Abstract
Elevations in matrix metalloproteinase 1 (MMP-1) and MMP-3 have been found in patients with Lyme arthritis and in in vitro models of Lyme arthritis using cartilage explants and chondrocytes. The pathways by which B. burgdorferi, the causative agent of Lyme disease, induces the production of MMP-1 and MMP-3 have not been elucidated. We examined the role of the extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK) and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways in MMP induction by B. burgdorferi. Infection with B. burgdorferi results in rapid phosphorylation of p38 and JNK within 15 to 30 min. Inhibition of JNK and p38 MAPK significantly reduced B. burgdorferi-induced MMP-1 and MMP-3 expression. Inhibition of ERK1/2 completely inhibited the expression of MMP-3 in human chondrocytes following B. burgdorferi infection but had little effect on the expression of MMP-1. B. burgdorferi infection also induced phosphorylation and nuclear translocation of STAT-3 and STAT-6 in primary human chondrocytes. Expression of MMP-1 and MMP-3 was significantly inhibited by inhibition of JAK3 activity. Induction of MMP-1 and -3 following MAPK and JAK/STAT activation was cycloheximide sensitive, suggesting synthesis of intermediary proteins is required. Inhibition of tumor necrosis factor alpha (TNF-α) significantly reduced MMP-1 but not MMP-3 expression from B. burgdorferi-infected cells; inhibition of interleukin-1β (IL-1β) had no effect. Treatment of B. burgdorferi-infected cells with JAK and MAPK inhibitors significantly inhibited TNF-α induction, consistent with at least a partial role for TNF-α in B. burgdorferi-induced MMP-1 expression in chondrocytes.
Oligoarticular arthritis is a prominent feature of late stage Lyme disease caused by infection with the spirochete Borrelia burgdorferi sensu stricto (26). In the absence of antibiotic therapy, infection with B. burgdorferi frequently progresses to an intermittent or chronic arthritis primarily affecting the knees and large joints. Over time, Lyme arthritis evolves into an erosive arthritis that is histologically similar to rheumatoid arthritis (27). The clinical progression of Lyme arthritis appears to be relatively unique among common bacterial causes of septic arthritis. While septic arthritis caused by bacteria such as Staphylococcus aureus and group A streptococci can progress very rapidly (hours to days) to cause permanent joint destruction, Lyme arthritis typically progresses very slowly (months to years) despite the longstanding presence of the organism in the joint. This difference appears to be due to differences in the mechanisms of cartilage degradation. It has been previously shown that B. burgdorferi, unlike most other bacteria that cause septic arthritis, does not produce collagenases or other enzymes capable of destroying joint extracellular matrix (5, 18). Instead, we have shown that cartilage degradation in Lyme arthritis is due to induction of a class of host enzymes called matrix metalloproteinases (MMPs) (13, 20). All MMPs share a common function of extracellular matrix degradation (8). In normal tissues, they are thought to play a major role in tissue growth and remodeling. They have also been shown to play a role in disease states such as periodontal disease, cancer metastases and rheumatoid arthritis and osteoarthritis. Two MMPs in particular, collagenase-1 (MMP-1) and stromelysin-1 (MMP-3), were found to be elevated in the synovial fluid of patients with Lyme arthritis (20). In vitro models of Lyme arthritis confirmed the ability of B. burgdorferi to induce these MMPs from human chondrocytes (HCs) and the ability of MMP inhibitors to block B. burgdorferi-induced cartilage degradation (13).
Many different signaling pathways have been shown to be involved in MMP induction in different in vitro and animal models of arthritis. The signaling pathways by which MMPs are induced vary depending upon the cell type and the stimulus. For example, while c-Jun N-terminal kinase (JNK) appears to play the major role in MMP induction from rheumatoid arthritis synoviocytes (12), MMP-1 and MMP-3 gene expression in endothelial cells is dependent on p38 mitogen-activated protein kinase (MAPK) (22). The activation of differing signaling pathways by different stimuli is likely to determine the specific patterns of MMP induction and the phenotype of MMP-dependent diseases. The signaling pathways involved in B. burgdorferi-stimulated MMP induction have not been previously examined. Here, we report the involvement of MAPKs (JNK, p38 MAPK, and extracellular signal-regulated kinase 1/2 [ERK1/2]) and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways in B. burgdorferi-induced MMP expression from primary HCs.
MATERIALS AND METHODS
Reagents.
All reagents were purchased from Sigma Co. (St. Louis, Mo.) unless otherwise stated. The MAPK and JAK/STAT pathway inhibitors SP600125, SB203580, U0126, and JAK3I were purchased from Calbiochem (San Diego, Calif.).
Primary cultures of HCs and infection with B. burgdorferi.
Primary HCs from a healthy donor were purchased from Cell Applications (San Diego, Calif.) and maintained in chondrocyte growth medium (Cell Applications) containing 10% fetal calf serum (FCS) at 37°C with 5% CO2. One day prior to infection HCs were washed and the cultured medium was replaced with chondrocyte medium without FCS. A clonal isolate of low passage (passages 4 to 7) B. burgdorferi sensu stricto strain N40 was cultured in Barbour-Stoenner-Kelly (BSKH) medium (1, 14). Spirochetes were washed three times and resuspended in chondrocyte medium without FCS. Cell cultures at 70 to 85% confluence were infected with B. burgdorferi at a cell/spirochete ratio of 1:10 for various time periods. For inhibitor experiments, various inhibitors of MAPK and JAK/STAT pathways were added to the cells in fresh serum-free medium 2 h prior to infection with B. burgdorferi and harvested at 18 h postinfection. The concentrations (20 μM SP600125, 3 μM SB203580, 10 μM U0126, and JAK3I [30 μg/ml]) of the inhibitors used were in conformity with earlier published reports and had no visible cytotoxic effect on the HC as judged by trypan blue exclusion. Cells were washed and harvested in cold phosphate-buffered saline (PBS), and the cell pellets were stored at −70°C until use.
MMP assay.
MMP-1 and MMP-3 were detected from cell culture extracts in culture medium using enzyme-linked immunosorbent assay (ELISA) kits from Oncogene Research (Boston, Mass.) used as per the manufacturer's instructions.
MAPK assay.
After incubation with B. burgdorferi, total cellular protein extracts were prepared from chondrocyte cultures. Cell culture plates were transferred to ice, and then washed twice with cold PBS. Cells were mechanically dislodged into cold PBS to which leupeptin (10 μg/ml), 1 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol were added. Cells were pelleted by centrifugation at 4°C, the supernatant was removed, and pelleted cells were resuspended in cell lysis buffer (25 mM HEPES [pH 7.5], 300 mM NaCl, 1.5 mM MgCl2, 2 mM EDTA pH 8.0, 0.05% Triton X-100, 0.1 mM Na3VO4, 20 mM β-glycerol phosphate, leupeptin [10 μg/ml], 1 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol). The resuspended cells were gently rocked at 4°C for 30 min; cellular debris was removed by centrifugation at 4°C. The cell extracts were collected and frozen at −80°C. The concentrations of the cell extracts were determined using the Bradford method (Bio-Rad, Hercules, Calif.). Two hundred micrograms of total protein was used for immunoprecipitation using phospho-specific antibodies to ERK1/2, p38 MAPK, and JNK, and the kinase assays were performed using specific kits from Cell Signaling (Beverly, Mass.) as per the manufacturer's instructions.
Immunoblot analysis.
Cells were harvested in cell lysis buffer as described above. Equal amounts of protein were separated by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. Immunoblotting was performed with polyclonal antibodies to phospho-STAT-3 and phospho-STAT-6 (Cell Signaling) and STAT-3 and STAT-6 (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.).
Quantitative reverse transcriptase PCR.
After incubation with B. burgdorferi, chondrocytes were washed with phosphate buffered saline and lysed in the presence of Trizol (Invitrogen, Carlsbad, Calif.). Total RNA was purified as per the manufacturer's instructions. First-strand synthesis of cDNA from total RNA was performed using a Superscript kit (Invitrogen) as per the manufacturer's instructions. Quantitation of cDNA from specific mRNA transcripts was accomplished by real-time, quantitative PCR (ABI7700; Applied Biosystems, Foster City, Calif.) using Sybr-green technology (Sybr Green Master Mix; Applied Biosystems). Cycling parameters were 50°C for 5 min and 95°C for 15 min, followed by 40 cycles of 95°C for 30 s and 55°C for 1 min. The following primers were used for PCR amplification: MMP-1 forward (5′-CTGAAGGTGATGAAGCAGCC-3′) and reverse (5′-AGTCCAAGAGAATGGCCGAG-3′), MMP-3 forward (5′-TGTAGAAGGCACAATATGGGCAC-3′) and reverse (5′-CAGTCACTTGTCTGTTGCACACG-3′), IL-1β forward (5′-ATGGCAGAAGTACCTGAGCTCGC-3′) and reverse (5′-CCATGGCCACAACAACTGACG-3′), TNF-α forward (5′-AACGGAGGCTGAACAATAGGC-3′) and reverse (5′-AGCAACCTTTATTTCTCGCCAC-3′), and β-actin forward (5′-CCACACCTTCTACAATGAGCTGCG-3′) and reverse (5′-CGGAGTCCATCACGATGCCA-3′. The specificity of each reaction was checked by melt curve analysis and electrophoresis of PCR products on agarose gels. Expression of MMP-1 and MMP-3 was normalized to that of β-actin. Calculations of expression were normalized using the ΔΔCt method where the amount of target, normalized to an endogenous reference and relative to a calibrator, is given by 2−ΔΔCt, where Ct is the cycle number of the detection threshold.
Immunocytochemistry.
B. burgdorferi-infected or uninfected cells were fixed in chilled acetone for 10 min and air dried. Cells were blocked with isotype matched serum and stained overnight at 4°C with rabbit anti-phospho-STAT-3 and -6 polyclonal antibodies (Cell Signaling). The unbound antibodies were removed by washing three times in PBS-Tween 20 (0.1%) buffer, pH 7.4 (PBST), and further incubated with secondary antibody goat anti-rabbit immunoglobulin G-fluorescein isothiocyanate (FITC) conjugate (Southern Biotech, Birmingham, Ala.). The slides were washed again three times with PBST, air-dried and mounted with VectaShield mounting medium containing the nuclear stain DAPI (Vector Laboratories, Burlingame, Calif.) and observed under fluorescent microscope.
Statistical analysis.
Each experiment was repeated three to five times as indicated. Statistical significance was analyzed using the nonparametric Mann-Whitney U test. Differences were considered statistically significant when the P value was less than 0.05.
RESULTS
B. burgdorferi induces expression of MMP-1 and MMP-3 in primary HCs.
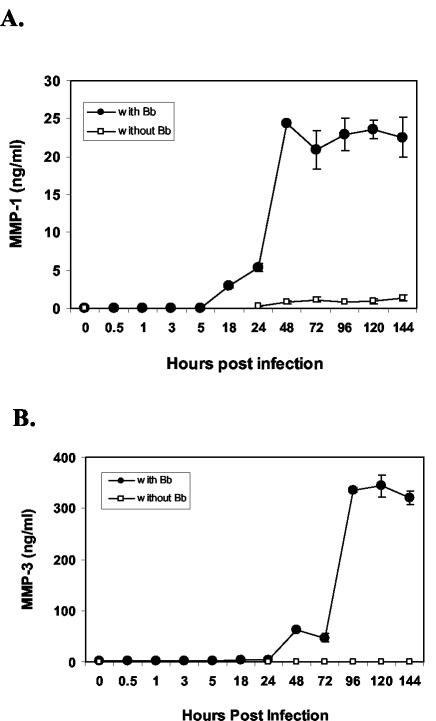
To examine the expression pattern of MMP-1 and MMP-3 in HCs following B. burgdorferi infection, primary HCs in serum-free medium were infected with B. burgdorferi and harvested at various time points. Levels of MMP-1 and MMP-3 expression in cellular lysates were measured by ELISA. Levels of both MMP-1 and MMP-3 were detectable by 18 h postinfection (hpi) (Fig. 1) and peaked on day 2 and day 4, respectively.
FIG. 1.
Expression of MMP-1 and MMP-3 in B. burgdorferi (Bb)-infected human primary chondrocytes. Primary HC cultures were either uninfected or infected with B. burgdorferi (107 organisms) for various lengths of time. Expression of MMP-1 (A) and MMP-3 (B) was measured by ELISA. The experiment was repeated twice in duplicate and a representative experiment is shown. Error bars represent standard deviations.
B. burgdorferi induces activation of p38 MAPK and JNK pathways in chondrocytes in a time-dependent manner.
In order to examine the effect of B. burgdorferi on the activation of MAPKs in primary HCs, cells were infected with B. burgdorferi and harvested at different time points.
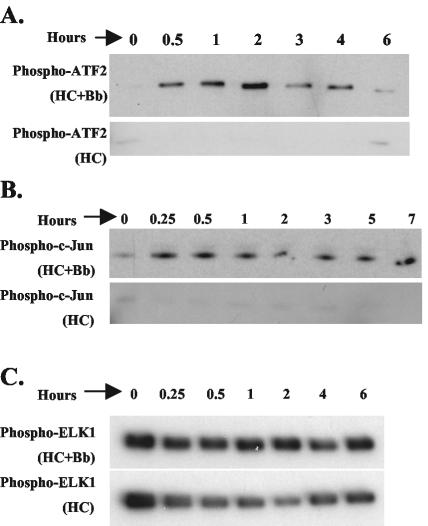
Activity of p38 MAPK was examined by immunoprecipitating the active p38 from cell lysate using phopho-p38 specific antibody. The immunoprecipitate was then incubated with its substrate, ATF-2 in the presence of ATP, followed by immunoblotting using antibody specific to phospho-ATF-2 (Fig. 2A). p38 activation in response to B. burgdorferi infection began increasing at 30 min following B. burgdorferi infection, peaked at 2 h and decreased thereafter.
FIG. 2.
Activation of ERK1/2, p38 MAPK, and JNK in human primary chondrocytes following B. burgdorferi infection. Primary HC cultures were infected with B. burgdorferi (Bb) for various lengths of time. Uninfected HCs harvested at the same time points were used as controls (lower panels). All cellular lysates were normalized to a total of 200 μg of protein prior to performing immunoprecipitation. (A) Activity of p38 MAPK was measured by immunoprecipitation of phospho-p38 MAPK, determining its activity by its ability to phosphorylate its substrate ATF-2. (B) Activity of JNK MAPK was measured by immunoprecipitating phospho-JNK, determining its activity by its ability to phosphorylate its substrate c-Jun. (C) The activity of ERK1/2 was measured by immunoprecipitation of phospho-ERK1/2, determining its kinase activity by phosphorylation of its substrate ELK-1. The experiments were repeated three to five times and representative experiments are shown.
Similarly, activity of JNK was examined by immunoprecipitating active JNK from cell lysates using immobilized c-Jun protein as bait and incubating the immunoprecipitate in the presence of ATP followed by immunoblotting using phospho-specific c-Jun antibody (Fig. 2B). JNK activation starts increasing at 15 min following B. burgdorferi infection and remains high through 7 h postinfection.
We also examined the effect of B. burgdorferi infection on ERK1/2 activity. Immobilized anti-phospho-ERK1/2 antibody was used to precipitate the protein from the cellular lysates. Activity was determined by phosphorylation of the ERK1/2 substrate ELK-1 in the presence of ATP followed by immunoblotting with phospho-ELK-1 antibody (Fig. 2C). Unlike p38 and JNK, no differences in ERK1/2 activity were seen in infected versus uninfected HCs.
Role of p38 MAPK, ERK1/2, and JNK on B. burgdorferi-induced MMP-1 and MMP-3 expression in primary HCs.
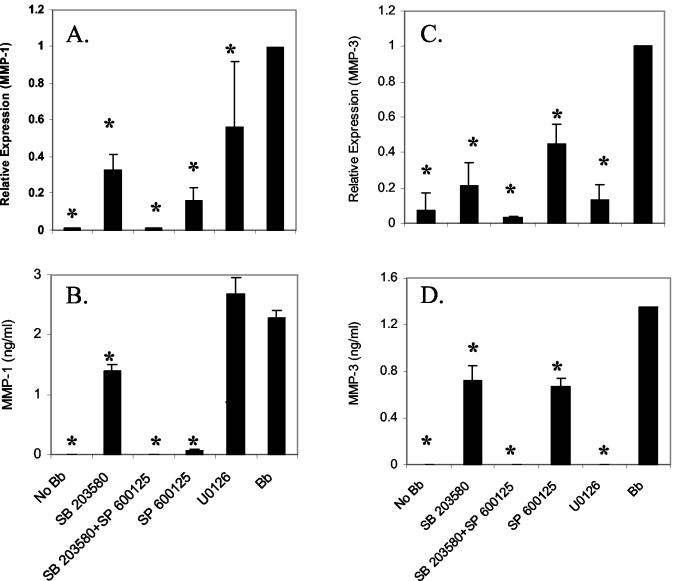
To confirm the role of p38 MAPK, ERK1/2 and JNK on MMP-1 and MMP-3 induction by B. burgdorferi, we examined expression of MMP-1 and MMP-3 in the presence of specific inhibitors for each pathway. Concentrations of inhibitors were selected based on prior studies of these inhibitors with chondrocytes (9, 15, 16, 21, 25, 28, 29, 31, 33, 34). Cells were infected in the presence of the p38-specific inhibitor SB203580, the JNK inhibitor SP600125, and the ERK kinase-specific inhibitor U0126. Cells were harvested at 18 hpi to assess the effect on the earliest time point when the expression of MMP-1 and MMP-3 are detectable. We examined MMP-1 and MMP-3 gene transcription in the presence or absence of inhibitors using real-time reverse transcriptase PCR (RT-PCR) (Fig. 3A and C). To ensure that RT-PCR data accurately reflected actual protein expression, all experiments were subsequently confirmed by ELISA. Results with ELISA were similar to results seen with RT-PCR (Fig. 3B and D). By ELISA, inhibition of JNK with SP600125 reduced B. burgdorferi-induced MMP-1 expression by 97% (Fig. 3B). The p38 inhibitor SB230580 inhibited MMP-1 protein expression by 67 and 39% at mRNA and protein levels, respectively. The combination of SP600125 and SB230580 completely inhibited B. burgdorferi-induced MMP-1 with levels equivalent to those of uninfected control chondrocytes. In contrast, the ERK pathway inhibitor U0126 showed only slight inhibition by RT-PCR (44%) and no inhibition by ELISA.
FIG. 3.
Role of p38 MAPK, ERK1/2, and JNK on B. burgdorferi-induced MMP-1 and MMP-3 expression in primary HCs. Primary HC cultures were treated either with p38 MAPK inhibitor (SB203580); JNK inhibitor (SP600125); or ERK1/2 inhibitor (U0126) for 2 h and subsequently infected with B. burgdorferi. Cells were harvested at 18 hpi and MMP-1 and -3 expression was examined by real-time RT-PCR (A and C) and by ELISA (B and D). The real-time RT-PCR experiments were repeated three to five times and the ELISAs were repeated twice in duplicate. Error bars represent standard deviations. *, P < 0.05.
The contribution of the JNK MAPK pathway to MMP-3 induction by B. burgdorferi appears to be less than that seen for MMP-1. SP600125 inhibited 50% of B. burgdorferi-induced MMP-3 expression (Fig. 3D). Inhibition of p38 with SB230580 produced similar decreases in MMP-3 expression as with MMP-1, and the combination of SP600125 and SB230580 again completely inhibited B. burgdorferi-induced expression of this MMP (Fig. 3D). However, compared with the minimal contribution of the ERK pathway to MMP-1 induction by B. burgdorferi, MMP-3 induction was completely inhibited by U0126 with levels similar to those for uninfected control cells (Fig. 3D).
B. burgdorferi induces activation of STAT-3 and STAT-6 in primary HCs in a time dependent manner.
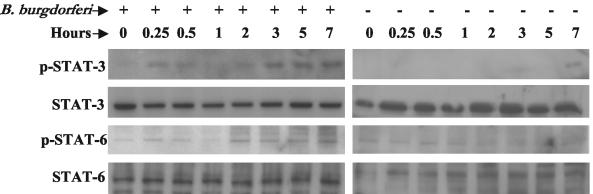
To examine the activation of STATs following B. burgdorferi infection in HCs, cells were again infected with B. burgdorferi and harvested at different time points. Expression of phospho-STAT-1, phospho-STAT-3 and phospho-STAT-6 was examined by immunoblotting using phospho-specific antibody to each of the STATs. Low-level, constitutive expression of phospho-STAT-3 and phospho-STAT-6 was observed in noninfected HCs; however, the expression of each of these increased by 2 hpi and remained high through 7 hpi (Fig. 4). Expression of phospho-STAT-1 could not be detected at any time point (data not shown).
FIG. 4.
Activation of STAT molecules in primary HCs following B. burgdorferi infection. Primary HC cultures were either uninfected or infected with B. burgdorferi for various amounts of time and whole cell protein extracts were analyzed by immunoblotting for the expression of phospho-STAT-3 and phospho-STAT-6 using specific antibodies. Immunoblots for nonphosphorylated STAT-3 and STAT-6 are shown as internal controls. Experiments were performed three times and representative experiments are shown.
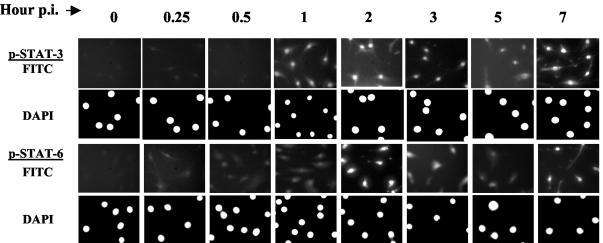
Phosphorylated STATs dimerize and translocate to the nucleus, where they bind to the specific STAT binding element (SBE) present in the promoter of various STAT regulated genes, and thus regulate their expression. To identify nuclear translocation of phospho-STAT molecules, we employed immunofluorescence techniques using individual phospho-specific STAT antibodies. Results demonstrated that phospho-STAT-3 and phospho-STAT-6 were translocated in a time-dependent manner following B. burgdorferi infection, which coincided with the immunoblot results (Fig. 5). Electrophoretic mobility shift assays further confirmed increased DNA binding to both STAT-3 and STAT-6 in the nuclear extracts from B. burgdorferi-infected cells when compared to that from uninfected cells (data not shown).
FIG. 5.
Nuclear translocation of phospho-STAT molecules in HC following B. burgdorferi infection. Primary HC were infected with B. burgdorferi for various periods of time. Nuclear translocation of STAT-3 and STAT-6 was examined by immunofluorescence using phospho-specific antibodies to individual STAT proteins and subsequently probed with FITC-labeled secondary antibody. The nucleus was counter stained with DAPI and photographed under fluorescence microscopy. The upper and lower panels show FITC and DAPI stain, respectively, for each of the STAT proteins. Experiments were performed two to three times and representative experiments are shown.
Role of JAK/STAT pathway on B. burgdorferi-induced MMP-1 and MMP-3 expression in primary HCs.
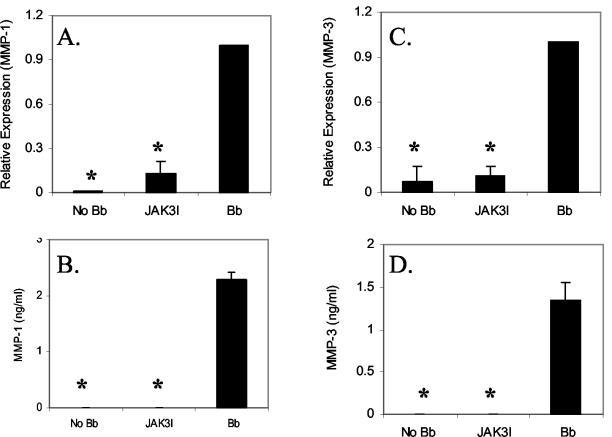
The contribution of JAK/STAT signaling cascades on B. burgdorferi-induced MMP-1 and MMP-3 expression was examined using a specific inhibitor of JAK3 (JAK3I). The concentration of JAK3I was based on previous studies with chondrocytes (19). Similar to our MAPK studies, HCs were infected with B. burgdorferi for 18 h in the presence or absence of the inhibitors. Levels of MMP-1 and MMP-3 gene expression were again measured first by RT-PCR and then confirmed using ELISA. JAK3I showed almost 100% inhibition of both MMP-1 and MMP-3 mRNA induction (Fig. 6A and C). Similar results were obtained for protein expression level by ELISA (Fig. 6B and D).
FIG. 6.
Role of JAK/STAT pathway on B. burgdorferi-induced MMP-1 and MMP-3 expression in primary HCs. HCs were treated with JAK3 inhibitor (JAK3I) for 2 h followed by infection with B. burgdorferi. Cells were harvested at 18 hpi and the expression of MMP-1 and -3 was examined by real-time PCR (A and C) and ELISA (B and D). The real-time RT-PCR experiments were repeated three times and the ELISAs were repeated twice. Bar represents standard deviation. *, P < 0.05.
Mechanism of activation of MMP-1 and MMP-3 following MAPK and STAT activation.
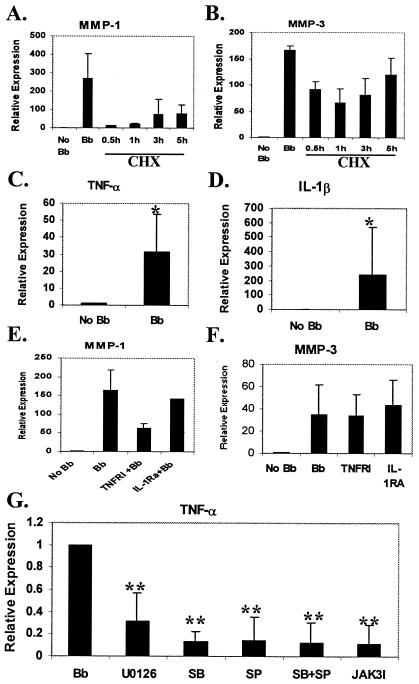
Due to the significant temporal lag between activation of MAPKs and JAK/STAT pathways and MMP-1 and -3 expression following B. burgdorferi infection, we examined the potential for involvement of intermediary molecules by treating the cells with protein synthesis inhibitor, cycloheximide (CHX) at 10 μg/ml. CHX at this concentration was shown previously to inhibit protein synthesis in HCs (3). Cells were treated with CHX after 30 min to 5 h post-B. burgdorferi infection. These time-points cover the times at which MAPK and JAK/STAT pathways are fully activated. Expression of MMP-1 and -3 was examined by real-time RT-PCR at 24 h postinfection. There was reduction in MMP-1 and MMP-3 transcription in CHX-treated cells at all time points (Fig. 7A and B), suggesting that synthesis of an intermediary protein is required for induction of MMP transcription. The largest inhibition was seen at 30 min and 1 h with gradually decreasing inhibition over time, suggesting that the intermediary molecules are beginning to be formed during this time span. The inhibition of MMP-1 expression following CHX treatment was greater than that for MMP-3 at all time points.
FIG. 7.
Mechanism of activation of MMP-1 and MMP-3 following MAPK and STAT activation. HC were treated with CHX (10 μg/ml) at different time point as indicated following B. burgdorferi infection. Cells were harvested at 24 hpi and real-time RT-PCR was performed for MMP-1 (A) and MMP-3 (B). (C and D) Real-time RT-PCR analysis of TNF-α (C) and IL-1β (D) expression in HC following 24 h of B. burgdorferi infection. (E and F) Effect of inhibition of TNF-α and IL-1β expression by TNFRI and IL-1RA on MMP-1 and MMP-3 expression. HC were treated with TNFRI (2 μg/ml) and of IL-1RA (1 μg/ml) for 2 h and subsequently infected with B. burgdorferi. Cells were harvested at 24 hpi and real-time RT-PCR was done examine the expression of MMP-1 (E) and MMP-3 (F). (G) Effect of inhibitors of MAPKs and JAK/STAT pathways on TNF-α expression. HC were treated either with p38 MAPK inhibitor (SB) SB203580; JNK inhibitor (SP) SP600125; ERK1/2 inhibitors (U0126), or JAK3 inhibitor (JAK3I) for 2 h and subsequently infected with B. burgdorferi. Cells were harvested at 18 hpi, and the expression of MMP-1 and -3 was examined by real-time PCR. All the experiments were repeated two to four times in duplicate. Bar represents standard errors of the means. Symbols: *, P < 0.05; **, P < 0.01.
The cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) have been implicated in the activation of MMPs in rheumatoid arthritis and are known to be induced from multiple cell types by the presence of B. burgdorferi. Expression of both TNF-α and IL-1β was significantly increased in HCs following B. burgdorferi infection (Fig. 7C and D). Inhibition of TNF-α expression following B. burgdorferi infection by soluble TNF receptor I (TNFRI) reduced MMP-1 expression by 61% (Fig. 7E), but had no effect on MMP-3 expression (Fig. 7F). However, inhibition of IL-1β by IL-1 receptor antagonist (IL-1RA) had no significant effect on either MMP-1 or -3 expression (14% reduction) (Fig. 7F). In order to determine whether the effects of the MAPK and JAK/STAT inhibitors could be due in part to effects on TNF-α induction, we measured TNF-α expression in the presence of specific inhibitors of each pathway. Significant reduction (P < 0.01) in TNF-α expression was observed following inhibition with each of the individual MAPK and JAK/STAT inhibitors (Fig. 7G). No significant reduction in IL-1β expression was observed in inhibitor-treated cells (data not shown).
DISCUSSION
As a family, the MMPs are capable of digesting all of the important structural components of extracellular matrix (8). Accordingly, in healthy states, MMP expression and activation are tightly regulated at multiple different levels. Elevations of MMPs have been observed in many pathological diseases including osteoarthritis and rheumatoid arthritis. However, studies of the role of MMPs in arthritis have been hampered by the lack of realistic in vitro and animal models for many of the diseases. Lyme arthritis, with its clearly identified initiating agent, serves as an excellent model for furthering the understanding of the mechanisms involved in MMP dysregulation leading to joint destruction and clinical arthritis. While the role of cytokines in the pathogenesis of Lyme arthritis has been well studied, the signal transduction pathways that determine matrix degradation are only partially understood.
The promoter regions of both MMP-1 and MMP-3 contain various transcription factor binding sites including several proximal AP-1 sites that bind members of the Fos and Jun family of transcription factors, STAT binding sites, GATA binding factors, and heat shock factors. AP-1 is regulated by MAPKs and is a pivotal transcription factor that regulates cytokine production and expression of MMPs (32), whereas STAT proteins are involved in mediating cytokine-induced intracellular signals leading to expression of inflammatory molecules (17). The relative importance of AP-1 and STAT proteins in MMP expression has varied depending upon cell type, stimulus and MMP being studied.
In this study, we have demonstrated that the MAPK and JAK/STAT pathways are activated rapidly by the presence of B. burgdorferi. Using pathway-specific inhibitors, we have defined the roles of the different signaling pathways in B. burgdorferi-induced HC MMP-1 and MMP-3 expression. There are three major groups of MAPKs in mammalian cells—the extracellular signal regulated protein kinases (ERK1/2) (23), the p38 MAPKs (11), and the c-Jun NH2 terminal kinases (JNK) (4, 6). ERKs are activated by mitogens and growth factors via Ras dependent pathway, while the JNK and p38 MAPKs are activated in response to pro-inflammatory cytokines such as TNF-α and IL-1 and by cellular stress. Separate inhibition of JNK and p38 MAPKs by their specific inhibitors significantly reduced the expression of MMP-1 and -3, both at mRNA and protein levels following B. burgdorferi infection. However, dual inhibition of both p38 MAPK and JNK resulted in enhanced inhibition, completely blocking expression of both MMP-1 and MMP-3. This indicates that JNK and p38 MAPKs may have separate contributions in the induction of MMP-1 and -3 following B. burgdorferi infection.
ERK1/2 inhibition had minimal effect on MMP-1 induction by B. burgdorferi. However, inhibition of ERK1/2 substantially reduced the expression of MMP-3 despite the fact that no change in ERK1/2 activity was seen in the presence of B. burgdorferi by our kinase assays. There are several possible explanations for this. First, there was high constitutive phospho-ERK1/2 expression in our cells prior to the addition of B. burgdorferi. If the activation of ERK1/2 plays a necessary but not sufficient role in MMP-3 induction (i.e., activation of another factor is required in addition to ERK1/2), then the presence of the constitutively activated ERK1/2 may be adequate for MMP-3 induction. Another possibility is that due to the high sensitivity of the kinase assay used and the background constitutive activity, small inductions of ERK1/2 activation may have been missed. In fact, in Western blots of cell lysates using a phospho-ERK1/2 antibody, small increases in phospho-ERK1/2 were seen at 30 min (data not shown). Regardless of the mechanism, the differential effects of ERK inhibition on MMP-1 and MMP-3 demonstrate that there are separate pathways involved in the B. burgdorferi-mediated induction of these two proteases.
STAT proteins are critical in mediating intracellular effects of various cytokines and are implicated in the expression of many inflammatory effector genes. Active STAT-3 has been detected in cells from inflamed joints (30), and synovial fluids from rheumatoid arthritis patients can activate STAT-3 but not STAT-1 in monocytes (24). Similarly, we found that B. burgdorferi infection resulted in activation of STAT-3 and STAT-6, but not STAT-1. Inhibition of B. burgdorferi-induced MMP-1 and MMP-3 expression by JAK3I suggests that JAK3 is important in mediating STAT activation in B. burgdorferi-induced chondrocytes.
The inhibition of B. burgdorferi-induced MMP-1 and MMP-3 production by either MAPK or JAK/STAT inhibitors suggests that activation of both pathways is critical in this process. The most-direct mechanism to account for this finding would be that binding of transcription factors to both AP-1 and STAT binding sites is required for maximal induction of MMP-1 and MMP-3. However, B. burgdorferi-induced expression of MMP-1 and MMP-3 lags behind MAPK and JAK/STAT activation. The temporal difference between activation of the signaling pathways and detectable production of MMPs suggests that there may be an intermediary step (i.e., induction of cytokines, growth factors, etc.) that occurs between the two processes. Treatment of cells with the protein synthesis inhibitor CHX following B. burgdorferi-induced activation of MAPKs and JAK/STAT pathways confirms that new protein synthesis is required for the induction of MMP-1 and -3 transcription, suggesting the role of intermediary molecules. The effect of CHX appeared to be greater on MMP-1 expression than MMP-3 expression.
B. burgdorferi has previously been shown to induce the expression of multiple cytokines which are known to induce certain matrix metalloproteinases, including but not limited to TNF-α, IL-1β, IL-2, and interferon-γ (2, 7, 10). Each of these has also been previously shown to play a role in induction of MMPs in other systems. While B. burgdorferi infection of HC results in induction of both TNF-α and IL-1β, it appears that only TNF-α, and not IL-1β, is involved in the subsequent induction of MMP-1. ERK, p38, JNK and JAK inhibitors all showed a significant effect on TNF-α induction but not on IL-1β induction. However, inhibition of TNF-α decreased MMP-1 induction by only 61%, which is less than the effect seen with many of the MAPK and JAK inhibitors, suggesting that other processes are likely to be involved. For MMP-3, inhibition of TNF-α did not affect its induction, suggesting that the effects of the pathway inhibitors acted through other mechanisms. It remains to be determined whether the effects of the individual inhibitors on MMP expression are due to their effects on other intermediary molecules or through direct activation by transcription factors.
In summary, our results demonstrate that B. burgdorferi-induced expression of MMP-1 and MMP-3 in HCs is regulated by both MAPK and JAK/STAT signaling pathways. The effect of these signaling pathways on MMP-1 expression is at least partially through control of TNF-α induction. A better understanding of the interactions between the receptors, signaling pathways, and transcription factors involved in MMP induction by B. burgdorferi may lead to further insights into the role of these enzymes in the shaping the phenotypic course of different arthritides and the eventual development of more specific inhibitors to alter the clinical course of arthritis.
Acknowledgments
We thank Douglas Golenbock for invaluable discussions. We also thank Jenifer Coburn, Melisa Medrano, and Carla Cugini for their technical expertise and advice with real-time PCR.
This work was supported by NIAID R01 AI44240 (L.T.H.), R01 AI50043 (L.T.H), U01 AI058266 (L.T.H.), and K08 AI-01715 (C.M.T.).
Editor: J. T. Barbieri
REFERENCES
- 1.Barbour, A. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57:521-525. [PMC free article] [PubMed] [Google Scholar]
- 2.Beck, G., J. L. Benach, and G. S. Habicht. 1989. Isolation of interleukin 1 from joint fluids of patients with Lyme disease. J. Rheumatol. 16:800-806. [PubMed] [Google Scholar]
- 3.Borzì, R. M., I. Mazzetti, L. Cattini, M. Uguccioni, M. Baggiolini, and A. Facchini. 2000. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 43:1734-1741. [DOI] [PubMed] [Google Scholar]
- 4.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 5.Coleman, J. L., T. J. Sellati, J. E. Testa, R. R. Kew, M. B. Furie, and J. L. Benach. 1995. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect. Immun. 63:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 7.Defosse, D. L., and R. C. Johnson. 1992. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect. Immun. 60:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fini, E. M., J. R. Cook, R. Mohan, and C. E. Brinckerhoff. 1998. Regulation of matrix metalloproteinase gene expression, p. 299-356. In W. C. Parks and R. P. Mecham (ed.), Matrix metalloproteinases. Academic Press, San Diego, Calif.
- 9.Gemba, T., J. Valbracht, S. Alsalameh, and M. Lotz. 2002. Focal adhesion kinase and mitogen-activated protein kinases are involved in chondrocyte activation by the 29-kDa amino-terminal fibronectin fragment. J. Biol. Chem. 277:907-911. [DOI] [PubMed] [Google Scholar]
- 10.Habicht, G. S., G. Beck, and J. L. Benach. 1988. The role of interleukin-1 in the pathogenesis of Lyme disease. Ann. N. Y. Acad. Sci. 539:80-86. [DOI] [PubMed] [Google Scholar]
- 11.Han, J., and R. J. Ulevitch. 1999. Emerging targets for anti-inflammatory therapy. Nat. Cell Biol. 1:E39-E40. [DOI] [PubMed] [Google Scholar]
- 12.Han, Z., D. L. Boyle, L. Chang, B. Bennett, M. Karin, L. Yang, A. M. Manning, and G. S. Firestein. 2001. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Investig. 108:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu, L. T., M. A. Eskildsen, C. Masgala, A. C. Steere, E. C. Arner, M. A. Pratta, A. J. Grodzinsky, A. Loening, and G. Perides. 2001. Host metalloproteinases in Lyme arthritis. Arthritis Rheum. 44:1401-1410. [DOI] [PubMed] [Google Scholar]
- 14.Hu, L. T., G. Perides, R. Noring, and M. S. Klempner. 1995. Binding of human plasminogen to Borrelia burgdorferi. Infect. Immun. 63:3491-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumi, Y., S. Kim, M. Namba, H. Yasumoto, H. Miyazaki, M. Hoshiga, Y. Kaneda, R. Morishita, Y. Zhan, and H. Iwao. 2001. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ. Res. 88:1120-1126. [DOI] [PubMed] [Google Scholar]
- 16.Kim, S. J., J. W. Ju, C. D. Oh, Y. M. Yoon, W. K. Song, J. H. Kim, Y. J. Yoo, O. S. Bang, S. S. Kang, and J. S. Chun. 2002. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J. Biol. Chem. 277:1332-1339. [DOI] [PubMed] [Google Scholar]
- 17.Kisseleva, T., S. Bhattacharya, J. Braunstein, and C. W. Schindler. 2002. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285:1-24. [DOI] [PubMed] [Google Scholar]
- 18.Klempner, M. S., R. Noring, M. P. Epstein, B. McCloud, R. Hu, S. A. Limentani, and R. A. Rogers. 1995. Binding of human plasminogen and urokinase-type plasminogen activator to the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 171:1258-1265. [DOI] [PubMed] [Google Scholar]
- 19.Li, W. Q., F. Dehnade, and M. Zafarullah. 2001. Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J. Immunol. 166:3491-3498. [DOI] [PubMed] [Google Scholar]
- 20.Lin, B., J. M. Kidder, R. Noring, A. C. Steere, M. S. Klempner, and L. T. Hu. 2001. Differences in synovial fluid levels of matrix metalloproteinases suggest separate mechanisms of pathogenesis in Lyme arthritis before and after antibiotic treatment. J. Infect. Dis. 184:174-180. [DOI] [PubMed] [Google Scholar]
- 21.Murakami, S., M. Kan, W. L. McKeehan, and B. de Crombrugghe. 2000. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc. Natl. Acad. Sci. USA 97:1113-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridley, S. H., S. J. Sarsfield, J. C. Lee, H. F. Bigg, T. E. Cawston, D. J. Taylor, D. L. DeWitt, and J. Saklatvala. 1997. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J. Immunol. 158:3165-3173. [PubMed] [Google Scholar]
- 23.Schaeffer, H. J., and M. J. Weber. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengupta, T. K., A. Chen, Z. Zhong, J. E. Darnell, Jr., and L. B. Ivashkiv. 1995. Activation of monocyte effector genes and STAT family transcription factors by inflammatory synovial fluid is independent of interferon gamma. J. Exp. Med. 181:1015-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakibaei, M., G. Schulze-Tanzil, P. de Souza, T. John, M. Rahmanzadeh, R. Rahmanzadeh, and H. J. Merker. 2001. Inhibition of mitogen-activated protein kinase kinase induces apoptosis of human chondrocytes. J. Biol. Chem. 276:13289-13294. [DOI] [PubMed] [Google Scholar]
- 26.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 27.Steere, A. C., P. H. Duray, and E. C. Butcher. 1988. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis: comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 31:487-495. [DOI] [PubMed] [Google Scholar]
- 28.Sung, Y. J., M. Povelones, and R. T. Ambron. 2001. RISK-1: a novel MAPK homologue in axoplasm that is activated and retrogradely transported after nerve injury. J. Neurobiol. 47:67-79. [DOI] [PubMed] [Google Scholar]
- 29.Thomas, B., S. Thirion, L. Humbert, L. Tan, M. B. Goldring, G. Bereziat, and F. Berenbaum. 2002. Differentiation regulates interleukin-1beta-induced cyclo-oxygenase-2 in human articular chondrocytes: role of p38 mitogen-activated protein kinase. Biochem. J. 362:367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang, F., T. K. Sengupta, Z. Zhong, and L. B. Ivashkiv. 1995. Regulation of the balance of cytokine production and the signal transducer and activator of transcription (STAT) transcription factor activity by cytokines and inflammatory synovial fluids. J. Exp. Med. 182:1825-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe, H., M. P. de Caestecker, and Y. Yamada. 2001. Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J. Biol. Chem. 276:14466-14473. [DOI] [PubMed] [Google Scholar]
- 32.Whitmarsh, A. J., and R. J. Davis. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589-607. [DOI] [PubMed] [Google Scholar]
- 33.Yosimichi, G., T. Nakanishi, T. Nishida, T. Hattori, T. Takano-Yamamoto, and M. Takigawa. 2001. CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK). Eur. J. Biochem. 268:6058-6065. [DOI] [PubMed] [Google Scholar]
- 34.Zhen, X., L. Wei, Q. Wu, Y. Zhang, and Q. Chen. 2001. Mitogen-activated protein kinase p38 mediates regulation of chondrocyte differentiation by parathyroid hormone. J. Biol. Chem. 276:4879-4885. [DOI] [PubMed] [Google Scholar]