Abstract
Background
Numerous methods have been reported for the determination of artemether (ARM) and its metabolite dihydroartemisinin (DHA) in plasma. However, stability issues in patient plasma have not received enough attention.
Results
An LC–MS/MS method for simultaneous determination of ARM and DHA in human plasma (K3EDTA) turned out to be problematic: ARM and DHA were degraded partially or completely in some patient plasma samples as indicated by the stable isotope-labeled internal standards. We postulated iron II (Fe2+) in hemoglobin or its derived products from malaria patients causes degradation of the drugs, and found that hydrogen peroxide (H2O2) protected the drugs from degradation. Acidifying plasma increased recovery of ARM significantly. Using only 50 µl of plasma sample, the method has a LLOQ at 0.5 ng/ml for both ARM and DHA.
Conclusion
H2O2 is a stabilizing agent for artemisinin derivatives. The modified method is reliable and sensitive.
Numerous methods have been reported for the determination of artemether (ARM) and its metabolite dihydroartemisinin (DHA) in plasma from malaria patients [1–6]. Few of the reported methods have addressed stability issues when quantitating the drugs within plasma generated from patients infected with the malaria parasite. For the first time, Lindegardh et al. reported degradation of artemisinin derivatives during sample preparation, as indicated by the stable isotope-labeled internal standard (IS), and the issue was solved by using SPE and dissolving IS in 50% plasma [7]. At around the same time, Keiser et al. reported the stability issue of artesunate and DHA in hemolyzed rat plasma, and they solved the problem by using sodium nitrite to pre-treat the samples [8]. Hodel et al. confirmed the findings using direct injection of supernatant from protein precipitation, but they reported that evaporation and reconstitution following protein precipitation prevents ARM, DHA and artesunate from degrading [9]. Wiesner et al. utilized a liquid–liquid extraction method that seems to withstand the degradation pitfall [6]. Our method utilized SPE, similar to that used in Lindegardh’s group. Degradation of ARM and DHA was also found in some patient plasma samples. Furthermore, we identified a new stabilization agent, hydrogen peroxide (H2O2), and the LLOQ of the method was approximately threefold lower than that reported by Lindegardh’s group, using the same plasma volume.
Experimental
Chemicals & reagents
ARM and DHA were purchased from AK Scientific, Inc., (CA, USA). ARM-13CD3 (isotopic purity >99%) was obtained from Novartis (Basel, Switzerland) as a gift and DHA-d3 (isotopic purity >99%) was purchased from Toronto Research Chemicals, Inc. (Toronto, Canada). K3-EDTA was used as the anticoagulant for human plasma samples. All solvents and common reagents were purchased from Thermo-Fisher Scientific, Inc (MA, USA).
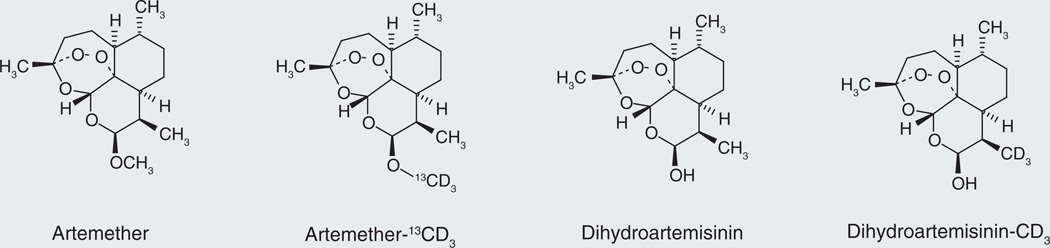
The structures of ARM, ARM-13CD3, DHA and DHA-d3 are shown in Figure 1. Stable isotope-labeled analytes are considered to be the best choice of IS for quantification methods using a mass spectrometer as the detector, because they are generally co-eluted and ionized similarly with the analytes. Signal variation for analytes (due to sample preparation, instrument and matrix effect) could be best compensated by this kind of IS. In general, 13C labeling is better than deuterium labeling in terms of co-elutability and ionization similarity with the corresponding analyte. In this assay we chose deuterated IS, solely based on availability. One disadvantage is that the deuterated ISs are not completely overlapped with the corresponding peaks of ARM and DHA (~0.2 min separation).
Figure 1. Artemether, dihydroartemisinin and their corresponding internal standards.
Sample preparation
Plasma samples (50 µl) were mixed with 50 µl IS solution (5 ng/ml ARM-13CD3 and DHA-d3 in 5% acetonitrile with 1% formic acid and 1% H2O2) in wells of Oasis HLB µElution plate (2 mg sorbent per well, 30 µm particle size, Waters, Inc., MA, USA). The mixtures were drained slowly under mild vacuum (2–5 inHg). The wells were washed with water 200 µl once and 5% acetonitrile 200 µl once subsequently under vacuum (5–8 inHg). The receiver plate was emptied at each step. The wells were eluted with acetonitrile-methyl acetate (9:1) 25 µl twice under mild vacuum (2–5 inHg). The combined eluent was injected (10 µl) for LC–MS/MS analysis.
LC–MS/MS analysis
AB Sciex API5000 (MA, USA) coupled with Shimadzu Prominence UFLC 20ADXR (Kyoto, Japan) pumps and a SIL-20ACXR AutoSampler (Shimadzu) was used. A LC–MS gas generator (Source 5000™, Parker Balston, MA, USA) was connected to the LC–MS/MS system. Separation was achieved on Acquity HSS C18 column (100 × 2.1 mm, 1.8 µm, Waters, Inc) eluted in a gradient mode with 10 mM ammonium formate at pH = 4.0 and acetonitrile (0.1% formic acid) at a flow rate of 0.3 ml/min. Acetonitrile % (time, min): 50 (0 min) → 50 (0.5 min) → 100 (6.5 min) → 100 (7.5 min) → 50 (7.6 min) → 50 (9.0 min). The mass spectrometer was set at ESI+ and SRM mode. The optimized MS parameters are listed in Tables 1 & 2.
Table 1.
MS source parameters for artemether and dihydroartemisinin.
| Source temperature (°C) |
Ionspray voltage (V) |
Curtain gas (psi) | Nebulizer gas (psi) | Auxiliary (turbo) gas (psi) |
Collision-activated dissociation (psi) |
|---|---|---|---|---|---|
| 300 | 5500 | 30 | 90 | 20 | 7 |
Table 2.
MS compound parameters for artemether and dihydroartemisinin.
| Compound parameters | Declustering potential (v) |
Entrance potential (v) |
Collision energy (v) |
Collision cell exit potential (v) |
|---|---|---|---|---|
| Artemether, 316/267† | 46 | 10 | 12 | 22 |
| Dihydroartemisinin, 302/163† | 31 | 10 | 21 | 18 |
Precursor/product ion pair.
The internal standards artemether-13CD3 (320/267†) and dihydroartemisinin-d3 (305/166†) have the same parameters as their corresponding analytes.
Results & discussion
Our laboratory previously developed a method to quantify ARM and DHA in human plasma based on a API2000 MS/MS [5]. In that method, 0.5 ml plasma sample was used and the LLOQ was 2 ng/ml. The method was applied to clinical studies on healthy subjects. We realized that a LLOQ below 1 ng/ml is better, in order to collect sufficient data for estimation of the elimination half-life. To support pediatric PK studies, a more sensitive method is needed. Initially we intended to transfer the method to the API5000 system with the following modifications:
-
▪
Plasma sample volume reduced to 50 µl;
-
▪
Micro-elution HLB plate (2 mg sorbent per well), suitable for small sample volume, used to replace HLB cartridge (10 mg sorbent) for SPE;
-
▪
Analytical column Acquity UPLC® HSS C18 (2.1 × 100 mm, 1.8 µm) used instead of Symmetry C18 (4.6 × 150 mm, 5 µm);
-
▪
IS utilized their corresponding stable isotope-labeled drugs instead of artemisinin.
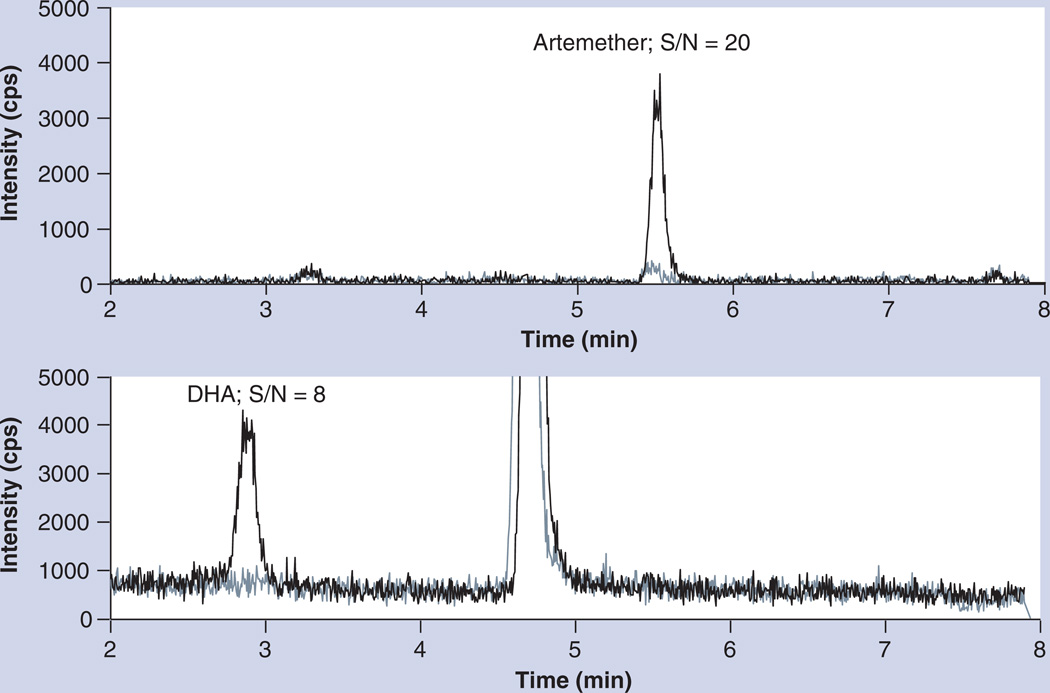
The LLOQ of the new method was 0.5 ng/ml for both ARM and DHA (S/N is 20 and 8, respectively), reduced by fourfold compared with our previous method, and plasma sample volume was reduced by tenfold. The representative chromatograms of blank plasma and LLOQ are shown in Figure 2. The method was validated (see Supplementary Data) according to the AIDS Clinical Trials Group network guidelines [10], which was based on US FDA guidelines.
Figure 2. Chromatograms of blank plasma (light line) and samples at LLOQ level (dark line).
DHA: Dihydroartemisinin.
However, when the method was applied to clinical samples from malaria patients, the IS signal diminished or even disappeared in some hemolyzed plasma samples as well as in some samples without obvious hemolysis; a phenomenon also observed by Lindegardh et al. and Keiser et al. Furthermore, we also observed that IS was not degraded in some hemolyzed samples (Supplementary Table 1). These results suggest it is not hemolysis itself but the specific hemolytic product that causes degradation of ARM and DHA. We believe that it is the Fe2+-containing hemolytic products that cause the degradation of artemisinin and its derivatives. Interestingly, these analytical issues have not been acknowledged by other groups. Wiesner et al. reported a liquid–liquid extraction method with ethyl acetate [6]. No degradation of ARM and DHA was observed when tested with 1–2% hemolyzed plasma samples, and the IS levels were consistent when the method was applied to clinical samples. Hodel et al. noticed approximately 20% degradation of artemisinin using 0.2% hemolyzed plasma samples and over 50% degradation in samples from some malaria patients, but evaporation and reconstitution steps reportedly helped to stabilize the drugs [7]. In reality, clinical samples could be contaminated by hemolytic products at various levels, and percentage degradation of artemisinins also depends on the concentration of the drugs. Since digestion of hemoglobin by malaria parasites releases Fe2+-containing products, in plasma samples from malaria patients, the hemolytic products that contain Fe2+ might also be varied in the context of different disease stages. In our previous method based on API2000, the degradation issue indicated by the IS artemisinin was not common; we only noticed a decrease of the IS signal in rare cases (ten out of 250 samples from healthy subjects with API2000 vs 61 out of 95 samples from patients with API5000) and the decrease of signal was typically no more than 50%. The most likely reason may be that this method has only been used for samples from healthy subjects, and the impact of the malaria parasite and heme generation through the pathologic process should not be present. It is also possible that the difference may be attributed to the instrument system used. API 2000 is much less sensitive, and a higher concentration of IS (100 ng/ml) was used in the assay. The degradation of ARM and DHA we have observed using our current API5000 method when analyzing these compounds in plasma from malaria-infected patients has confirmed the findings reported by Lindegardh’s group.
Based on the hypothesis that degradation of ARM and DHA during sample analysis is caused by Fe2+-containing products, we selected H2O2 to remove Fe2+. In acidic conditions, H2O2 converted Fe2+ to Fe3+ according to the following reaction:
| (Equation 1) |
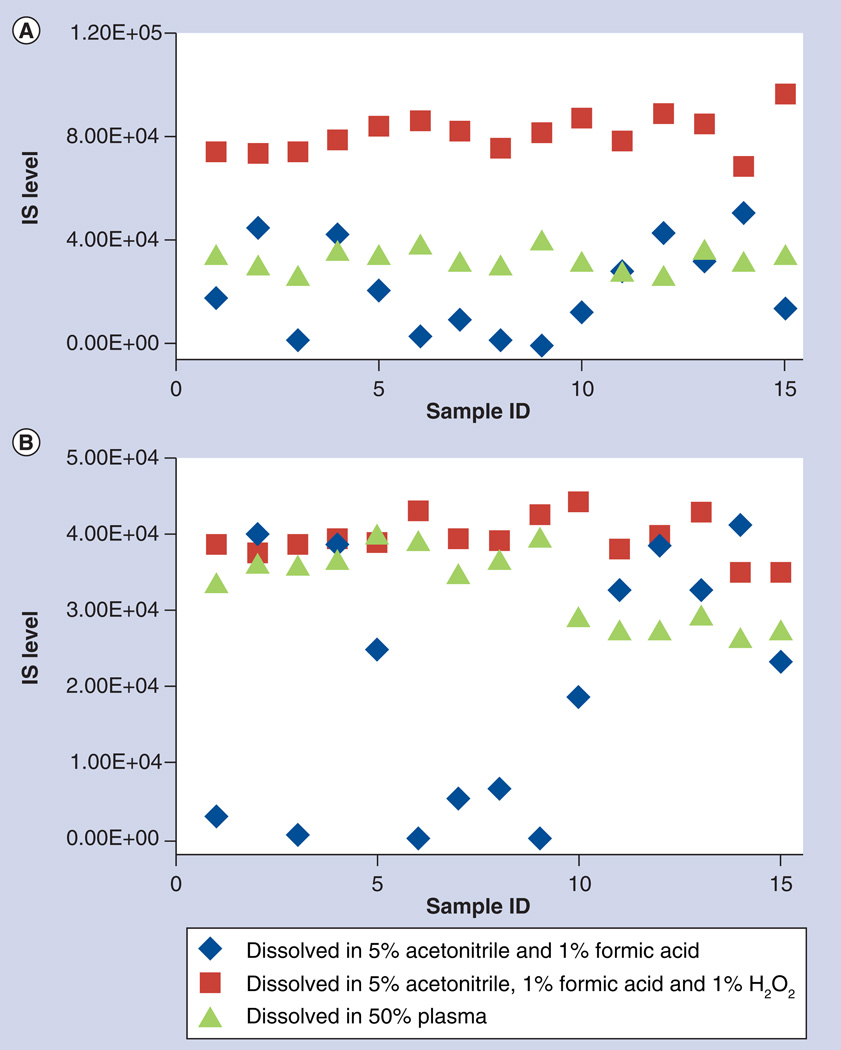
The IS (ARM-13CD3 and DHA-d3) was dissolved in 5% acetonitrile, 1% formic acid and 1% H2O2 (1:30 dilution from 30% H2O2). A subset of patient samples was reanalyzed with this new IS solution. The signals for the IS were now consistent and deviation from the mean IS signal is less than 25% (square markers in Figure 3), in contrast with the results with initial solution (diamonds in Figure 3). The signals for ARM and DHA in those problematic samples were also higher and could be quantified (Supplementary Table 1). The same samples were also analyzed with the IS dissolved in 50% plasma (plasma diluted with equal volume of water), a method used by Lindegardh’s group. Similar results were obtained (triangles in Figure 3); however, the IS signals were lower than that in our method, suggesting that partial degradation of artemisinin derivatives still occurred even with IS dissolved in 50% plasma (Figure 3). Nevertheless, this should not affect quantification because stable isotope-labeled drugs used as the IS would be degraded similarly to the drugs. The concentrations determined with the latter two IS solutions agree with each other with less than 20% difference for ARM in all 15 samples and less than 20% difference for DHA in 13 of 15 samples (Supplementary Table 1). Our method provided a better protection for the analysis of artemisinin derivatives in hemolyzed samples and samples from malaria patients. Interestingly, Keiser et al. used sodium nitrite to pre-treat the plasma samples from rat and achieved the same protection effect [8].
Figure 3. Comparison of signal intensity of internal standard for patient samples when added in three different solutions.
(A) Artemether-13CD3 and (B) dihydrosrtemisinin-d3.
IS: Internal standard.
Of note, the new IS solution is not stable. Over time, a residual DHA peak was formed from DHA-d3. The conversion was less than 20% of LLOQ in 6 h but more than 20% after overnight standing on a bench (29.7% after 22 h at 20°C). ARM-13CD3 was not converted to ARM and no significant degradation observed when standing at room temperature overnight. The IS solution was stable for at least 1 month when stored at −70°C. Therefore, the IS solution was stored at −70°C in small aliquots (1–2 ml) and used within 1 day if thawed.
Our second finding is that acidification of plasma increased the recovery of ARM significantly. Without acidification of the plasma samples, the recovery of ARM was lower than DHA (52.2–62.7% vs 83.1–98.1%; Table 3). This difference may be attributed to higher plasma protein binding of ARM (95–98%) compared with DHA (47–93%) [11,12]. Acidification of plasma increased the recovery of ARM, probably due to disruption of drug–protein interactions. However, this also exposed the drugs to Fe2+ in plasma of certain patients, resulting in drug and stable isotope-labeled IS degradation. By adding H2O2, Fe2+ was oxidized to Fe3+ and, thus, shielded the peroxide bridge of the artemisinin derivatives from Fe2+.
Table 3.
Recovery of artemether and dihydroartemisinin.
| Analyte | Concentration (ng/ml) |
Recovery (%) | |
|---|---|---|---|
| With 1% formic acid | Without acidification | ||
| ARM | 1.5 | 116 | 52.2 |
| 170 | 122 | 62.7 | |
| DHA | 1.5 | 100 | 83.1 |
| 170 | 103 | 98.1 | |
Recovery with 1% formic acid was obtained by mixing low and high QC samples in triplicate with internal standard solution containing 1% formic acid and then processing with HLB micro-elution plate. Recovery without acidification was obtained by mixing low and high QC samples in triplicate with internal standard solution that was not acidified.
ARM: Artemether; DHA: Dihydroartemisinin.
Compared with the assay reported by Lindegardh’s group, our assay has a lower LLOQ (0.5 ng/ml vs 1.43 ng/ml). This is mostly attributed to the smaller elution volume (50 µl vs 100 µl + 50 µl). As the S/N ratio for ARM was 20 at 0.5 ng/ml, its LLOQ could be lower. Higher sensitivity for ARM is attributed to acidification of plasma samples, leading to higher recovery. The sensitivity could also be improved if a better UPLC system was utilized (maximum system pressure ≥15,000 psi), enabling higher flow rate (0.6–0.8 ml/min).
In summary, ARM, DHA and their corresponding stable isotope-labeled IS – ARM-13CD3 and DHA-d3 – were found to be degraded in some plasma samples from malaria patients during sample analysis, and we found that H2O2 protected ARM, DHA, ARM-13CD3 and DHA-d3 from degradation. Acidification of plasma during sample preparation increased recovery of ARM significantly. The new H2O2 stabilization method, combined with a modified sample preparation method, enables us to develop a sensitive assay for ARM and DHA quantification, with a small sample volume (50 µl) and an LLOQ at 0.5 ng/ml.
Future perspective
It is important to stabilize artemisinin derivatives in clinical studies [13]. Our results demonstrated that ARM and DHA are degraded in certain patient samples and hemolyzed samples during analysis. What exactly causes the degradation? Is there degradation of artemisinins during blood sample collection and storage? How can the blood samples be stabilized? Further studies are needed to answer these questions. H2O2 is a promising stabilization agent for artemisinin derivatives. Although only ARM and DHA were tested in this work, it may be applied to other artemisinin derivatives. In this report only plasma samples were tested. Future study can be extended to blood samples.
Supplementary Material
Executive summary.
Method development
-
▪
Sample preparation utilized a micro-elution SPE HLB plate, suitable for small sample volume.
-
▪
Acidification of plasma prior to extraction increased sensitivity of artemether (ARM) significantly.
Stability issues in application
-
▪
Degradation of ARM and dihydroartemisinin was found in some plasma samples from malaria patients, including both hemolyzed and nonhemolyzed samples.
-
▪
Hydrogen peroxide is an efficient stabilization agent to protect ARM and dihydroartemisinin from degradation.
Acknowledgments
This work was supported by NIH (R01 HD068174).
Key Terms
- Micro-elution HLB plate
Kind of SPE plate designed for small sample volume and small elution volume.
- Hemolysis
Breakdown of red blood cells and release of hemoglobin into plasma.
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.future-science.com/doi/full/10.4155/BIO.13.91
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Peys E, Vandenkerckhove J, Van Hemel J, Sas B. Simultaneous determination of β-artemether and its metabolite dihydroartemisinin in human plasma and urine by a high-performance liquid chromatography-mass spectrometry assay using electrospray ionisation. Chromatographia. 2005;61:637–641. [Google Scholar]
- 2.Shi B, YU Y, Li Z, et al. Quantitative analysis of artemether and its metabolite dihydroartemisinin in human plasma by LC with tandem mass spectrometry. Chromatographia. 2006;64:523–530. [Google Scholar]
- 3.Souppart C, Gauducheau N, Sandrenan N, Richard F. Development and validation of a high-performance liquid chromatography–mass spectrometry assay for the determination of artemether and its metabolite dihydroartemisinin in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;774:195–203. doi: 10.1016/s1570-0232(02)00207-6. [DOI] [PubMed] [Google Scholar]
- 4.Hanpithakpong W, Kamanikom B, Singhasivanon P, White NJ, Day NPJ, Lindegardh N. A liquid chromatographic–tandem mass spectrometric method for determination of artemether and its metabolite dihydroartemisinin in human plasma. Bioanalysis. 2009;1:37–46. doi: 10.4155/bio.09.6. [DOI] [PubMed] [Google Scholar]
- 5.Huang L, Jayewardene AL, Li X, Marzan F, Lizak PS, Aweeka FT. Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method for determination of artemether and its active metabolite dihydroartemisinin in human plasma. J. Pharm. Biomed. Anal. 2009;50:959–965. doi: 10.1016/j.jpba.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wiesner L, Govender K, Meredith SA, Norman J, Smith PJ. A liquid-liquid LC/MS/MS assay for the determination of artemether and DHA in malaria patient samples. J. Pharm. Biomed. Anal. 2011;55:373–378. doi: 10.1016/j.jpba.2011.01.036. ▪ Reports no degradation of artemether and dihydroartemisinin.
- 7. Lindegardh N, Hanpithakpong W, Kamanikom B, et al. Major pitfalls in the measurement of artemisinin derivatives in plasma in clinical studies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;876:54–60. doi: 10.1016/j.jchromb.2008.10.021. ▪▪ Reports degradation of artemisinin derivatives in plasma from some malaria patients.
- 8. Keiser J, Gruyer MS, Perrottet N, Zanolari B, Mercier T, Decosterd LA. Pharmacokinetics parameters of artesunate and dihydroartemisinin in rats infected or not by Fasciola hepatica. J. Antimicrob. Chemother. 2009;63:543–549. doi: 10.1093/jac/dkn550. ▪ Reports some protection of artemether by sodium nitrite.
- 9. Hodel EM, Zanolari B, Mercier T, et al. A single LC tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009;877:867–886. doi: 10.1016/j.jchromb.2009.02.006. ▪ Reports controversial results that protein precipitation can withstand degradation if evaporation and reconstitution are applied.
- 10. AIDS Clinical Trials Group network guidelines for method development and validation based on (and including) FDA guidelines dated by 2001. Version. 2005;2 ▪ A revision was made in 2012 and posted in the Clinical Pharmacology Quality Assurance and Quality Control Program (available at: www.fstrf.org).
- 11.Colussi D, Parisot C, Legay F, Lefèvre G. Binding of artemether and lumefantrine to plasma proteins and Erythrocytes. Eur. J. Pharm. Sci. 1999;9:9–16. doi: 10.1016/s0928-0987(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 12.Batty KT, Ilett KF, Davis TM. Protein binding and α:β anomer ratio of dihydroartemisinin in vivo. Br. J. Clin. Pharmacol. 2004;57:529–533. doi: 10.1046/j.1365-2125.2003.02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blessborn D, Skölld K, Zeeberg D, Kaewkhao K, Sköld O Ahnoff M. Heat stabilization of blood spot samples for determination of metabolically unstable drug compounds. Bioanalysis. 2013;5(1):31–39. doi: 10.4155/bio.12.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.