Abstract
Despite extensive study, the molecular mechanisms that lead to multinucleation and cell enlargement (hypertrophy) remain poorly understood. Here, we show that a single bacterial virulence protein, EspF, from the human pathogen enteropathogenic E. coli induces extreme multi-nucleation in small intestinal epithelial cells. Ectopic expression of EspF induced cell-cell internalization events, presumably responsible for the enlarged multinucleated cells. These extreme phenotypes were dependent on a C-terminal polyproline-rich domain in EspF and not linked to the targeting of mitochondria or the nucleolus. The subversive functions of EspF may provide valuable insight into the molecular mechanisms that mediate cell fusion, multinucleation and cell hypertrophy.
Keywords: Caco-2, EPEC, EspF, N-WASP, Syncytium, TC-7, bacterial effector, cell fusion, enteropathogenic E. coli, hypertrophy, intestinal, monolayer, multi-nucleation
Introduction
Multinucleation and cell hypertrophy are important cellular processes that generally arise from the fusion of mononucleated cells, giving rise to an enlarged multinucleated cell called a syncytium. Syncytia are specialist cells types that play important roles in normal physiology and disease including multinucleated giant cells that derive from macrophage fusion at the sites of inflammation,1,2 osteoclasts in bone tissue,3 trophoblasts of the placenta4 and skeletal muscle cells.5 Enlarged multinucleated cells have been associated with many different types of cancer6-10 — involving cell-cell fusion events11 or cell-in-cell internalization processes.12 Some important disease agents induce multinucleation in host cells including viruses such as HIV,13 bacterial pathogens such as Mycobacterium tuberculosis14 and lesser known pathogens like microsporidia.15 However, despite the involvement of multinucleated cells in a broad range of physiological processes, little is known about the underlying molecular mechanisms that lead to their formation, although it is believed to be a complex and tightly controlled event.16
The human enteric pathogen enteropathogenic E. coli (EPEC) delivers over 20 virulence-related proteins, termed effectors, into epithelial cells lining the small intestine resulting in severe diarrhea.17 Most EPEC effectors have been functionally characterized, leading to the prevailing view that effector proteins are highly multifunctional.18,19 EspF is a relatively small but well-studied EPEC effector20 that displays a broad range of biological activities including the targeting of host mitochondria21 and nucleoli leading to their dysfunction.22 EspF is a modular protein with several defined domains including a mitochondrial targeting sequence in the N-terminus, a nucleolar targeting domain directly downstream, and a C-terminal polyproline rich repeat (PRR) region.20 This latter region has been implicated in membrane remodeling and modulation of the cytoskeleton within host cells.23,24
Here, we show that EspF induces overt phenotypical and behavioral changes when expressed ectopically within human small intestinal cells. We show that EspF-induced multinucleation and cell hypertrophy occur concomitantly with cell-in-cell fusion events as we observed a marked induction in this process. EspF variants revealed that the observed cellular phenotypes were dependent on the C-terminal proline-rich repeat region. Taken together, this study identifies a single bacterial protein that induces extreme alterations in epithelial cell behavior leading to the induction of a multinucleated syncytium-like intestinal cell.
Materials and Methods
Plasmids
The plasmids used in this study were derived from pEGFP-N1 (Clontech) and encode mutated variants of EspF fused to EGFP as described previously.22 The source of EspF was the enteropathogenic E.coli strain E2348/69. Plasmids were purified to ~2mg/mL using the Qiagen midiprep kit according to the manufacturer’s instructions.
Small intestinal model system
The Caco-2 clonal cell line TC-7 is a homogeneous small intestinal model that has been well characterized since its isolation.25 TC-7 cells were maintained in tissue culture flasks at 37°C as described previously.26 Routinely, the cells were fed fresh Dulbecco’s minimal Eagle medium (DMEM; Invitrogen) supplemented with 1 × penicillin/streptomycin and 10% (v/v) heat inactivated fetal calf serum (Gibco).
Transfection of TC-7 cells with pEGFP-N1-EspF variants
Following trypsinization, TC-7 cells were diluted in fresh DMEM (without supplements) to a concentration of 2 × 106 cells/mL. Lipofectamine 2000 (Invitrogen) was mixed with plasmid DNA according to the manufacturer’s instructions and added to the cell suspension. Cells were then rotated at 37°C for 30 min and then transferred to 24-well plates (Corning) and centrifuged at 500 g for 5 min onto 13 mm sterile glass coverslips. Cells were left for 6h at 37°C and the medium was replaced with fresh complete DMEM. By 24h post-transfection, the cells had attached to the glass coverslip and were confluent.
Staining of transfected cells and confocal microscopy
Transfected TC-7 were fixed in 4% (w/v) para-formaldehyde in PBS for 15 min, permeabilized for 5 min with 0.2% (w/w) Triton X-100 and stained as described.27 Briefly, fixed cells were stained with TRITC-labeled phalloidin (Invitrogen) to stain filamentous actin and DAPI to stain cell nuclei. Cells were mounted in Mowiol containing p-phenylenediamine and visualized on a Leica SP2 confocal microscope with a x63 objective lens. Maximal cell diameter and cell area were determined using phallodin staining to indicate cell periphery and measured using Leica confocal software, typically from 8 randomly selected fields of view per experiment at × 63 magnification. Cells exhibiting low EspF-GFP expression were visualized by empirically increasing the optical gain of the confocal microscope, while cells expressing much higher levels of EspF-GFP (above maximal saturation intensity at this optical gain) were not included in this study as they have been described elsewhere.22
Statistical analysis
All experiments were repeated three times, unless otherwise stated. Data are expressed as mean ± SD and was analyzed by the Student's t-test using the statistical software package SPSS.
Results and Discussion
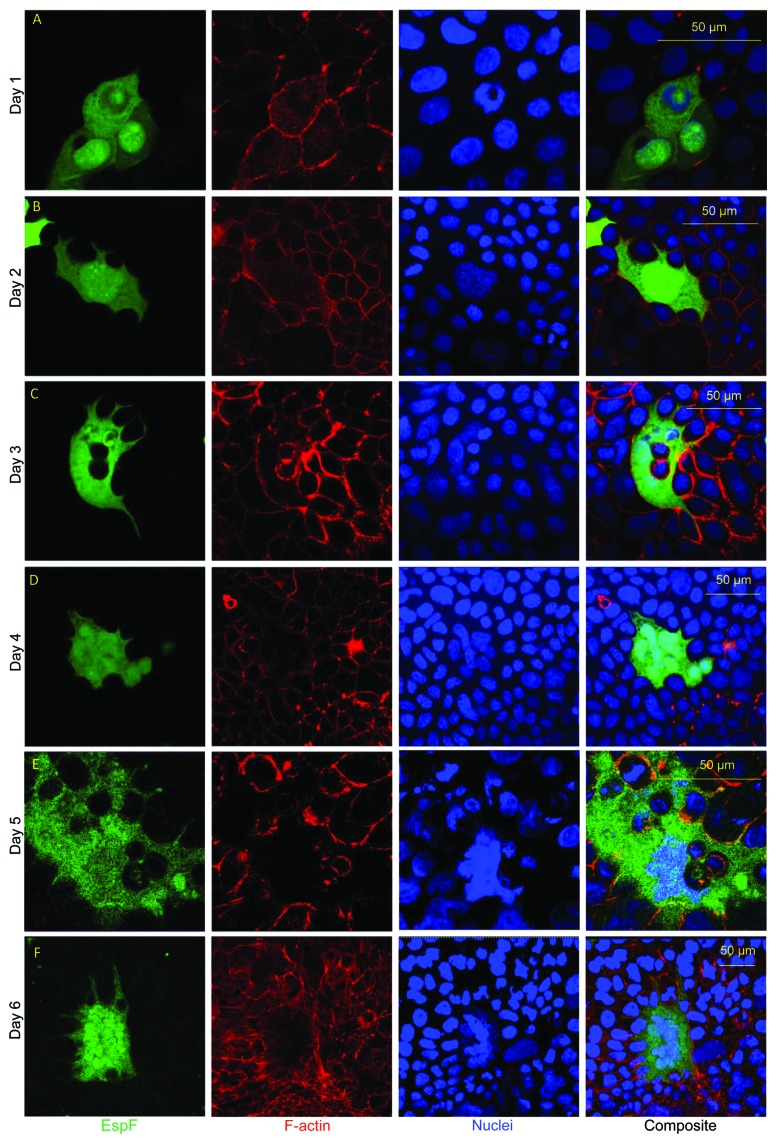
EspF targets the mitochondrion, nucleolus and cytoplasm of a range of human host cells.21,22,28 Its predominant target site is the mitochondrion, thus removal or mutation (L16E) of the N-terminal mitochondrial targeting sequence of EspF enables a better assessment of its cytoplasmic and nucleolar functions.22,28 Our previous work on EspF, looked at the effects of a variant of EspF (L16E)-tagged EGFP expressed within the small intestinal cell line TC-7 — a clonal line of the more commonly used Caco-2 model. TC-7 cells provide a homogeneous population of enterocytes that enables a better assessment of phenotypes and cell behavior, particularly of individual cells. A transfection protocol was developed for TC-7 cells in which monolayers expressing a protein of interest were ~100% confluent on day 1 post-transfection (that were detected as described in Materials and Methods section). Microscopy analysis of TC-7 cells expressing EspF(L16E)-EGFP (herein termed EspF-GFP) revealed the protein targeted the cytoplasm and nucleolus (Fig. 1A) as described previously.22 However, we also noticed that cells expressing EspF-GFP at much lower levels (see Materials and Methods) exhibited a marked increase in the number of nuclei and cell size, unlike those expressing higher levels of EspF above the threshold level (see Materials and Methods). Thus, a time course experiment was performed to investigate the linkage between low EspF expression and these phenotypes.
Figure 1. Expression of EspF(L16E)-GFP in small intestinal cells induces extreme cell hypertrophy and multinucleation. TC-7 intestinal cells were transfected in suspension and seeded at high density. Cells were then visualized on different days post infection as described in Materials and Methods.
TC-7 cells expressing EspF-GFP on day 1 post-transfection remained mononuclear (unless dividing), similar to adjacent untransfected cells in the same well (Fig. 1A and Fig. 2A). Cells expressing EspF-GFP exhibited a progressive increase in cell hypertrophy (maximal diameter) and multinucleation (counted as 3 or more nuclei) from day 2 to day 6 post-transfection (Fig. 1A-F and Fig. 2). Untransfected cells or cells expressing EGFP alone (not shown) were unchanged — with a slight decrease in cell diameter/nuclear size due to increases in cell confluency (Fig. 1 and Fig. 2). Indeed, comparing day 1 with day 5 post-transfection revealed that the EspF-induced multinucleated cells, had a ~22-fold mean increase in cell size (Fig. 2C) and a 14-fold mean increase in nuclei (Fig. 2A) with some cells displaying ~50 nuclei per cell. As the doubling time of TC-7 cells is approximately 26 h,29 this data suggested that multinucleation in cells that contained 40–50 nuclei was unlinked to the cell cycle but may have arose by another mechanism. Taken together, the data shows that ectopic expression of the cytoplasmic variant of EspF induces multinucleation and cell hypertrophy in an epithelial model.
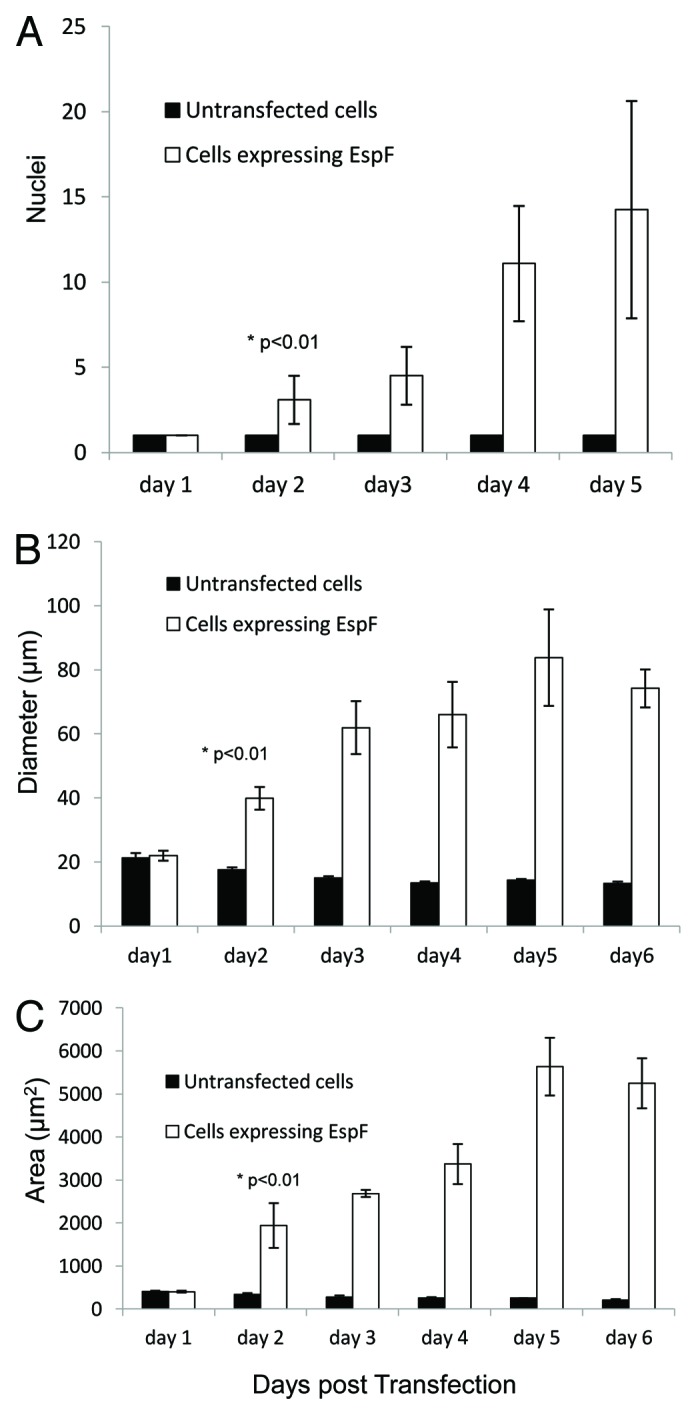
Figure 2. Quantification of phenotypical changes in TC-7 cells induced by EspF. TC-7 cells expressing EspF(L16E)-GFP were assessed for (A) nuclei number (B) maximal cell diameter and (C) cell area. Data shows mean ± SD; n = 3 with 8 fields of view per experiment. * p value on day 2 relates to a comparison of transfected and untransfected cells.
A striking feature of the EspF-expressing TC-7 cells was the high levels of internalization of adjacent non-transfected cell types (Fig. 1B-F and Fig. 3). Confocal sectioning of cells along the x-z axis revealed complete internalization of engulfed cells (bound by an actin peripheral signal) within the EspF-expressing cells (Fig. 3A), using the phalloidin signal to discern cell periphery. Intact internalized cells were negative for EspF-GFP in their cytoplasm and nucleus, revealing they were not daughter cells of the EspF-expressing cell but were indeed from a neighboring cell location. Moreover, all EspF-expressing multinucleated cells possessed long cytoplasmic extensions that penetrated between the cell-cell interfaces of adjacent epithelial cells (Fig. 3B). These extensions in EspF-expressing cells were consistent throughout the monolayer reaching lengths of over 50 µm (Fig. 1F) and provided an explanation for how the smaller cell types were being internalized. Quantification of cells on day 4 post-transfection revealed that ~90% ± 4.65 (mean ± SD) of EspF-expressing cells were in the process of engulfing or had engulfed 2 or more non-transfected cells (Fig. 3C), whereas non-transfected cells displayed near-zero levels of internalization (Fig. 3C). Thus, it is evident that expression of EspF-GFP within this small intestinal model induces extensive cell-in-cell internalization events.
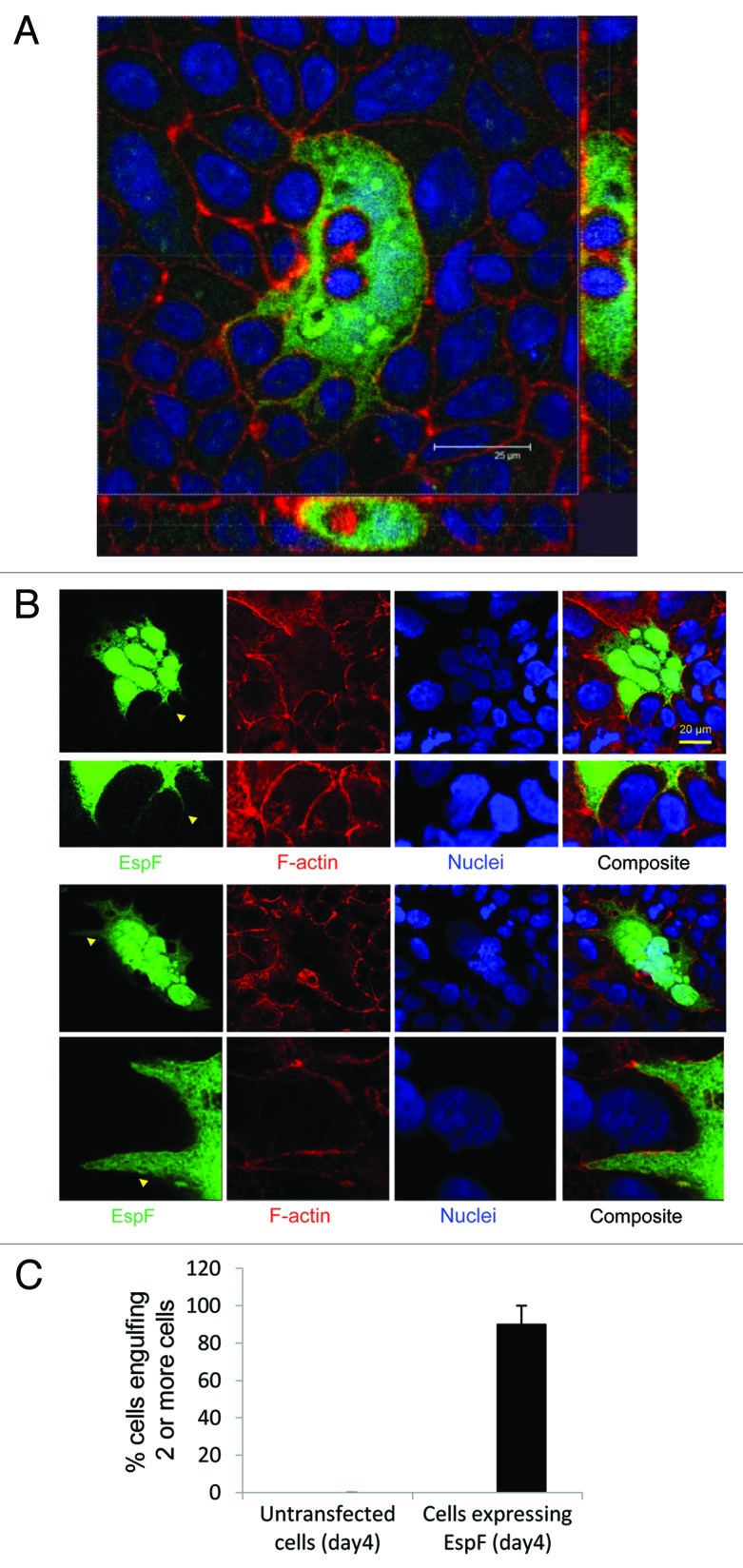
Figure 3. EspF expression induces cell internalization in an epithelial monolayer. (A) TC-7 cells expressing EspF(L16E)-GFP were visualized by confocal microscopy and sectioned along the x-z axis. (B) Cytoplasmic extensions (yellow arrow) in EspF-expressing cells (day 4 post transfection) were consistently found surrounding non-transfected cell types. (C) Quantification of cell engulfment in which only cells engulfing 2 or more cells were included in the analysis. Data shows mean ± SD, n = 3.
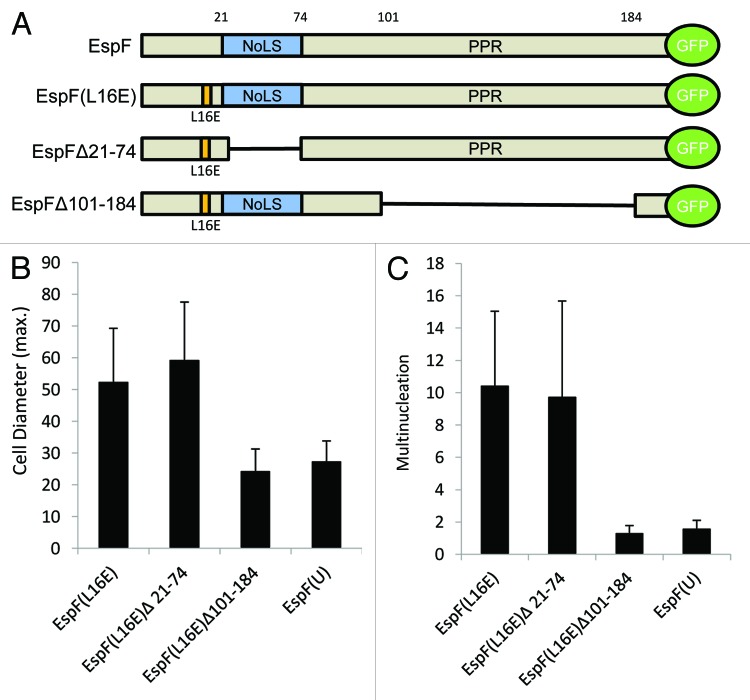
EspF targets host organelles and also exhibits several cytoplasmic functions.20 The EspF protein has a modular architecture (Fig. 4) and can be broadly divided into 3 sub-domains that include the mitochondrial localization sequence, nucleolar targeting domain and a C-terminal proline rich repeat (PRR; Fig. 4A). As the L16E version of EspF was used throughout this study, the targeting of mitochondria by EspF was not responsible for the phenotypes described. Deletion analysis of EspF ruled out a role for nucleolar targeting in multinucleation (Fig. 4C; p = 0.95) and cell hypertrophy (Fig. 4B; p = 0.676) but revealed an essential role for the C-terminal PRR domain (Fig. 4B and C; p < 0.01). Indeed, the EspF variant Δ21–74, which possesses the intact C-terminal PRR half of the protein, was similar to the full length EspF in inducing multinucleation and hypertrophy. While the first 21 amino acids of EspF were also encoded by this variant, they are also encoded by EspF Δ101–184, which was defective in inducing the phenotypes. The first 21 amino acids are also known to be required for bacterial secretion and mitochondrial targeting (Holmes et al., 2010), the latter of which was ruled out because of the L16E mutation. While we cannot rule out a contributory role for the N-terminal domain, the data demonstrates an essential role for C-terminal PRR region for inducing the phenotypes described. Intriguingly, the PRR region of this effector protein has been linked with membrane remodeling and cytoskeletal rearrangements, both of which are presumably essential for the cell-cell internalization events described here. Myoblast fusion, which gives rise to skeletal muscle cell syncytia, has been shown to depend on N-WASP, a protein that binds EspF via its C-terminal PRR domain.30 It seems likely that EspF acts through N-WASP to elicit cell fusion events and future studies will elucidate whether this is the case. Finally, studies with the effector EspF(U)-EGFP, which possesses a homologous PRR region to EspF (Alto et al., 2007), including a variant of the N-WASP binding site, showed no induction of multinucleation or cell-cell fusion when expressed in TC-7 cells (Fig. 4B-C) although EspF(U)-EGFP was notably more toxic than EspF-EGFP in TC-7 cells. As EspF(U), contains a variation of the N-WASP binding site, this may also explain these differences. Alternatively, EspF may encode an unknown protein-protein interaction site in its C-terminal repeat region that could be mediating the cell fusion events.
Figure 4. The proline rich repeat region in EspF is responsible for multinucleation and cell hypertrophy. (A) The modular architecture of EspF is shown in top schematic with the different constructs used in this study shown beneath. Constructs have an L16E substitution, or have been deleted for the nucleolar localization sequence (NoLS) or the proline rich repeat (PRR). EspF(U) is an effector that contains a homologous PRR to EspF. Maximal cell diameter (B) and levels of multinucleation (3 or more nuclei; C) were measured in cells expressing the different constructs (day 4 post-transfection). Data shows mean ± SD, n = 3.
In this study, the clonal cell line TC-7 was used for the purpose of removing heterogeneity in epithelial populations, thus it is very unlikely that the multinucleated cells were a subset within the TC-7 population. No enlargement or increases in nucleation were found in untransfected control cells during the time course experiment, thus these phenotypes were directly linked to ectopic EspF expression. Multinucleation and cell hypertrophy in response to EPEC infection has not been previously reported although this is very difficult to determine in vivo. Thus, it is unclear whether or not EspF possesses such functions during the infection process or whether they are being suppressed due to the concerted actions of other EPEC effector proteins. Furthermore, whether these cell types exist within the gastrointestinal tract is unknown although this study shows that multinucleation in epithelial cells can clearly be induced. Interestingly, enlarged multinucleated cells have been reported in hyperplastic polyps in several epithelial tissue types suggesting that they can, under certain conditions, display these extreme phenotypes.31 Nonetheless, multinucleation, cell enlargement and cell-cell internalization are consistent features of infectious diseases, cancers, inflammation, and normal physiological processes, yet we know little of the molecular events unpinning them. The documented examples that N-WASP is essential for cell-cell fusion events29 suggests the enticing possibility that EspF is functioning through N-WASP via its N-WASP binding site to induce cell fusion. As bacterial effector proteins commonly mimic vertebrate host proteins, it seems plausible that the EspF PRR may have counterparts in higher eukaryotes to regulate the cellular responses described in this study.
Acknowledgments
We would like to thank Sabine Quitard for technical assistance. This work was supported by a Faculty Fellowship awarded to P.D. from the University of Newcastle and a Wellcome Trust senior fellowship awarded to B.K.
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/22639
References
- 1.Vignery A. Macrophage fusion: molecular mechanisms. Methods Mol Biol. 2008;475:149–61. doi: 10.1007/978-1-59745-250-2_9. [DOI] [PubMed] [Google Scholar]
- 2.Vignery A. Macrophage fusion: the making of osteoclasts and giant cells. J Exp Med. 2005;202:337–40. doi: 10.1084/jem.20051123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards JR, Mundy GR. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol. 2011;7:235–43. doi: 10.1038/nrrheum.2011.23. [DOI] [PubMed] [Google Scholar]
- 4.Huppertz B, Gauster M. Trophoblast fusion. Adv Exp Med Biol. 2011;713:81–95. doi: 10.1007/978-94-007-0763-4_6. [DOI] [PubMed] [Google Scholar]
- 5.Simionescu A, Pavlath GK. Molecular mechanisms of myoblast fusion across species. Adv Exp Med Biol. 2011;713:113–35. doi: 10.1007/978-94-007-0763-4_8. [DOI] [PubMed] [Google Scholar]
- 6.Attanoos RL, Papagiannis A, Suttinont P, Goddard H, Papotti M, Gibbs AR. Pulmonary giant cell carcinoma: pathological entity or morphological phenotype? Histopathology. 1998;32:225–31. doi: 10.1046/j.1365-2559.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- 7.Baschinsky DY, Frankel WL, Niemann TH. Gastric carcinoma with osteoclast-like giant cells. Am J Gastroenterol. 1999;94:1678–81. doi: 10.1111/j.1572-0241.1999.01162.x. [DOI] [PubMed] [Google Scholar]
- 8.Douglas-Jones AG, Barr WT. Breast carcinoma with tumor giant cells. Report of a case with fine needle aspiration cytology. Acta Cytol. 1989;33:109–14. [PubMed] [Google Scholar]
- 9.Guiter GE, DeLellis RA. Multinucleate giant cells in papillary thyroid carcinoma. A morphologic and immunohistochemical study. Am J Clin Pathol. 1996;106:765–8. doi: 10.1093/ajcp/106.6.765. [DOI] [PubMed] [Google Scholar]
- 10.Weihua Z, Lin Q, Ramoth AJ, Fan D, Fidler IJ. Formation of solid tumors by a single multinucleated cancer cell. Cancer. 2011;117:4092–9. doi: 10.1002/cncr.26021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosaka M, Hatori M, Smith R, Kokubun S. Giant cell formation through fusion of cells derived from a human giant cell tumor of tendon sheath. J Orthop Sci. 2004;9:581–4. doi: 10.1007/s00776-004-0825-0. [DOI] [PubMed] [Google Scholar]
- 12.Krajcovic M, Johnson NB, Sun Q, Normand G, Hoover N, Yao E, et al. A non-genetic route to aneuploidy in human cancers. Nat Cell Biol. 2011;13:324–30. doi: 10.1038/ncb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildreth JE, Orentas RJ. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989;244:1075–8. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- 14.Gasser A, Möst J. Generation of multinucleated giant cells in vitro by culture of human monocytes with Mycobacterium bovis BCG in combination with cytokine-containing supernatants. Infect Immun. 1999;67:395–402. doi: 10.1128/iai.67.1.395-402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wittner M, Weiss LM. The Microsporidia and Microsporidiosis. Washington: ASM Press, 1999. [Google Scholar]
- 16.Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–93. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 17.Dean P, Kenny B. The effector repertoire of enteropathogenic E. coli: ganging up on the host cell. Curr Opin Microbiol. 2009;12:101–9. doi: 10.1016/j.mib.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dean P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol Rev. 2011;35:1100–25. doi: 10.1111/j.1574-6976.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- 19.Galán JE. SnapShot: effector proteins of type III secretion systems. Cell. 2007;130:192. doi: 10.1016/j.cell.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 20.Holmes A, Mühlen S, Roe AJ, Dean P. The EspF effector, a bacterial pathogen’s Swiss army knife. Infect Immun. 2010;78:4445–53. doi: 10.1128/IAI.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nougayrède JP, Donnenberg MS. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell Microbiol. 2004;6:1097–111. doi: 10.1111/j.1462-5822.2004.00421.x. [DOI] [PubMed] [Google Scholar]
- 22.Dean P, Scott JA, Knox AA, Quitard S, Watkins NJ, Kenny B. The enteropathogenic E. coli effector EspF targets and disrupts the nucleolus by a process regulated by mitochondrial dysfunction. PLoS Pathog. 2010;6:e1000961. doi: 10.1371/journal.ppat.1000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alto NM, Weflen AW, Rardin MJ, Yarar D, Lazar CS, Tonikian R, et al. The type III effector EspF coordinates membrane trafficking by the spatiotemporal activation of two eukaryotic signaling pathways. J Cell Biol. 2007;178:1265–78. doi: 10.1083/jcb.200705021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campellone KG, Cheng HC, Robbins D, Siripala AD, McGhie EJ, Hayward RD, et al. Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly. PLoS Pathog. 2008;4:e1000191. doi: 10.1371/journal.ppat.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chantret I, Rodolosse A, Barbat A, Dussaulx E, Brot-Laroche E, Zweibaum A, et al. Differential expression of sucrase-isomaltase in clones isolated from early and late passages of the cell line Caco-2: evidence for glucose-dependent negative regulation. J Cell Sci. 1994;107:213–25. doi: 10.1242/jcs.107.1.213. [DOI] [PubMed] [Google Scholar]
- 26.Dean P, Mühlen S, Quitard S, Kenny B. The bacterial effectors EspG and EspG2 induce a destructive calpain activity that is kept in check by the co-delivered Tir effector. Cell Microbiol. 2010;12:1308–21. doi: 10.1111/j.1462-5822.2010.01469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean P, Kenny B. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol Microbiol. 2004;54:665–75. doi: 10.1111/j.1365-2958.2004.04308.x. [DOI] [PubMed] [Google Scholar]
- 28.Nagai T, Abe A, Sasakawa C. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J Biol Chem. 2005;280:2998–3011. doi: 10.1074/jbc.M411550200. [DOI] [PubMed] [Google Scholar]
- 29.Grès MC, Julian B, Bourrié M, Meunier V, Roques C, Berger M, et al. Correlation between oral drug absorption in humans, and apparent drug permeability in TC-7 cells, a human epithelial intestinal cell line: comparison with the parental Caco-2 cell line. Pharm Res. 1998;15:726–33. doi: 10.1023/A:1011919003030. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee P, Gildor B, Shilo BZ, VijayRaghavan K, Schejter ED. The actin nucleator WASp is required for myoblast fusion during adult Drosophila myogenesis. Development. 2011;138:2347–57. doi: 10.1242/dev.055012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambie DL, Brown IS. Multinucleate epithelial change in colorectal hyperplastic polyps: a review of 27 cases. J Clin Pathol. 2008;61:611–4. doi: 10.1136/jcp.2007.050849. [DOI] [PubMed] [Google Scholar]