9.38)b | 7.61 (7.19-7.92)b | 1 (0-1.62) | 20 (16-23) |
| rOPA | 7 | 2,780 (1,625-4,225) | 9,464 (7,550-11,550) | 9.33 (8.67-9.72) | 7.82 (7.32-8.16) | 1 (0-2.13) | 21 (17-25) |
P < 0.001.
P < 0.005.
Immunological parameters.
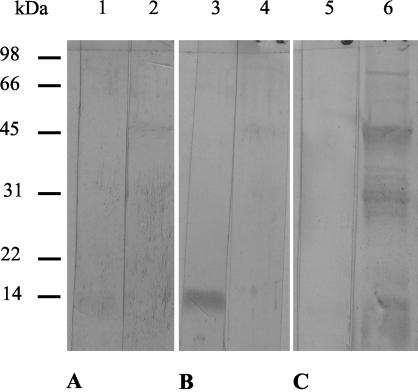
To monitor the antibody response to the vaccine, Western blots of nOPA and rOPA were developed with serum taken from all animals 1 week after the second immunization (Fig. 5). No antibodies to nOPA or rOPA were detected in serum from the control QuilA group (Fig. 5A). While serum from nOPA-vaccinated animals specifically recognized nOPA (Fig. 5B), serum antibodies from rOPA-vaccinated animals bound only to the rOPA protein bands (Fig. 5C). There was no cross-recognition between the nOPA- and rOPA-vaccinated groups.
FIG. 5.
Serum antibody responses to the vaccine. Western blots of nOPA (lanes 1, 3, and 5) and rOPA (lanes 2, 4, and 6) were developed with pooled sera collected 1 week after the second immunization of control animals (A), nOPA-vaccinated animals (B), and rOPA-vaccinated animals (C).
The experiments in which we determined the levels of abomasal immunoglobulin against total L3 ES products and nOPA by ELISA produced similar results. Both nOPA- and rOPA-vaccinated animals had significantly higher IgG1 titers in the mucus than the adjuvant control group had (Table 3). Elevated IgG1 levels were negatively correlated with the cumulative number of eggs per gram (Spearman's rho value, −0.448) and the lengths of male and female adult worms (Spearman's rho values, −0.654 and −0.630, respectively). Moreover, significantly greater IgG2 responses were evident in the protected animals, and these responses were negatively correlated with the length of the female adult parasites (Spearman's rho values, −0.374 and −0.429, respectively). There was not a statistically significant difference in the IgA, IgE, and IgM levels among the three groups (Table 3).
TABLE 3.
Immunological parameters
| Group | n | Mucosal nOPA-specific levels (mean ± SD) ofa:
|
No. (mean ± SD) ofb:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| IgG1 | IgG2 | IgA | IgE | IgM | Mucosal mast cells | Globular leukocytes | Eosinophils | ||
| Control | 7 | 0.145 ± 0.018 | 0.151 ± 0.017 | 0.399 ± 0.234 | 0.230 ± 0.027 | 0.228 ± 0.042 | 9.624 ± 2.925 | 3.769 ± 3.473 | 7.921 ± 3.950 |
| nOPA | 7 | 0.257 ± 0.092c | 0.315 ± 0.147c | 0.527 ± 0.347 | 0.232 ± 0.014 | 0.247 ± 0.024 | 10.02 ± 3.924 | 2.35 ± 4.197d | 2.23 ± 4.345c |
| rOPA | 7 | 0.183 ± 0.027c | 0.256 ± 0.109d | 0.488 ± 0.176 | 0.225 ± 0.027 | 0.258 ± 0.036 | 9.65 ± 2.877 | 1.87 ± 2.916d | 2.79 ± 3.090d |
Determined by ELISA.
Number of cells per 100,000 μm2 of microscopic field.
P < 0.01.
P < 0.05.
The numbers of mast cells in the vaccinated animals and the control group were not significantly different (Table 3). Both the mean numbers of globular leukocytes and the mean numbers of eosinophils were significantly lower in the vaccinated animals than in the adjuvant control group. No correlations between effector cell types and parasitological parameters were found (data not shown).
DISCUSSION
In this paper we describe molecular characterization of OPA, an Ostertagia polyprotein, and its potential to induce protection against homologous infection in cattle.
The organization of the coding sequence of opa in genomic DNA of Ostertagia could indicate that there is only one copy of the opa gene. This is the case for the genes encoding NPAs of other nematodes, such as Dirofilaria immitis (6), Brugia pahangi (27), Brugia malayi (27), and D. viviparus (1). The nOPA antigen was located in the intestinal cells of Ostertagia L3 and in the cuticle and hypodermis of L4 and adult parasites. Similarly, immunostaining of Di5 antigen, the OPA homologue of D. immitis, was apparent in the hypodermis and the cuticle of the parasite (22). In Caenorhabditis elegans, NPAs have been found to bind fatty acids and retinol (reviewed in reference 15). In parasitic nematodes however, the major function of NPAs is to alter the host tissue environment to the parasites' advantage (16). The results of the immunolocalization analysis together with the Western blot results indeed suggest that OPA is secreted. Release of OPA into the environment could occur directly from the gut with the L3 stage or from the cuticle via the hypodemis with Ostertagia L4 and adults.
Surprisingly, the protein profile of rOPA determined by reducing SDS-PAGE and Coomassie blue staining contained three bands (at 14, 30, and 45 kDa) instead of the one protein band expected at 45 kDa. OPA and NPAs in general are composed of a series of direct repeats with regularly spaced proteolytic cleavage sites such that they are processed into multiple polypeptides that are approximately 14 kDa long. These proteolytic consensus cleavage sites consisting of four amino acids match the motif K/R-X-K/R-R. It is known that the use of certain codons (especially those coding for arginine) is rare in E. coli, so it is possible that peptide synthesis is retarded or even retained at the cleavage sites, resulting in three partial recombinant proteins. This hypothesis is strengthened by the fact that the peptides generated from the 14-kDa protein band in the MALDI-TOF mass spectrometry analysis originated from the first 100 amino acids of OPA.
Despite the protective properties of the nOPA, no protective immune response was generated by vaccination with the rOPA expressed in E. coli. Failure to induce protection may have been due to the absence of specific posttranslational modifications of nematode antigens which are essential for the protective capacity of the antigens, and glycosylation is very important (18). For example, IgE and IgA recognition of a glycoprotein expressed on the surface of Teladorsagia circumcincta L3 is correlated with protective immunity but is almost totally directed against the glycan component (18). It is also possible that a full-length OPA is necessary for appropriate conformation and thus for protection. Of course, we cannot rule out the possibility that the second protein (EMBL accession no. AJ318472), although present at only minor levels, may have contributed to the protection obtained with nOPA.
Our experiment confirms that systemic (intramuscular) vaccination in combination with QuilA as an adjuvant can induce a mucosal immune response (11). A clear abomasal IgG1 and IgG2 response was observed in both the nOPA- and rOPA-vaccinated groups; however, the antibody titers were highest in the nOPA-vaccinated animals. Because there was a negative correlation between IgG titers and cumulative number of eggs per gram or worm length, it is likely that a threshold level of local antibodies is required for protection. In contrast to what was expected, significantly lower numbers of globular leukocytes and eosinophils were found in the vaccinated groups. Surprisingly, vaccination with nOPA did not lead to higher IgE levels, suggesting that nOPA is not intrinsically allergenic.
It is important to stress that an Ostertagia vaccine in cattle needs to be an antifecundity vaccine (30). As the number of worm eggs shed during the first part of the grazing season determines the number of infective larvae in the pasture in the second half of the grazing season, reduction in egg excretion should be the target. Also, because the fecundity of Ostertagia is highly regulated by host immunity (23) and fecal egg output can be strongly reduced without a reduction in the number of worms (9, 4), the number of eggs per gram is the best parameter for evaluation of the protective efficacy of Ostertagia antigens (30). Analogous with observations on the effects of antihelminthic boli on gastrointestinal nematodes in cattle (8), it can be stated that vaccination with nOPA that results in a 60% reduction in the number of eggs per gram for at least 2 months is sufficient to protect first-grazing-season calves from ostertagiosis without interfering with the development of natural immunity.
Acknowledgments
We thank N. Dierickx, S. Casaert, L. Braem, and K. Hugelier for their excellent technical assistance. We thank F. N. Kooyman for providing anti-sheep IgE.
This research was supported by grant G.0229.02 from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (Flanders, Belgium). I.V. is indebted to the Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen (Flanders, Belgium) for a postdoctoral fellowship. K.G. is a postdoctoral fellow of the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (Flanders, Belgium).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Britton, C., J. Moore, J. S. Gilleard, and M. W. Kennedy. 1995. Extensive diversity in repeat unit sequences of the cDNA encoding the polyprotein antigen/allergen from the bovine lungworm Dictyocaulus viviparus. Mol. Biochem. Parasitol. 72:77-88. [DOI] [PubMed] [Google Scholar]
- 2.Burger, H. J., and A. Pfeiffer. 1969. Experimental vaccination of calves with irradiated larvae of Ostertagia ostertagi and Cooperia oncophora. Zentralbl. Veterinarmed. Reihe B 16:357-367. [PubMed] [Google Scholar]
- 3.Christie, J. F., B. Dunbar, and M. W. Kennedy. 1993. The ABA-1 allergen of the nematode Ascaris suum: epitope stability, mass spectrometry, and N-terminal sequence comparison with its homologue in Toxocara canis. Clin. Exp. Immunol. 92:125-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claerebout, E., and J. Vercruysse. 2000. The immune response and the evaluation of acquired immunity against gastrointestinal nematodes in cattle: a review. Parasitology 120:S25-S42. [DOI] [PubMed] [Google Scholar]
- 5.Coles, G. C., C. L. Watson, and O. S. Anziani. 2001. Ivermectin-resistant Cooperia in cattle. Vet. Rec. 148:283-284. [PubMed] [Google Scholar]
- 6.Culpepper, J., R. B. Grieve, L. Friedman, M. Mika-Grieve, G. R. Frank, and B. Dale. 1992. Molecular characterization of a Dirofilaria immitis cDNA encoding a highly immunoreactive antigen. Mol. Biochem. Parasitol. 54:51-62. [DOI] [PubMed] [Google Scholar]
- 7.de Graaf, D. C., L. J. Peelman, E. Claerebout, H. Hilderson, H. D. Schallig, and J. Vercruysse. 1995. Cloning and sequencing of an excretory/secretory antigen from Ostertagia ostertagi fourth-stage larvae containing multiple tandem repeats. Mol. Biochem. Parasitol. 72:239-241. [DOI] [PubMed] [Google Scholar]
- 8.Dorny, P., P. Berghen, J. Vercruysse, and K. Frankena. 1986. Some observations on the use of the morantel sustained-release bolus in first season-grazing calves on a Belgian dairy cattle farm. Vet. Q. 8:189-194. [DOI] [PubMed] [Google Scholar]
- 9.Gasbarre, L. C. 1997. Effects of gastrointestinal nematode infection on the ruminant immune system. Vet. Parasitol. 72:327-337. [DOI] [PubMed] [Google Scholar]
- 10.Geldhof, P., E. Claerebout, D. P. Knox, J. Agneessens, and J. Vercruysse. 2000. Proteinases released in vitro by the parasitic stages of the bovine abomasal nematode Ostertagia ostertagi. Parasitology 121:639-647. [DOI] [PubMed] [Google Scholar]
- 11.Geldhof, P., E. Claerebout, D. Knox, I. Vercauteren, A. Looszova, and J. Vercruysse. 2002. Vaccination of calves against Ostertagia ostertagi with cysteine proteinase enriched protein fractions. Parasite Immunol. 24:263-270. [DOI] [PubMed] [Google Scholar]
- 12.Herlich, H., and F. W. Douvres. 1979. Gastrointestinal nematode immunization trials in cattle. Am. J. Vet. Res. 40:1781-1782. [PubMed] [Google Scholar]
- 13.Hilderson, H., P. Berghen, D. C. de Graaf, E. Claerebout, and J. Vercruysse. 1995. Immunisation of calves with Ostertagia ostertagi fourth stage larval antigens failed to protect calves from infection. Int. J. Parasitol. 25:757-760. [DOI] [PubMed] [Google Scholar]
- 14.Howard, C. V., and M. G. Reed. 1998. Random sampling and random geometry, p. 19-37. In C. V Howard and M. G Reed (ed.), Unbiased stereology, three-dimensional measurement in microscopy, 1st ed. BIOS Scientific Publichers Limited, Oxford, United Kingdom.
- 15.Kennedy, M. W. 2000. The polyprotein lipid binding proteins of nematodes. Biochim. Biophys. Acta 1476:149-164. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy, M. W. 2000. The nematode polyprotein allergens/antigens. Parasitol. Today 16:373-380. [DOI] [PubMed] [Google Scholar]
- 17.Knox, D. P. 2000. Development of vaccines against gastrointestinal nematodes. Parasitology 120:S43-S61. [DOI] [PubMed] [Google Scholar]
- 18.Knox, D. P., D. L. Redmond, P. J. Skuce, and G. F. Newlands. 2001. The contribution of molecular biology to the development of vaccines against nematode and trematode parasites of domestic ruminants. Vet. Parasitol. 101:311-335. [DOI] [PubMed] [Google Scholar]
- 19.Kooyman, F. N., A. P. Yatsuda, H. W. Ploeger, and M. Eysker. 2002. Serum immunoglobulin E response in calves infected with the lungworm Dictyocaulus viviparus and its correlation with protection. Parasite Immunol. 24:47-56. [DOI] [PubMed] [Google Scholar]
- 20.McSharry, C., Y. Xia, C. V. Holland, and M. W. Kennedy. 1999. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect. Immun. 67:484-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore, J., L. McDermott, N. C. Price, S. M. Kelly, A. Cooper, and M. W. Kennedy. 1999. Sequence-divergent units of the ABA-1 polyprotein array of the nematode Ascaris suum have similar fatty-acid- and retinol-binding properties but different binding-site environments. Biochem. J. 340:337-343. [PMC free article] [PubMed] [Google Scholar]
- 22.Poole, C. B., A. G. Grandea 3rd, C. V. Maina, R. E. Jenkins, M. E. Selkirk, and L. A. McReynolds. 1992. Cloning of a cuticular antigen that contains multiple tandem repeats from the filarial parasite Dirofilaria immitis. Proc. Natl. Acad. Sci. 89:5986-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, G., B. T. Grenfell, and R. M. Anderson. 1987. The regulation of Ostertagia ostertagi populations in calves: density-dependent control of fecundity. Parasitology 95:373-388. [DOI] [PubMed] [Google Scholar]
- 24.Smith, W. D., S. K. Smith, and D. Pettit. 2000. Evaluation of immunization with gut membrane glycoproteins of Ostertagia ostertagi against homologous challenge in calves and against Haemonchus contortus in sheep. Parasite Immunol. 22:239-247. [DOI] [PubMed] [Google Scholar]
- 25.Spence, H. J., J. Moore, A. Brass, and M. W. Kennedy. 1993. A cDNA encoding repeating units of the ABA-1 allergen of Ascaris. Mol. Biochem. Parasitol. 57:339-343. [DOI] [PubMed] [Google Scholar]
- 26.Thienpont, D., F. Rochette, and O. F. J. Van Parijs. 1979. Diagnosing helminthiasis by coprological examination. Janssen Research Foundation, Beerse, Belgium.
- 27.Tweedie, S., W. A. Paxton, L. Ingram, R. M. Maizels, L. A. McReynolds, and M. E. Selkirk. 1993. Brugia pahangi and Brugia malayi: a surface-associated glycoprotein (gp15/400) is composed of multiple tandemly repeated units and processed from a 400-kDa precursor. Exp. Parasitol. 76:156-164. [DOI] [PubMed] [Google Scholar]
- 28.Vercauteren, I., P. Geldhof, I. Peelaers, E. Claerebout, G. Berx, and J. Vercruysse. 2003. Identification of excretory-secretory products of larval and adult Ostertagia ostertagi by immunoscreening of cDNA libraries. Mol. Biochem. Parasitol. 126:201-208. [DOI] [PubMed] [Google Scholar]
- 29.Vercauteren, I., E. Van Der Schueren, M. Van Montagu, and G. Gheysen. 2001. Arabidopsis thaliana genes expressed in the early compatible interaction with root-knot nematodes. Mol. Plant-Microbe Interact. 14:288-299. [DOI] [PubMed] [Google Scholar]
- 30.Vercruysse, J., and E. Claerebout. 2003. Assessment of the efficacy of helminth vaccines. J. Parasitol. 89:S202-S209. [Google Scholar]
- 31.Vermunt, J. J., D. M. West, and W. E. Pomroy. 1995. Multiple resistance to ivermectin and oxfendazole in Cooperia species of cattle in New Zealand. Vet. Rec. 137:43-45. [DOI] [PubMed] [Google Scholar]
- 32.Xia, Y., H. J. Spence, J. Moore, N. Heaney, L. McDermott, A. Cooper, D. G. Watson, B. Mei, R. Komuniecki, and M. W. Kennedy. 2000. The ABA-1 allergen of Ascaris lumbricoides: sequence polymorphism, stage and tissue-specific expression, lipid binding function, and protein biophysical properties. Parasitology 120:211-224. [DOI] [PubMed] [Google Scholar]
- 33.Yahiro, S., G. Cain, and J. E. Butler. 1998. Identification, characterization and expression of Toxocara canis nematode polyprotein allergen TBA-1. Parasite Immunol. 20:351-357. [DOI] [PubMed] [Google Scholar]