Abstract
Background & Aims
Pancreatitis is the most common serious complication of endoscopic retrograde cholangiopancreatography (ERCP). We performed a pilot study to determine whether aggressive peri-procedural hydration with lactated Ringer’s solution reduces the incidence of pancreatitis following ERCP.
Methods
Patients who underwent first-time ERCP were randomly assigned to groups (2:1) that received aggressive hydration with lactated Ringer’s solution (3 cc/kg/hr during the procedure, a 20 cc/kg bolus after the procedure, and 3cc/kg/hr for 8 hours following the procedure, n=39) or standard hydration with the same solution (1.5 cc/kg/hr during and for 8 hrs after procedure, n=23). Serum levels of amylase, visual analog pain scores (scale of 0–10), and volume overload were assessed at baseline and 2, 8, and 24 hrs after ERCP. The primary endpoint, post-ERCP pancreatitis was defined as hyperamylasemia (level of amylase > 3 times the upper limit of normal) and increased epigastric pain (≥3 points on visual analog scale) persisting for ≥24 hrs after the procedure. Secondary endpoints included hyper-amylasemia, increased pain, and volume overload.
Results
None of the patients who received aggressive hydration developed post-ERCP pancreatitis, compared with 17% of patients who received standard hydration (P=.016).
Hyperamylasemia developed in 23% of patients who received aggressive hydration vs 39% of those who received standard hydration (P=.116, non-significant); increased epigastric pain developed in 8% of patients who received aggressive hydration vs 22% of those who received standard hydration (P=.146, non-significant). No patients had evidence of volume overload.
Conclusions
Based on a pilot study, aggressive intravenous hydration with lactated Ringer’s solution appears to reduce the development of post-ERCP pancreatitis and is not associated with volume overload. ClinicalTrials.gov number, NCT 01758549
Keywords: Pancreas, outcome, endoscopic retrograde cholangiopancreatography, inflammation, clinical trial
INTRODUCTION
Pancreatitis is the leading complication of endoscopic retrograde cholangiopancreatography (ERCP), resulting in considerable morbidity and, rarely, in death. Numerous trials studying a variety of agents (e.g., octreotide, corticosteroids, protease inhibitors), have failed to demonstrate a significant benefit in reduction of post-ERCP pancreatitis1-2, although rectal indomethacin has recently been shown to significantly decrease post-ERCP pancreatitis in high-risk patients3.
Hydration is a mainstay of treatment for acute pancreatitis, independent of etiology4. Experiments in animal models demonstrate that pancreatic microvascular hypoperfusion leads to necrosis5. Clinical studies of fluid resuscitation in patients with acute pancreatitis suggest that hemoconcentration and decreased systemic perfusion are associated with increased risk of pancreas necrosis and unfavorable outcome6.
We hypothesized that aggressive intravenous hydration with lactated Ringer’s solution would diminish the risk of post-ERCP pancreatitis, and performed a randomized controlled trial to test this hypothesis.
METHODS
Study Design
Patients were enrolled at the Los Angeles County + University of Southern California Medical Center following approval by the University of Southern California Health Sciences Institutional Review Board. The study was registered with clinical trials.gov (NCT 01758549). Informed consent was obtained from all patients prior to enrollment and randomization. All authors had access to the study data and reviewed and approved the final submission.
Patients
Only inpatients were included to ensure close follow-up and a controlled environment. Those undergoing ERCP for standard clinical indications such as choledocholithiasis, bile duct leak, and biliary obstruction were included. Patients with active cholangitis or sepsis were excluded as they required aggressive intravenous hydration. Patients with chronic pancreatitis and active gallstone pancreatitis were also excluded as it would not have been possible to interpret the outcomes in these patients.
Patients at increased risk of fluid overload were also excluded. This included patients with cardiac insufficiency (New York Heart Association Class II or greater heart failure), renal insufficiency (creatinine clearance <40ml/minute), liver dysfunction, or respiratory insufficiency (room air oxygen saturation <90%). Patients with clinical signs of fluid overload including peripheral or pulmonary edema or those with electrolyte disturbances such as hypernatremia (Na+ >150mEq/L) or hyponatremia (Na+<130mEq/L) were excluded. Additionally, pregnant women were excluded as they are prone to retention of sodium and water, and patients greater than 70 years old were excluded as they are at greater risk of undiagnosed cardiac or renal insufficiency. To eliminate patients at very low risk for post-ERCP pancreatitis those who had previously undergone ERCP with sphincterotomy were excluded.
Intervention
Immediately prior to ERCP, subjects were randomly assigned in a 2:1 ratio to receive aggressive intravenous hydration versus standard peri-procedural fluids. A concealed computer-generated block randomization schedule was used. Randomization was stratified by patient sex and the protocol called for the randomization of 30 women and 30 men. Patients in the aggressive hydration arm received intravenous lactated Ringer’s solution at a rate of 3.0cc/kg/hour during the procedure, a bolus of 20cc/kg immediately after the procedure, followed by a post-procedure rate of 3cc/kg/hr for 8 hours. Those in the standard group received intravenous lactated Ringer’s solution at a rate of 1.5cc/kg/hour during the procedure and for 8 hours after the procedure without a bolus. Patients in the standard hydration arm who developed post-ERCP pancreatitis at 2 or 8 hours were given a bolus of 20cc/kg followed by a rate of 3cc/kg/hour. Patients in the aggressive arm who reported no pain at 8 hours had their fluids decreased to 1.5cc/kg/hour and diet advanced. Fluids in both groups were stopped when the patients tolerated a normal diet.
Endpoints
The primary endpoint was post-ERCP pancreatitis, defined as hyperamylasemia (amylase > three times the upper limit of normal (390 U/L)) and pancreatic pain (epigastric abdominal pain radiating to the back scored by patient as development of or increase of pain ≥3 on a 0-10 visual analog pain scale and persisting for ≥24 hours after ERCP). In those who had pain prior to the procedure, pancreatic pain was defined as an increase of ≥3 on the 0-10 visual-analogue scale. Secondary endpoints included the components of the post-ERCP pancreatitis criteria, hyperamylasemia and pancreatic pain. Computed tomography or other imaging studies were not routinely performed in patients diagnosed with post-ERCP pancreatitis.
The total length of hospitalization of all patients, whether or nor they developed pancreatitis was also compared. Additionally, a physical assessment for fluid overload, which was defined by the development of ankle or upper extremity edema, ascites, pulmonary rales, and/or decreased oxygen saturation, was performed at 2, 8, and 24 hours following ERCP. Patient demographics, procedure details, vital signs, laboratories, and fluid and analgesic administration post-ERCP were prospectively collected.
Statistical Analysis
As this is the first study to examine the impact of fluids to prevent post-ERCP pancreatitis the analytic goal was to assess feasibility and safety and estimate the size of the treatment effect to power future trials. Based on previous multicenter trials of therapeutic ERCP in teaching programs we assumed that the rate of post-ERCP pancreatitis would be 15.1%.1 The Institutional Review Board limited total enrollment to 60 patients. Thus, we adopted a 2:1 randomized design to test our hypothesis that aggressive hydration with lactated Ringer’s solution would prevent post-ERCP pancreatitis. According to our a priori calculations, this sample size allowed us to demonstrate a significant difference assuming an absolute decrease of post-ERCP pancreatitis of 15% with a two-sided alpha = 0.05 and a power of 80%. A total of 62 patients were enrolled because 2 patients who were enrolled had unsuspected prior sphincterotomy, in violation of the enrollment criteria. These 2 patients were included in the primary intention-to-treat analysis but not in a secondary per-protocol analysis.
Descriptive statistics for participant characteristics and outcomes were computed. Due to skew in data medians and interquartile ranges were computed for continuous outcomes. A Fisher’s exact test was used to test the primary outcome, post-ERCP pancreatitis, and secondary outcomes, hyperamylasemia, and pancreatic pain. Given a non-normal distribution and small sample size, a nonparametric Mann-Whitney U test was used to test differences in continuous data (days of hospitalization, amylase at 2 and 8 hours). Hodges-Lehman estimates were computed for the confidence interval of the difference of medians.
RESULTS
Patients
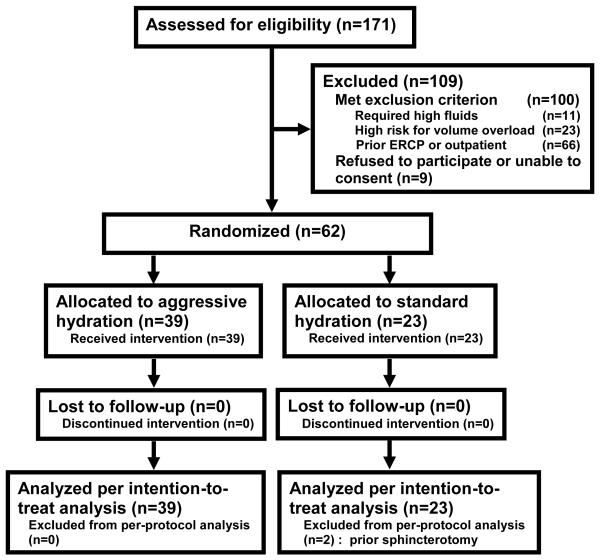
Among the 171 patients screened, 71 met the inclusion criteria: 9 declined participation and 62 were enrolled (Figure 1). Two patients were found after randomization to standard fluids to have an unsuspected prior sphincterotomy but were included in the intention-to-treat analysis. Baseline characteristics of the study groups were similar (Table 1). The mean age was 44+15 years, 78% were Hispanic, and 75% were undergoing ERCP for bile duct stones.
FIGURE 1.
CONSORT DIAGRAM OF PATIENT FLOW THROUGH THE TRIAL
TABLE 1.
Selected Baseline characteristics
| Standard Hydration (N=23) |
Aggressive Hydration (N=39) |
|
|---|---|---|
| Age (mean ± SD) | 45 ± 17 | 43 ± 14 |
| Comorbidities N (%) | 8 (34.8%) | 11 (28.2%) |
| Hispanic ethnicity N (%) | 18 (78.3%) | 34 (78.3%) |
| Indication: bile duct stone N (%) |
17 (73.9%) | 29 (74.4%) |
| Female gender | 13 (56.5%) | 19 (48.7%) |
| Total bilirubin (mean ± SD) |
4.2 ± 5.9 | 3.7 ± 3.8 |
| Hematocrit (mean ± SD) | 36 ± 4 | 37 ± 5 |
| Creatinine (mean ± SD) | 0.7 ± 0.2 | 0.7 ± 0.2 |
p > 0.25 for all comparisons
Risk Factors for Post-ERCP Pancreatitis
Risk factors for post-ERCP pancreatitis, as defined by the current American Society for Gastrointestinal Endoscopy guidelines, were similar in the two groups.7 None of the patients had the following four risk factors: suspected Sphincter of Oddi dysfunction, a history of prior post-ERCP pancreatitis, pancreatic sphincterotomy, or balloon dilatation of the biliary sphincter. The proportion of patients with each of the other four high-risk factors for post-ERCP pancreatitis in each study group are shown in Table 2. In addition, difficult cannulation, defined as >10 cannulation attempts, occurred in 3 (13%) of the standard hydration group and 4 (10%) of the aggressive hydration group. Pancreatic stents were used in 1 (4%) of the patients in the standard hydration group and 2 (5%) of those in the aggressive hydration group. Trainees were involved in every case and participated in all aspects of the procedures.
TABLE 2.
Risk Factors for Post-ERCP Pancreatitis based on 2012 ASGE Guidelines7
| Standard Hydration (N=23) |
Aggressive Hydration (N=39) |
|
|---|---|---|
| Normal bilirubin (≤1mg/dl) | 6 (26.1%) | 9 (23.1%) |
| Pancreatic duct injection | 4 (17.4%) | 10 (25.6%) |
| Precut Sphincterotomy | 1 (4.3%) | 1 (2.6%) |
| Young age (<30 yrs) | 3 (13.0%) | 4 (10.3%) |
No patients in either group had prior post-ERCP pancreatitis, suspected Sphincter of Oddi dyfuncion, pancreatic sphincterotomy, or balloon dilation of the biliary sphincter.
p > 0.25 for all comparisons
Primary Outcome
Patients in the aggressive hydration group received a median of 3.8 liters of lactated Ringer’s solution compared to 2.2 liters in the standard group (p <0.001)(Table 3). Post-ERCP pancreatitis developed in 0 of 39 patients in the aggressive hydration group vs. 4 (17%) of the 23 patients in the standard hydration group (95% CI of difference 5.8, 35.9%; p=0.016). The duration of hospitalization was prolonged to at least 2 days in all patients with post-ERCP pancreatitis. Two episodes of pancreatitis were mild, one moderate, and one severe, based on the consensus grading system for post-ERCP pancreatitis reported by Cotton et al8.
TABLE 3.
Results in the Study Groups
| Standard Hydration (N=23) |
Aggressive Hydration (N=39) |
||
|---|---|---|---|
| N (%) | N (%) | P value | |
| Post-ERCP Pancreatitis |
4 (17%) | 0 (0%) | 0.016 |
| Hyper- amylasemia |
9 (39.1%) | 9 (23.1%) | 0.146 |
| Pancreatic pain | 5 (21.7%) | 5 (7.7%) | 0.116 |
| Median (IQR a ) | Median (IQR) | P value | |
| 2-Hour amylase (U/L) |
172 (596) | 162 (296) | 0.42 |
| 8-Hours amylase (U/L) |
200 (639) | 138 (190) | 0.10 |
| Total fluids during first 24 hours (L) |
2.2 (2.1) | 3.8 (1.5) | <0.001 |
| Hospitalization (days) |
4 (6) | 3 (3) | 0.41 |
Interquartile Range
Hyperamylasemia (>390 U/L) was seen in 9 (23%) of 39 patients who received aggressive hydration compared to 9 (39%) of 23 who received standard fluids (95% CI of difference −8, 40%; p=0.15). Median amylase values at 8 hours were 200 U/L in the standard fluids group and 138 U/L in the aggressive hydration group (95% CI of difference −9 U/L, 317 U/L; p =0.10). Pancreatic pain occurred in 22% of the standard hydration group and 8% of those who received aggressive hydration (95% CI of difference −5%, 33%; p=0.12). None of the patients in either group developed clinical evidence of fluid overload. Median duration of hospitalization was not significantly different between the study groups (Table 3).
DISCUSSION
This study, the first randomized trial of aggressive hydration to reduce the incidence of post-ERCP pancreatitis, found that aggressive hydration with lactated Ringer’s solution was associated with a significant decrease in post-ERCP pancreatitis. Hyperamylasemia and persistent epigastric pain, the individual criteria used to define post-ERCP pancreatitis, were also less frequent in those who received aggressive hydration, though the difference was not statistically significant. Variation in the results for these primary and secondary endpoints are not surprising because patients may meet individual criteria without meeting both criteria for post-ERCP pancreatitis.
ERCP is one of the few events which reliably induce pancreatitis in a substantial number of patients. Clinical trials have shown that markers of inadequate fluid resuscitation including elevated hematocrit, creatinine, and BUN are associated with the development of organ failure6. Aggressive fluid resuscitation to restore volume status is recommended in patients with pancreatitis4. In experimental models of pancreatitis, regional hypoperfusion correlates with more severe inflammation5. Agents which improve pancreatic microcirculation reduce histopathologic damage in animal models of pancreatitis9. Prophylactic administration of these agents is even more effective in preventing tissue damage than administration after initiation of pancreatitis.
Recent randomized work suggests that hydration with lactated Ringer’s solution as opposed to saline may decrease the likelihood of systemic inflammatory response syndrome10. An acidic environment favors trypsinogen activation and development of pancreatitis in experimental models11. Lactated Ringer’s solution is less likely to induce metabolic acidosis than saline, which may explain its potential protective effect. Additionally, lactate may stimulate an anti-inflammatory immune response12.
Prior medical therapy of post-ERCP pancreatitis has aimed to curb exocrine stimulation and the inflammatory cascade with mixed results. Recently, Elmunzer et al demonstrated that rectal indomethacin decreases the risk of post-ERCP pancreatitis in high-risk patients3.At the time of the study rectal indomethacin was not yet available on our hospital formulary. Additionally, investigators have shown that wire-guided cannulation and the use of pancreatic stents in high-risk cases decrease the risk of pancreatitis13-14. Both of these technical approaches were used in our trial with no differences between the two groups in the proportions receiving each technique. The wire-guided approach was used in all patients. However, given the uncertainty regarding which specific patients benefit most from pancreatic stent placement coupled with the fact that many patients in our underserved population fail to return for follow-up procedures to remove pancreatic stents, our practice is to place pancreatic stents only in the highest risk patients (e.g., precut sphincterotomy, repeated pancreatic duct injection).15-17 Thus, only about 5% of patients received pancreatic duct stents. Certainly, if a larger proportion of patients had received pancreatic stents it may have impacted our results, potentially decreasing the rate of pancreatitis in the standard hydration group. Future larger studies will be needed to assess the potential benefit of multiple interventions (e.g., aggressive hydration, pancreatic duct stent placement, rectal indomethacin).
Our results need to be interpreted cautiously given that this was a pilot study designed primarily to assess feasibility and safety of aggressive hydration and to inform decisions on design of future trials. Due to the small sample size and the relatively high rate of pancreatitis in the control group, we cannot rule out the possibility that the significant benefit of aggressive hydration reflects a Type 1 error, in which the null hypothesis (that the two treatments are not different) is rejected despite the fact that it is true. In addition, differences between study groups in characteristics that may impact the primary outcome may occur more frequently in small randomized trials. Potential confounding factors were relatively similar in the two study groups; p values were >0.25 for all comparisons and small differences of <10% were seen favoring both the aggressive hydration arm (e.g., normal bilirubin) and the control arm (e.g., pancreatic duct injections). Larger randomized trials are needed to confirm our findings and provide more precision on estimates of treatment effect with aggressive hydration. Larger trials also will help determine if aggressive hydration can reduce post-ERCP pancreatitis severity, as it is likely that aggressive fluids may act at least in part by attenuating pancreatic inflammation, and to assess the role of aggressive fluid hydration in the outpatient setting.
In conclusion, this prospective randomized trial suggests that aggressive hydration with lactated Ringer’s solution reduces the incidence of post-ERCP pancreatitis. Larger trials of this strategy are required to confirm our findings and provide more precise estimates of its efficacy.
Acknowledgments
Support: This publication was supported by NIH/NCRR SC CTSI Grant Number UL1TR000130. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no relevant conflict of interest to disclose
Author Contributions: Concept and Design: JB, CL, LL; Acquisition of Data: JB, AY, KY; Statistical Analysis: JB, CL, LL; Drafting and Revision of Manuscript: JB, AY, KY, CL, LL
ClinicalTrials.gov number, NCT 01758549
REFERENCES
- 1.Sherman S, Blaut U, Watkins JL, et al. Does prophylactic administration of corticosteroid reduce the risk and severity of post-ERCP pancreatitis: a randomized, prospective, multicenter study. Gastrointest Endosc. 2003;58:23–9. doi: 10.1067/mge.2003.307. [DOI] [PubMed] [Google Scholar]
- 2.Andriulli A, Leandro G, Federici T, et al. Prophylactic administration of somatostatin or gabexate does not prevent pancreatitis after ERCP: an updated meta-analysis. Gastrointest Endosc. 2007;65:624–32. doi: 10.1016/j.gie.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012;366:1414–22. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banks PA, Freeman ML. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379–400. doi: 10.1111/j.1572-0241.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 5.Kusterer K, Enghofer M, Zendler S, et al. Microcirculatory changes in sodium taurocholate-induced pancreatitis in rats. Am J Physiol. 1991;260:G346–51. doi: 10.1152/ajpgi.1991.260.2.G346. [DOI] [PubMed] [Google Scholar]
- 6.Muddana V, Whitcomb DC, Khalid A, et al. Elevated serum creatinine as a marker of pancreatic necrosis in acute pancreatitis. Am J Gastroenterol. 2009;104:164–70. doi: 10.1038/ajg.2008.66. [DOI] [PubMed] [Google Scholar]
- 7.Anderson MA, Fisher L, Jain R, et al. Complications of ERCP. Gastrointest Endosc. 2012;75:467–73. doi: 10.1016/j.gie.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–93. doi: 10.1016/s0016-5107(91)70740-2. [DOI] [PubMed] [Google Scholar]
- 9.Strate T, Mann O, Kleinhans H, et al. Microcirculatory function and tissue damage is improved after therapeutic injection of bovine hemoglobin in severe acute rodent pancreatitis. Pancreas. 2005;30:254–9. doi: 10.1097/01.mpa.0000157481.22155.2d. [DOI] [PubMed] [Google Scholar]
- 10.Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol. 2011;9:710–717. doi: 10.1016/j.cgh.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Seyama Y, Otani T, Matsukura A, et al. The pH modulator chloroquine blocks trypsinogen activation peptide generation in cerulein-induced pancreatitis. Pancreas. 2003;26:15–7. doi: 10.1097/00006676-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264.7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286:R686–92. doi: 10.1152/ajpregu.00564.2003. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary A, Bechtold ML, Arif M, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc. 2011;73:275–82. doi: 10.1016/j.gie.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Cennamo V, Fuccio L, Zagari RM, et al. Can a wire-guided cannulation technique increase bile duct cannulation rate and prevent post-ERCP pancreatitis?: A meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:2343–50. doi: 10.1038/ajg.2009.269. [DOI] [PubMed] [Google Scholar]
- 15.Freeman ML. Pancreatic stents for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Clin Gastroenterol Hepatol. 2007;5:1354–65. doi: 10.1016/j.cgh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Fazel A, Quadri A, Catalano MF, et al. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study. Gastrointest Endosc. 2003;57:291–4. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 17.Mazaki T, Masuda H, Takayama T. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2010;42:842–53. doi: 10.1055/s-0030-1255781. [DOI] [PubMed] [Google Scholar]
- 18.Testoni PA, Bagnolo F. Pain at 24 hours associated with amylase levels greater than 5 times the upper normal limit as the most reliable indicator of post-ERCP pancreatitis. Gastrointest Endosc. 2001;53:33–9. doi: 10.1067/mge.2001.111390. [DOI] [PubMed] [Google Scholar]