Abstract
The normal gastrointestinal bacterial flora is crucial for the maturation of acquired immunity via effects on antigen-presenting cells (APCs). Here we investigated how two types of APCs, monocytes and dendritic cells (DCs), react to different bacterial strains typical of the commensal intestinal microflora. Purified human monocytes and monocyte-derived DCs were stimulated with UV-inactivated gram-positive (Lactobacillus plantarum and Bifidobacterium adolescentis) and gram-negative (Escherichia coli and Veillonella parvula) bacterial strains. Monocytes produced higher levels of interleukin 12p70 (IL-12p70) and tumor necrosis factor (TNF), as detected by an enzyme-linked immunosorbent assay, in response to L. plantarum than in response to E. coli and V. parvula. In contrast, DCs secreted large amounts of IL-12p70, TNF, IL-6, and IL-10 in response to E. coli and V. parvula but were practically unresponsive to L. plantarum and B. adolescentis. The lack of a response to the gram-positive strains correlated with lower surface expression of Toll-like receptor 2 (TLR2) on DCs than on monocytes. The surface expression of TLR4 on DCs was undetectable when it was analyzed by flow cytometry, but blocking this receptor decreased the TNF production in response to V. parvula, indicating that TLR4 is expressed at a low density on DCs. Gamma interferon increased the expression of TLR4 on DCs and also potentiated the cytokine response to the gram-negative strains. Our results indicate that when monocytes differentiate into DCs, their ability to respond to different commensal bacteria dramatically changes, and they become unresponsive to probiotic gram-positive bacteria. These results may have important implications for the abilities of different groups of commensal bacteria to regulate mucosal and systemic immunity.
The normal gastrointestinal flora is in close and continuous contact with immune cells, and the resulting stimulation is essential for maturation of the immune system (44). The establishment of the gastrointestinal flora starts at birth and occurs in an ordered fashion (1). During the first days of life the flora is dominated by facultative bacteria, such as Escherichia coli and other enterobacteria, enterococci, and staphylococci, which in the absence of competition from anaerobes can reach very high levels. Along with this large population of facultative bacteria, infants have poorly developed acquired immunity, which allows bacteria to translocate over the epithelium and reach lymph nodes and peripheral blood (5, 8, 41). When the number of aerobic and facultative bacteria increases, oxygen is consumed, which enables anaerobes to colonize the gastrointestinal tract. The anaerobic bacteria that can be found in the intestinal flora of 1-week-old infants include bifidobacteria, lactobacilli, and Veillonella (1).
Antigen-presenting cells (APCs), such as monocytes, macrophages, and dendritic cells (DCs), are responsible for detecting microbes and presenting their antigenic structures to T cells, thus eliciting acquired immune responses. In addition, monocytes and macrophages kill microorganisms by phagocytosis and produce proinflammatory cytokines. DCs are more potent APCs with a special ability to prime naïve T lymphocytes to novel protein antigens (6). Immature DCs can be differentiated from CD14+ monocytes in vitro by incubation with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4) (35, 45). Studies with mice have indicated that monocytes may also differentiate into DCs in vivo (32). Upon activation initiated by signals from microorganisms or from other cells in the tissue, DCs migrate to the regional lymph nodes. During this process, they undergo maturation, up-regulate costimulatory molecules, and produce cytokines, which make them qualified to activate naïve T cells (6). There is accumulating evidence that DCs also can contribute to the differentiation of suppressive T cells that regulate other T cells (38).
Monocytes and DCs recognize conserved motifs in bacteria through Toll-like receptors (TLRs), along with other pattern recognition receptors. The triggering of APCs through TLRs initiates a signal transduction cascade that culminates in the activation of transcription factors, such as NF-κB, which leads to secretion of cytokines and expression of costimulatory molecules. Furthermore, CD14 plays an essential role in the response to microbial components from both gram-positive and gram-negative bacteria (4, 25, 36). CD14 lacks an intracellular domain and therefore has no signaling capacity, but it acts as a coreceptor for TLRs (4). Purified components of microbes have been used to study the specificity of individual TLRs. The gram-negative bacterial compound lipopolysaccharide (LPS) is recognized by TLR4 (23, 30, 37). TLR2 recognizes many different microbial compounds, including peptidoglycan and lipoproteins from both gram-positive and gram-negative bacterial cell walls (40). However, few studies have addressed how the complex structures of intact bacteria are recognized by pattern recognition receptors and affect the expression of these receptors.
It has previously been shown that gram-positive and gram-negative commensal bacteria have different capacities to induce cytokine production from blood mononuclear cells in both neonates and adults (20, 25) and that they also differ in the ability to trigger prostaglandin secretion (21). This indicates that the composition of the flora might be important for how APCs modulate acquired immunity. Therefore, we set out to study how a selection of commensal gram-positive bacteria (Lactobacillus plantarum and Bifidobacterium adolescentis) and gram-negative bacteria (E. coli and Veillonella parvula) induce cytokine responses and regulate the expression of pattern recognition receptors in purified monocytes and DCs.
MATERIALS AND METHODS
Bacterial strains.
Isolates of the commensal intestinal bacterial species B. adolescentis, E. coli, and V. parvula were obtained from the Culture Collection of the University of Göteborg (Göteborg, Sweden). A strain of L. plantarum was isolated from the rectal mucosa of a healthy volunteer (3). E. coli was cultured on blood agar plates aerobically at 37°C for 24 h, while L. plantarum, B. adolescentis, and V. parvula were cultured anaerobically at 37°C for 3 days. The bacteria were harvested by centrifugation (1,000 × g, 10 min), washed three times in phosphate-buffered saline, counted with a microscope, and suspended at a concentration of 109 cells per ml, as verified by viable counting. The strains were inactivated by exposure to UV light for 15 min to inhibit uncontrolled bacterial growth, which was confirmed by negative viable counting, and then stored at −70°C. UV-killed bacteria have a preserved structural integrity, in contrast to heat-inactivated bacteria. LPS from E. coli (serotype O127:B8) and peptidoglycan from Staphylococcus aureus were purchased from Sigma-Aldrich (St. Louis, Mo.).
Cell separation.
Peripheral blood mononuclear cells (PBMCs) were isolated from blood donor buffy coats by density gradient centrifugation with Lymphoprep (Nycomed, Oslo, Norway). CD14-positive monocytes were then purified from the mononuclear cells by magnetic cell sorting by using positive selection according to the manufacturer's protocol (Miltenyi Biotec, Bergich Gladbach, Germany). Briefly, the cells were incubated with magnetic microbeads conjugated with monoclonal mouse anti-human CD14 antibodies in MACS buffer for 15 min. After this, the cells were run through a MACS column (Miltenyi Biotec) in a magnetic field. The column was then removed from the magnet, and the positive cells were flushed out. Analysis by flow cytometry showed that more than 97% of the purified cells expressed CD14.
Generation of monocyte-derived DCs and macrophages.
Monocytes (106 cells/ml) were cultured in six-well plates in endotoxin-free RPMI 1640 medium (BioWhittaker, Cambrex Company, Verriers, Belgium) containing 5% heat-inactivated AB serum (Sigma-Aldrich), 1 mM l-glutamine, and 50 μg of gentamicin per ml (complete medium) supplemented with 500 U of recombinant IL-4 (R&D Systems, Minneapolis, Minn.) per ml and 300 U of recombinant GM-CSF (Schering-Plough, Kenilworth, N.J.) per ml. The cells were cultured in the presence of 5% CO2 at 37°C for 6 to 7 days, and fresh medium containing IL-4 and GM-CSF was added every second day. This resulted in generation of immature DCs that were positive for CD11c but negative for CD14 and CD83. Monocyte-derived macrophages were generated by the same procedure, but the medium was complete medium supplemented with 1.1 U of GM-CSF per ml as described by other workers (18).
Cell cultures.
The concentration of monocytes or immature DCs was adjusted to 106 cells per ml in complete medium, and the cells were transferred to 24-well plates. They were then stimulated with L. plantarum, B. adolescentis, E. coli, or V. parvula for 24 and 48 h with or without 10 ng of human recombinant gamma interferon (rIFN-γ) (R&D Systems) per ml. Initial dose-response experiments were performed with 0.5, 5, and 50 bacteria per cell. The optimal cytokine response was obtained with 50 bacteria per cell, and this ratio was used throughout the study, except for the blocking experiments. For blocking experiments, DCs (5 × 105 cells per ml) from three individuals were preincubated with anti-CD14 (UCHM-1; Serotec, Oxford, United Kingdom), anti-TLR2 (TL2.1; Alexis Biochemicals, San Diego, Calif.), anti-TLR4 (HTA125; Serotec), or isotype control antibody (10 μg per ml) for 1 h at 4°C, and this was followed by stimulation with V. parvula (5 × 106 cells per ml) for 7 h. Supernatants were collected, and the cells were analyzed for expression of different surface markers by flow cytometry.
Cytokine determination.
Concentrations of IL-12p70, tumor necrosis factor (TNF), IL-6, and IL-10 in cell culture supernatants were determined by enzyme-linked immunosorbent assays (ELISAs). A standard ELISA procedure was used as described in detail elsewhere (25). All antibodies and standards were purchased from Pharmingen (San Diego, Calif.). Costar plates (Invitrogen, San Diego, Calif.) were coated with the following capture monoclonal antibodies: anti-IL-12p70 (20C2), anti-TNF (MAb1), anti-IL-6 (MQ2-13A5), and anti-IL-10 (JES3-9D7). Standard curves were generated by using recombinant human IL-12p70, TNF, IL-6, and IL-10, respectively. The following biotinylated detection antibodies were used: anti-IL-12p40/p70 (C8.6), anti-TNF (MAb11), anti-IL-6 (MQ2-39C3), and anti-IL-10 (JES3-12G8). Samples, standards, biotinylated antibodies, and streptavidin-horseradish peroxidase were diluted in high-performance ELISA dilution buffer (Sanquin, Amsterdam, The Netherlands).
Flow cytometry.
Phenotypic analysis of cells was performed by flow cytometry. The cells were incubated with optimal concentrations of the following monoclonal antibodies and controls: allophycocyanin-conjugated anti-CD11c (B-ly6) and mouse immunoglobulin G1 (IgG1) (X40); phycoerythrin-conjugated anti-CD83 (HB15e), anti-CD14 (MoP9), and mouse IgG1 (X40); and biotinylated anti-TLR2 (TL2.1), anti-TLR4 (HTA125), and mouse IgG2a (PK136). Most antibodies and streptavidin-phycoerythrin were purchased from Becton-Dickinson (Erembodegum, Belgium); the only exceptions were the TLR antibodies, which were purchased from Serotec. We analyzed 10,000 to 20,000 cells with a FACSCalibur (Becton-Dickinson) equipped with CellQuest software. The percentage of positive cells and the geometric mean fluorescence intensity were recorded.
Phagocytosis assay.
L. plantarum, B. adolescentis, E. coli, and V. parvula were incubated with 0.1 mg of fluorescein isothiocyanate (FITC) per ml in 0.1 M NaHCO3 for 1 h at room temperature. Phagotest from Orpegen Pharma (Heidelberg, Germany) was used to analyze the phagocytic activities of monocytes and DCs. We incubated 5 × 105 cells with 2.5 × 107 FITC-labeled bacteria in complete medium for 60 to 120 min at 37°C. Quenching solution was added to extinguish the fluorescence of extracellular bacteria. The cells were washed and incubated with a DNA staining solution prior to a flow cytometry analysis in order to exclude aggregation artifacts. Control samples with no bacteria added were used to set the gate for positive cells. We analyzed the percentage of cells that had phagocytosed bacteria, as well as the mean fluorescence intensity as a measure of the number of ingested bacteria per cell. For microphotographs, monocytes or DCs (2.5 × 105 cells per ml) were cultured on four-well Permanox slides (Nunc, Naperville, Ill.) with FITC-labeled bacteria (1.25 × 106 cells per ml) in complete medium for 1 or 2 h at 37°C. The uptake of bacteria by monocytes and DCs was evaluated with a Leica microscope (Leica, Cambridge, United Kingdom). Leica QWin software was used to acquire and visualize the data.
Statistical analysis.
The Mann-Whitney test (GraphPad Prism) was used to compare the levels of cytokine production from DCs and monocytes in response to the various bacterial strains. A paired t test was used to analyze the inhibiting effect of blocking antibodies on TNF production.
RESULTS
Monocytes and DCs respond differently to stimulation with commensal bacteria.
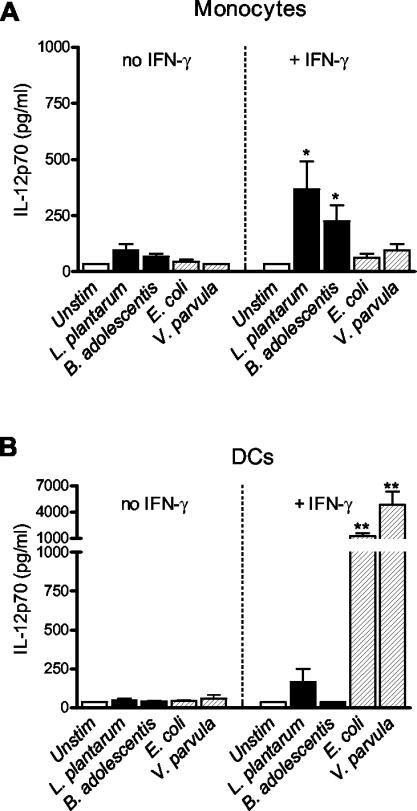
In this study we compared how different bacterial strains typical of the commensal intestinal microflora affected cytokine production in purified monocytes and monocyte-derived DCs. We exposed the cells to UV-inactivated gram-positive L. plantarum and B. adolescentis strains and gram-negative E. coli and V. parvula strains for 24 h and analyzed the production of cytokines. As shown in Fig. 1, bacterial stimulation induced low levels of IL-12p70 from both purified monocytes and DCs in the absence of IFN-γ. This contrasted with the high levels obtained from PBMCs or monocytes enriched by adherence, which might be explained by the finding that T cells and their secreted soluble factors primed the APCs for IL-12 production (20, 25). As T cells isolated from the lamina propria of the normal human gut spontaneously secrete IFN-γ, we investigated whether IFN-γ affected the responses of the APCs (26). When IFN-γ was added to the monocyte cultures, we observed that secretion of IL-12p70 in response to bacteria increased and that the gram-positive bacteria L. plantarum and B. adolescentis were stronger inducers of IL-12p70 than the gram-negative bacteria E. coli and V. parvula were (Fig. 1A). In contrast, DCs produced considerably higher levels of IL-12p70 in response to E. coli and V. parvula than in response to L. plantarum and B. adolescentis in the presence of IFN-γ (Fig. 1B). It should be noted that in the presence of IFN-γ, DCs were much more potent producers of IL-12p70 in response to the gram-negative bacteria than monocytes were in response to the gram-positive strains. Thus, monocytes and DCs primed with IFN-γ exhibited opposite patterns of reactivity to gram-positive and gram-negative bacteria.
FIG. 1.
IL-12p70 production from monocytes (A) and monocyte-derived DCs (B) (106 cells) after stimulation with the gram-positive organisms L. plantarum and B. adolescentis or the gram-negative organisms E. coli and V. parvula (5 × 107 bacteria) for 24 h in the absence or in the presence of 10 ng of rIFN-γ per ml. The bars indicate the mean cytokine production from six donors, and the error bars indicate the standard error of the mean. Statistical significance refers to the difference between the bacterium and the two gram-positive or two gram-negative organisms, respectively (one asterisk, P < 0.05; two asterisks, P < 0.01). Unstim, unstimulated.
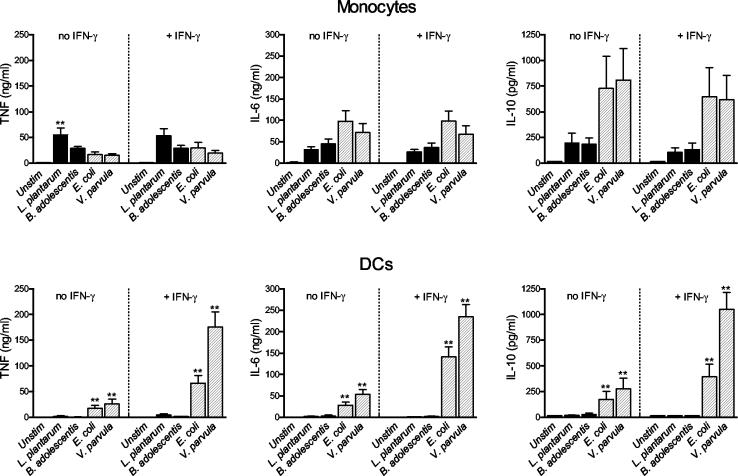
We also analyzed the production of TNF, IL-6, and IL-10 from monocytes and DCs after bacterial stimulation (Fig. 2). In monocytes, L. plantarum induced higher levels of TNF from monocytes than E. coli and V. parvula induced. On the other hand, the gram-negative strains tended to induce higher levels of IL-6 and IL-10 from monocytes than the gram-positive bacteria induced, although the difference was not statistically significant. Priming with IFN-γ had no effect on monocyte production of TNF, IL-6, or IL-10 in response to bacteria. In contrast, DCs produced considerably higher levels of TNF, IL-6, and IL-10 after stimulation with E. coli and V. parvula than after stimulation with L. plantarum and B. adolescentis (Fig. 2B). IFN-γ priming further potentiated this response. Furthermore, human macrophages differentiated in vitro from monocytes produced higher levels of TNF, IL-6, and IL-10 in response to gram-negative bacterial strains than they produced in response to gram-positive bacterial strains, even though the difference was not as pronounced as that observed with DCs (data not shown). In conclusion, while monocytes responded to both the gram-positive and gram-negative strains, albeit with different cytokine patterns, DCs produced high levels of cytokines only in response to the gram-negative bacteria. Furthermore, DCs were more dependent on IFN-γ for the capacity to produce cytokines than monocytes were. Experiments performed with live bacteria produced similar results (data not shown).
FIG. 2.
TNF, IL-6, and IL-10 production by monocytes and monocyte-derived DCs (106 cells) after stimulation with the gram-positive organisms L. plantarum and B. adolescentis or the gram-negative organisms E. coli and V. parvula (5 × 107 bacteria) for 24 h in the absence or in the presence of 10 ng of rIFN-γ per ml. The bars indicate the mean cytokine production from six donors, and the error bars indicate the standard error of the mean. Statistical significance refers to the difference between the bacterium and the two gram-positive or two gram-negative organisms, respectively (one asterisk, P < 0.05; two asterisks, P < 0.01). Unstim, unstimulated.
Bacteria are phagocytosed equally well by monocytes and DCs.
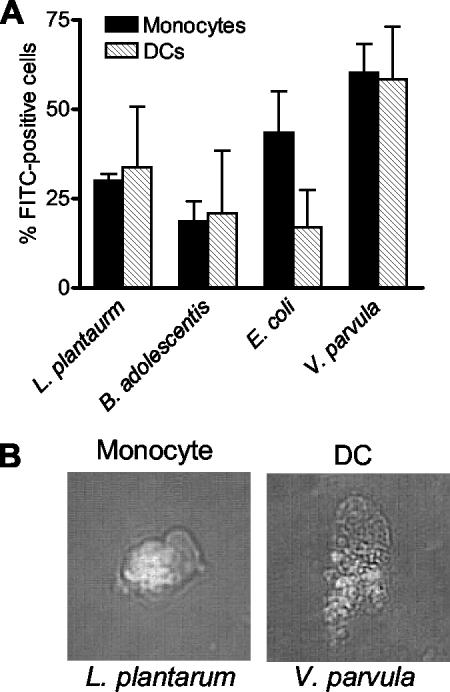
The difference in the capacities of monocytes and DCs to respond to the different bacterial strains might be due to differences in phagocytic capacity. To examine this, we incubated FITC-labeled bacteria with monocytes or monocyte-derived DCs and analyzed the uptake of bacteria by flow cytometry. As shown in Fig. 3A, monocytes and DCs had similar capacities to phagocytose bacteria, and there was no distinct difference between phagocytosis of the gram-positive strains and phagocytosis of the gram-negative strains. Figure 3B is a microphotograph of a monocyte and a DC that have taken up L. plantarum and V. parvula, respectively. The mean fluorescence intensity corresponding to the number of bacteria was highest for V. parvula, both in monocytes and in DCs (data not shown). Consequently, the differences in induction of cytokines by monocytes and DCs and the differences in the responses to gram-positive and gram-negative bacteria could not be explained by differences in phagocytosis.
FIG. 3.
(A) Phagocytosis of FITC-labeled bacteria by monocytes and DCs. The bars indicate the mean percentage of FITC-positive cells from three donors, and the error bars indicate the standard error of the mean. (B) Photomicrographs of a monocyte and a DC that have phagocytosed FITC-labeled bacteria. Original magnification, ×400.
Bacterial stimulation alters the expression of CD14, TLR4, and TLR2 on monocytes and DCs.
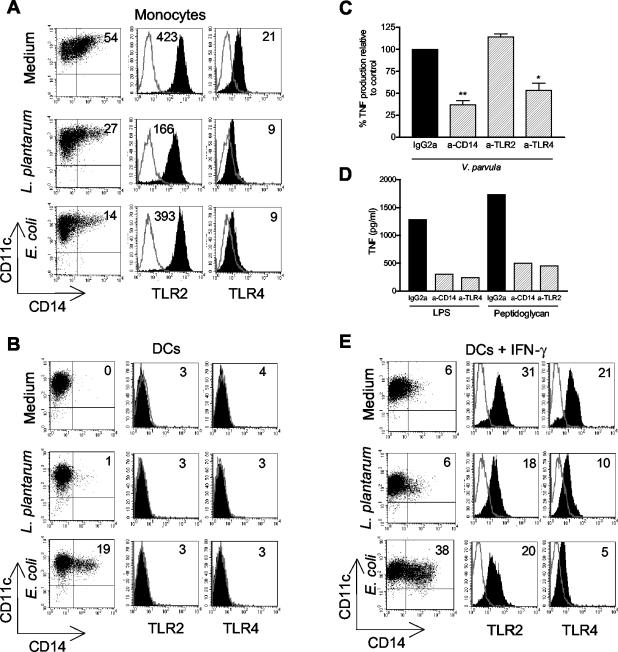
We next examined how intact bacteria regulate surface expression of microbial pattern recognition receptors on APCs. Figure 4A shows that after stimulation with either L. plantarum or E. coli the expression of CD14 and TLR4 was down-regulated on monocytes. The surface expression of TLR2 on monocytes was reduced only by stimulation with L. plantarum.
FIG. 4.
Expression of CD14, TLR2, and TLR4 on monocytes (A) and DCs and in the absence (B) or in the presence (E) of 10 ng of IFN-γ per ml after stimulation for 48 h with L. plantarum or E. coli. The percentages of CD14-positive cells and the geometric mean fluorescence intensity for TLR expression, respectively, are indicated in the upper right corners of the panels. The lines show the data for the isotype control antibodies, and the solid histograms show the expression of TLRs. The results shown are the results of one representative experiment of three experiments performed. (C) Effect on V. pavula-induced TNF production from DCs after blocking of CD14, TLR2, or TLR4. DCs (5 × 105 cells per ml) were incubated with 10 μg of blocking antibody per ml or 10 μg of isotype control antibody per ml for 1 h prior to stimulation with bacteria (5 × 106 cells per ml) for 7 h. The results after blocking were expressed as the percentage of TNF production relative to control production for each individual. The bars indicate the mean percentage of positive cells from three donors, and the error bars indicate the standard error of the mean. (D) Positive control for the effectiveness and specificity of the blocking antibodies used. The experiments were performed as described above, and the cells were stimulated with 0.1 μg of LPS per ml or 1 μg of peptidoglycan per ml. a-CD14, anti-CD14; a-TLR2, anti-TLR2; a-TLR4, anti-TLR4.
Immature DCs generated from monocytes did not express CD14. However, when cells were stimulated with E. coli for 48 h, CD14 appeared on the surface (Fig. 4B). In contrast, L. plantarum did not up-regulate CD14 expression on DCs. DCs cultured with bacteria or medium alone did not stain positively for either TLR2 or TLR4. However, it is well established that DCs generated in vitro do express these TLRs, at least if the medium is supplemented with fetal calf serum. Therefore, we analyzed whether these receptors are expressed on DCs generated in the presence of human serum by studying if they are involved in the response of DCs to intact gram-negative bacteria. We blocked CD14, TLR2, and TLR4 with specific monoclonal antibodies prior to stimulation with V. parvula and measured the production of TNF. As shown in Fig. 4C, blocking CD14 and TLR4 in fact decreased the production of TNF from DCs, whereas blocking of TLR2 did not have this effect. This shows that TLR4 is expressed on DCs and is important for proinflammatory responses to intact gram-negative bacteria. As a positive control, Fig. 4D shows the effectiveness and specificity of the blocking antibodies used.
Since IFN-γ potentiated the cytokine response of DCs to E. coli and V. parvula, we subsequently studied whether priming by IFN-γ affected the surface expression of pattern recognition receptors. Figure 4E shows that TLR2 and TLR4 were up-regulated on DCs in the presence of IFN-γ. However, it should be noted that the intensity of TLR2 expression was considerably higher on monocytes than on IFN-γ-primed DCs, whereas similar amounts of TLR4 were expressed on both types of cells (Fig. 4A and E). This could partially explain why monocytes exhibit much greater cytokine responses to L. plantarum and B. adolescentis than DCs elicit. Bacterial stimulation slightly decreased the expression of TLR2 on such IFN-γ-primed DCs. TLR4 was also down-regulated after stimulation with both L. plantarum and E. coli. The results obtained for B. adolescentis and V. parvula were similar to the results obtained for L. plantarum and E. coli, respectively (data not shown). In summary, when monocytes differentiated into DCs, the surface expression of CD14, TLR2, and TLR4 was down-regulated, but IFN-γ could effectively up-regulate the surface expression of TLR2 and TLR4 on DCs. However, bacterial stimulation decreased the expression of TLRs on both monocytes and DCs. Furthermore, the lack of a response of DCs to gram-positive bacteria could be associated with lower surface expression of TLR2 on DCs than on monocytes.
DISCUSSION
Colonization of the gastrointestinal tract of the newborn infant starts immediately after birth and is one of the major factor driving maturation of the immune system (1). In rodents the presence of a normal intestinal microflora seems to be required for proper maturation of the immune system and induction of oral tolerance (28, 39). For example, animals harboring a normal intestinal microflora have 10 times more IgA-producing cells in the intestinal lamina propria than germfree animals have (14). The large intestine of a human adult contains 1014 bacteria, and these organisms are in close and constant contact with gut-associated lymphoid tissues (44). These bacteria encounter DCs, which after maturation direct T-cell responses in lymphoid tissues. Viable bacteria may penetrate peripheral blood vessels and enter the circulation, where they are generally killed instantaneously by monocytes and granulocytes while they simultaneously generate a proinflammatory response. Gram-negative facultative bacteria, such as E. coli, translocate to a greater extent than obligate anaerobes and gram-positive bacteria translocate. However, gram-positive bacteria that are not strictly anaerobic, such as lactobacilli, may also translocate (34). Since the composition of the gut flora of allergic children and the composition of the gut flora of nonallergic children have been observed to differ, gastrointestinal exposure to microbes may be significant for protection against allergy (9, 10, 12, 27). However, it is still not clear how different groups or species of bacteria modulate the immune system of the host or activate various types of APCs.
How DCs and macrophages respond to bacterial cell wall components has been well documented. For example, activation of TLR4 by purified ligands, such as LPS, seems to induce a response predominated by IL-12 production, whereas TLR2 activation by lipopeptides, peptidoglycan, and Porphyromonas gingivalis LPS instead induces IL-10 production (2, 22, 31). However, intact microbes are likely to be recognized by a combination of innate recognition receptors as cell walls of both gram-positive and gram-negative bacteria are composed of various molecules with immunostimulatory properties. Only few studies have examined how clinical isolates of intact bacteria affect the immune system through the interaction with DCs and monocytes. It has previously been shown that enriched monocytes and PBMCs generally produce higher levels of IL-12 and TNF in response to a panel of gram-positive bacterial strains than in response to gram-negative strains (20, 25). Other workers have shown that murine macrophages elicit higher levels of IL-12 in response to intact gram-negative bacteria than in response to gram-positive organisms (29). In the present study we found that purified human monocytes and DCs respond differentially to a group of four gastrointestinal bacteria. As previously observed with PBMCs, purified monocytes produce higher levels of IL-12 and TNF when they are cultured with the gram-positive organism L. plantarum than when they are cultured with the gram-negative organisms E. coli and V. parvula. Murine splenocytes, with a repertoire of cells similar to that found in human PBMCs, also produce higher levels of IL-12 in response to L. plantarum than in response to E. coli (33). We found that in contrast to monocytes, immature DCs are practically unresponsive to L. plantarum and B. adolescentis but produce high levels of IL-12, TNF, IL-6, and IL-10 in response to E. coli and V. parvula. Veckman et al. have shown that the pathogenic gram-positive organism Streptococcus pyogenes potently induces IL-12 production by human DCs (42). This indicates that probiotic and pathogenic gram-positive bacteria differ in the capacity to induce cytokine secretion from DCs.
Whether monocyte-derived DCs represent the DCs located in the gut is questionable. However, in line with our results, experiments with human intestinal mucosal explants have shown that TNF, IL-8, and IL-10 are produced in response to E. coli but not in response to Lactobacillus casei (11). Furthermore, the gut mucosal DCs predominantly consist of myeloid DCs, which presumably are of monocyte origin (7). The differences in the cytokine responses of monocytes and DCs could not be explained by differences in phagocytic capacities, as the bacteria were equally well phagocytosed by monocytes and DCs. Rather, the variations are explained by differential expression of pattern recognition receptors on the APCs.
Our results indicate that direct exposure to both gram-positive and gram-negative bacteria down-regulates CD14, TLR2, and TLR4 surface expression on APCs. This could either be due to the fact that the pattern recognition receptors are internalized together with the microbe or be due to the fact that activation induces a negative feedback mechanism to control the inflammatory response. Other groups studying gene expression have shown that LPS transiently up-regulates TLR2 and TLR4 in monocytes and DCs, but a prolonged treatment led to down-regulation of the TLR message (43). The expression of CD14, TLR2, and TLR4 on monocytes is down-regulated during the differentiation into immature DCs, which has also been demonstrated by other workers (43). Since we were unable to detect the surface expression of TLR2 and TLR4 on immature and bacterium-stimulated DCs with flow cytometry, we concluded that the density of these receptors must be very low. However, as shown by our data, the cytokine responses to intact V. parvula are at least partially dependent on TLR4 and CD14. This indicates that low TLR4 surface expression is sufficient to mount proinflammatory responses to gram-negative bacteria.
The human gut mucosa seems to be in a state of controlled inflammation under normal conditions, since intestinal intraepithelial and lamina propria lymphocytes spontaneously secrete IFN-γ (13, 19, 26). We observed that IFN-γ dramatically increases the expression of TLRs and potentiates the capacity of DCs to secrete cytokines, but only in response to E. coli and V. parvula. It has previously been shown that TNF production by monocytes induced by L. plantarum is at least partially dependent on TLR2 (25). IFN-γ up-regulates TLR2 on DCs, but the cells remain unresponsive to L. plantarum and B. adolescentis. However, the intensity of TLR2 expression is considerably higher on monocytes than on IFN-γ-primed DCs. Other workers have shown that monocytes express higher levels of TLR2 mRNA than both monocyte-derived DCs and peripheral blood CD11c+ DCs express (24). Thus, the decreased ability to respond to L. plantarum and B. adolescentis when monocytes differentiate into DCs could be due to a reduction in TLR2 expression. Alternatively, DCs might express other receptors than monocytes that have inhibitory properties. C-type lectins are implicated in both enhancing and abrogating activation of TLRs. Dectin-1 collaborates with TLR2 and potentiates inflammatory responses to zymosan, whereas binding of mannose-capped lipoarabinomannan from Mycobacterium tuberculosis to dendritic cell-specific ICAM-grabbing nonintegrin (DC-SIGN) mediates immunosuppressive effects (16, 17). Moreover, L. casei has been shown to suppress TNF responses induced by E. coli from human gut mucosal explants, but it is still not known whether this suppressive effect involves C-type lectins (11). Nevertheless, microbes are likely to be recognized by a complex combination of receptors, which by acting together decide the outcome of the response.
In summary, the gram-positive probiotic bacteria L. plantarum and B. adolescentis may have a role in controlling local inflammation in the gut as they do not activate DCs, but they could also be important for activation of systemic immunity after translocation as they preferentially trigger activation of monocytes. Gram-negative bacteria, such as E. coli and V. parvula, are strong activators of DCs, which might lead to either tolerance or hypersensitivity to concomitantly presented innocuous antigens depending on the level of bacterial stimulation (15). Preliminary results obtained in our laboratory show that monocytes and DCs from neonates respond to gut bacteria with a cytokine pattern similar to the pattern observed for cells from adults. Thus, our results may have implications for the capacities of different groups of commensal bacteria to regulate mucosal and systemic immunity in newborns.
Acknowledgments
This study was funded by the Medical Faculty, Göteborg University, by the Swedish Research Council (grant K2002-745X-14455-01A), by the Vårdal Foundation, by the Swedish Asthma and Allergy Association Research Foundation, and by the Göteborg Medical Society Frimurare Barnhusdirektionen.
We acknowledge Anna-Carin Andersson for testing the effectiveness of blocking antibodies and Åsa Lindgren for performing experiments with live bacteria.
Editor: A. D. O'Brien
REFERENCES
- 1.Adlerberth, I., L. Å. Hansson, and A. E. Wold. 2000. Ontogeny of the intestinal flora, p. 279-292. In I. R. Sanderson and W. A. Walker (ed.), Development of the gastrointestinal tract. BC Decker Inc., Hamilton, Ontario, Canada.
- 2.Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. [DOI] [PubMed] [Google Scholar]
- 3.Ahrne, S., S. Nobaek, B. Jeppsson, I. Adlerberth, A. E. Wold, and G. Molin. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Appl. Microbiol. 85:88-94. [DOI] [PubMed] [Google Scholar]
- 4.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 5.Albers, W. H., C. W. Tyler, and B. Boxerbaum. 1966. Asymptomatic bacteremia in the newborn infant. J. Pediatr. 69:193-197. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 7.Bell, S. J., R. Rigby, N. English, S. D. Mann, S. C. Knight, M. A. Kamm, and A. J. Stagg. 2001. Migration and maturation of human colonic dendritic cells. J. Immunol. 166:4958-4967. [DOI] [PubMed] [Google Scholar]
- 8.Berg, R. D. 1995. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 3:149-154. [DOI] [PubMed] [Google Scholar]
- 9.Björksten, B., P. Naaber, E. Sepp, and M. Mikelsaar. 1999. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin. Exp. Allergy 29:342-346. [DOI] [PubMed] [Google Scholar]
- 10.Björksten, B., E. Sepp, K. Julge, T. Voor, and M. Mikelsaar. 2001. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108:516-520. [DOI] [PubMed] [Google Scholar]
- 11.Borruel, N., F. Casellas, M. Antolin, M. Llopis, M. Carol, E. Espiin, J. Naval, F. Guarner, and J. R. Malagelada. 2003. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. Am. J. Gastroenterol. 98:865-870. [DOI] [PubMed] [Google Scholar]
- 12.Böttcher, M. F., E. K. Nordin, A. Sandin, T. Midtvedt, and B. Björksten. 2000. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clin. Exp. Allergy 30:1590-1596. [DOI] [PubMed] [Google Scholar]
- 13.Carol, M., A. Lambrechts, A. Van Gossum, M. Libin, M. Goldman, and F. Mascart-Lemone. 1998. Spontaneous secretion of interferon gamma and interleukin 4 by human intraepithelial and lamina propria gut lymphocytes. Gut 42:643-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crabbe, P. A., H. Bazin, H. Eyssen, and J. F. Heremans. 1968. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int. Arch. Allergy Appl. Immunol. 34:362-375. [DOI] [PubMed] [Google Scholar]
- 15.Eisenbarth, S. C., D. A. Piggott, J. W. Huleatt, I. Visintin, C. A. Herrick, and K. Bottomly. 2002. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196:1645-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geijtenbeek, T. B., S. J. Van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. Vandenbroucke-Grauls, B. Appelmelk, and Y. Van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacomini, E., E. Iona, L. Ferroni, M. Miettinen, L. Fattorini, G. Orefici, I. Julkunen, and E. M. Coccia. 2001. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J. Immunol. 166:7033-7041. [DOI] [PubMed] [Google Scholar]
- 19.Hauer, A. C., M. Bajaj-Elliott, C. B. Williams, J. A. Walker-Smith, and T. T. MacDonald. 1998. An analysis of interferon gamma, IL-4, IL-5 and IL-10 production by ELISPOT and quantitative reverse transcriptase-PCR in human Peyer's patches. Cytokine 10:627-634. [DOI] [PubMed] [Google Scholar]
- 20.Hessle, C., B. Andersson, and A. E. Wold. 2000. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while gram-negative bacteria preferentially stimulate IL-10 production. Infect. Immun. 68:3581-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hessle, C., B. Andersson, and A. E. Wold. 2003. Gram-negative, but not Gram-positive bacteria are strong inducers of PGE2 production in human monocytes. Inflammation 329-332. 27: [DOI] [PubMed]
- 22.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2001. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 24.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlsson, H., C. Hessle, and A. Rudin. 2002. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 70:6688-6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDonald, T. T., and G. Monteleone. 2001. IL-12 and Th1 immune responses in human Peyer's patches. Trends Immunol. 22:244-247. [DOI] [PubMed] [Google Scholar]
- 27.Matricardi, P. M., F. Rosmini, S. Riondino, M. Fortini, L. Ferrigno, M. Rapicetta, and S. Bonini. 2000. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. Br. Med. J. 320:412-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreau, M. C., and G. Corthier. 1988. Effect of the gastrointestinal microflora on induction and maintenance of oral tolerance to ovalbumin in C3H/HeJ mice. Infect. Immun. 56:2766-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nau, G. J., A. Schlesinger, J. F. Richmond, and R. A. Young. 2003. Cumulative Toll-like receptor activation in human macrophages treated with whole bacteria. J. Immunol. 170:5203-5209. [DOI] [PubMed] [Google Scholar]
- 30.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 31.Qi, H., T. L. Denning, and L. Soong. 2003. Differential induction of interleukin-10 and interleukin-12 in dendritic cells by microbial Toll-like receptor activators and skewing of T-cell cytokine profiles. Infect. Immun. 71:3337-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 33.Repa, A., C. Grangette, C. Daniel, R. Hochreiter, K. Hoffmann-Sommergruber, J. Thalhamer, D. Kraft, H. Breiteneder, A. Mercenier, and U. Wiedermann. 2003. Mucosal co-application of lactic acid bacteria and allergen induces counter-regulatory immune responses in a murine model of birch pollen allergy. Vaccine 22:87-95. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez, A. V., M. D. Baigori, S. Alvarez, G. R. Castro, and G. Oliver. 2001. Phosphatidylinositol-specific phospholipase C activity in Lactobacillus rhamnosus with capacity to translocate. FEMS Microbiol. Lett. 204:33-38. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroder, N. W., S. Morath, C. Alexander, L. Hamann, T. Hartung, U. Zahringer, U. B. Gobel, J. R. Weber, and R. R. Schumann. 2003. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 278:15587-15594. [DOI] [PubMed] [Google Scholar]
- 37.Shimazu, R., S. Akashi, H. Ogata, Y. Nagai, K. Fukudome, K. Miyake, and M. Kimoto. 1999. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189:1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinman, R. M., D. Hawiger, and M. C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685-711. [DOI] [PubMed] [Google Scholar]
- 39.Sudo, N., S. Sawamura, K. Tanaka, Y. Aiba, C. Kubo, and Y. Koga. 1997. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J. Immunol. 159:1739-1745. [PubMed] [Google Scholar]
- 40.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 41.Van Camp, J. M., V. Tomaselli, and A. G. Coran. 1994. Bacterial translocation in the neonate. Curr. Opin. Pediatr. 6:327-333. [PubMed] [Google Scholar]
- 42.Veckman, V., M. Miettinen, J. Pirhonen, J. Sirén. S. Matikainen, and I. Julkunen. Streptococcus pyogenes and Lactobacillus rhamnosus differentially induce maturation and production of Th1 type cytokines and chemokines in human monocyte-derived dendritic cells. J. Leukoc. Biol., in press. [DOI] [PubMed]
- 43.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]
- 44.Wold, A. E., and I. Adlerberth. 2000. Pathologic effects of commensalism, p. 115-144. In J. P. Nataro, M. J. Blaser, and S. Cunningham-Rundles (ed.), Persistent bacterial infections. ASM Publishing Co., Washington, D.C.
- 45.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]