Abstract
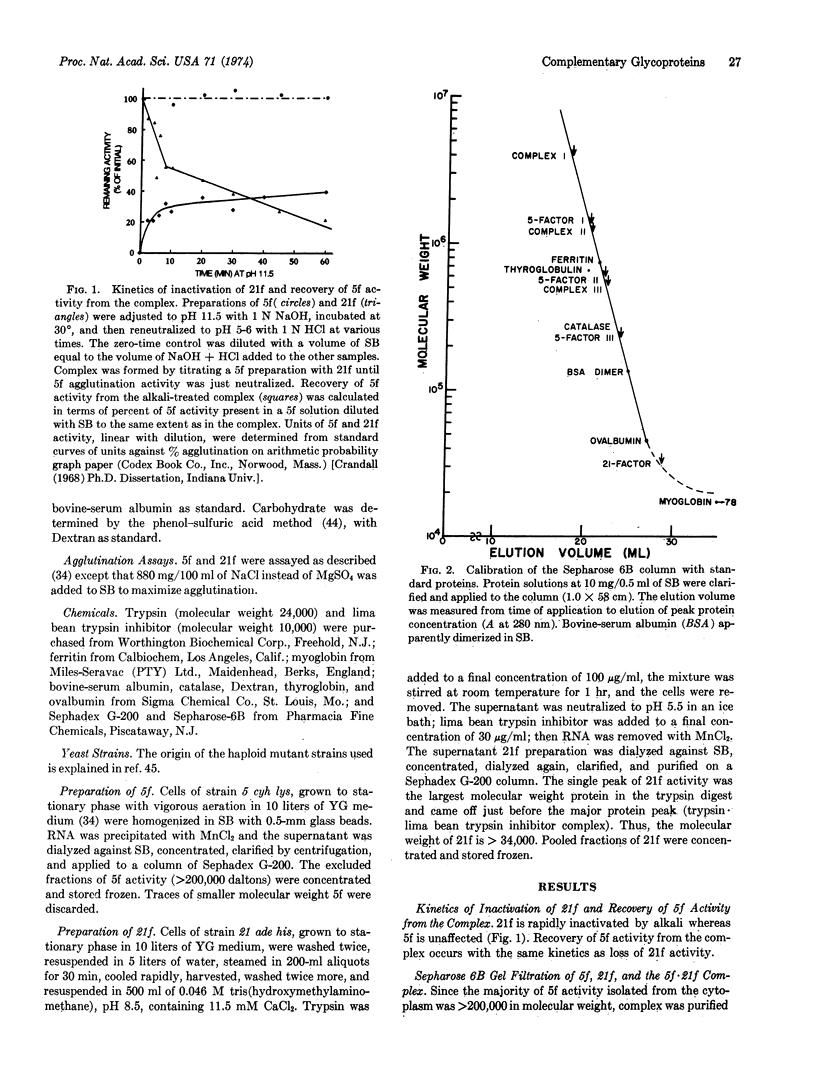
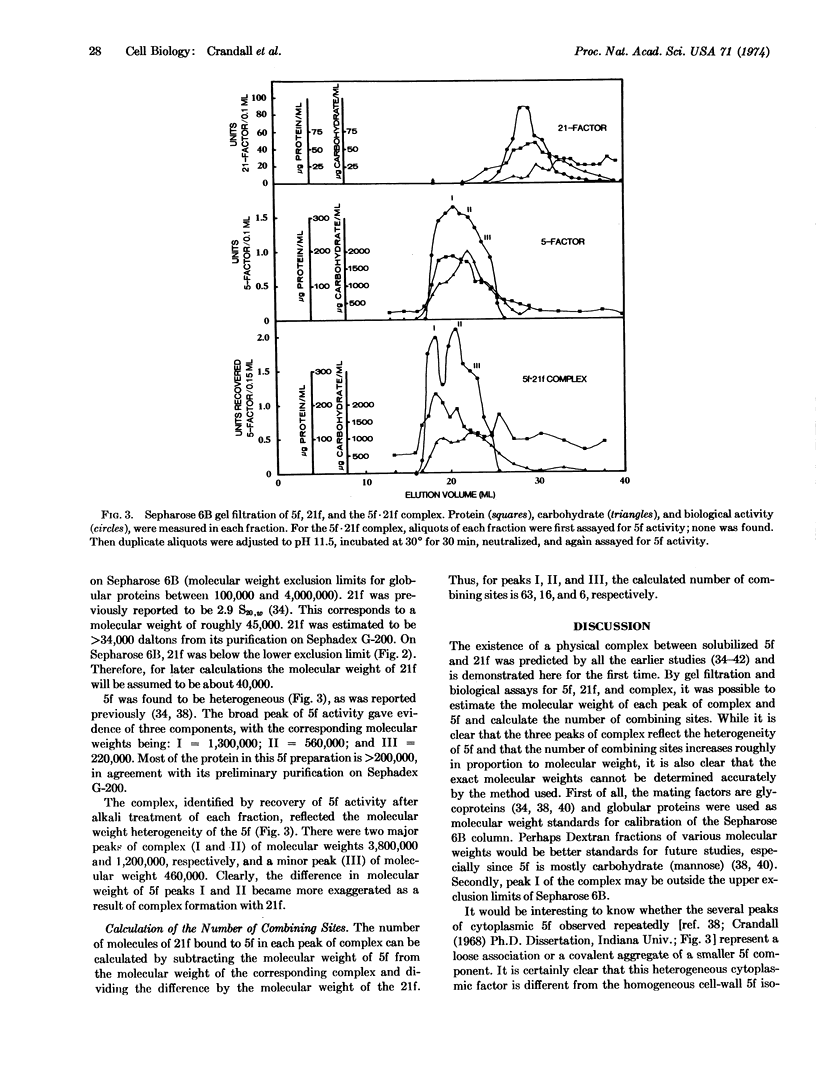
Cell fusion between opposite mating types 5 and 21 of the yeast Hansenula wingei is initiated by a strong sexual agglutination reaction. The mating factors responsible for the specificity of cellular recognition are complementary glycoproteins which form a physical complex in vitro. The complex is assayed by recovery of agglutination activity of the multivalent 5-factor after the univalent 21-factor has been inactivated by treatment of the complex with alkali. The 5-factor·21-factor complex, purified on Sepharose 6B, is large (several million daltons) and heterogeneous. The three peaks of 5-factor activity contain a number of combining sites proportional to molecular size.
Keywords: Hansenula wingei, cellular recognition, Sepharose gel filtration, complementary glycoprotein complex formation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D. The purification and characterization of an intracellular sex-specific mannan protein from yeast. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1104–1112. doi: 10.1073/pnas.54.4.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauldwell C. B., Henkart P., Humphreys T. Physical properties of sponge aggregation factor. A unique proteoglycan complex. Biochemistry. 1973 Jul 31;12(16):3051–3055. doi: 10.1021/bi00740a017. [DOI] [PubMed] [Google Scholar]
- Clark M. A. Control of mating type expression in Paramecium multimicronucleatum, syngen 2. J Cell Physiol. 1972 Feb;79(1):1–14. doi: 10.1002/jcp.1040790102. [DOI] [PubMed] [Google Scholar]
- Crandall M. A., Brock T. D. Molecular aspects of specific cell contact. Science. 1968 Aug 2;161(3840):473–475. doi: 10.1126/science.161.3840.473. [DOI] [PubMed] [Google Scholar]
- Crandall M. A., Brock T. D. Molecular basis of mating in the yeast hansenula wingei. Bacteriol Rev. 1968 Sep;32(3):139–163. doi: 10.1128/br.32.3.139-163.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall M. A., Brock T. D. Mutual repression of haploid genes in diploid yeast. Nature. 1968 Aug 3;219(5153):533–534. doi: 10.1038/219533a0. [DOI] [PubMed] [Google Scholar]
- Crandall M., Caulton J. H. Induction of glycoprotein mating factors in diploid yeast of Hansenula wingei by vanadium salts or chelating agents. Exp Cell Res. 1973 Nov;82(1):159–167. doi: 10.1016/0014-4827(73)90258-9. [DOI] [PubMed] [Google Scholar]
- Crandall M. Comparison of Hansenula wingei, a petite-negative, obligately aerobic yeast, to the petite-positive yeast Saccharomyces cerevisiae. J Gen Microbiol. 1973 Apr;75(2):363–375. doi: 10.1099/00221287-75-2-363. [DOI] [PubMed] [Google Scholar]
- Garber B. B., Moscona A. A. Reconstruction of brain tissue from cell suspensions. 2. Specific enhancement of aggregation of embryonic cerebral cells by supernatant from homologous cell cultures. Dev Biol. 1972 Feb;27(2):235–243. doi: 10.1016/0012-1606(72)90100-5. [DOI] [PubMed] [Google Scholar]
- Good R. J. Theory of the adhesion of cells and the spontaneous sorting-out of mixed cell aggregates. J Theor Biol. 1972 Dec;37(3):413–434. doi: 10.1016/0022-5193(72)90083-5. [DOI] [PubMed] [Google Scholar]
- Heinmets F. Cell-cell recognition and interaction. Curr Mod Biol. 1968 Jan-Feb;1(5):299–313. doi: 10.1016/0303-2647(68)90032-4. [DOI] [PubMed] [Google Scholar]
- Henkart P., Humphreys S., Humphreys T. Characterization of sponge aggregation factor. A unique proteoglycan complex. Biochemistry. 1973 Jul 31;12(16):3045–3050. doi: 10.1021/bi00740a016. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oppenheimer S. B., Humphreys T. Isolation of specific macromolecules required for adhesion of mouse tumour cells. Nature. 1971 Jul 9;232(5306):125–127. doi: 10.1038/232125a0. [DOI] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. An assay for intercellular adhesive specificity. J Cell Biol. 1971 Nov;51(21):525–535. doi: 10.1083/jcb.51.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., McGuire E. J., Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971 Nov;51(21):536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. S. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970 Apr;173(4):395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- TAYLOR N. W. SPECIFIC, SOLUBLE FACTOR INVOLVED IN SEXUAL AGGLUTINATION OF THE YEAST HANSENULA WINGEI. J Bacteriol. 1964 Apr;87:863–866. doi: 10.1128/jb.87.4.863-866.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. W., Orton W. L. Association constant of the sex-specific agglutinin in the yeast, Hansenula wingei. Biochemistry. 1970 Jul 7;9(14):2931–2934. doi: 10.1021/bi00816a027. [DOI] [PubMed] [Google Scholar]
- Taylor N. W., Orton W. L. Cooperation among the active binding sites in the sex-specific agglutinin from the yeast, Hansenula wingei. Biochemistry. 1971 May 25;10(11):2043–2049. doi: 10.1021/bi00787a012. [DOI] [PubMed] [Google Scholar]
- Taylor N. W., Orton W. L. Sexual agglutination in yeast. VII. Significance of the 1.7S component from reduced 5-agglutinin. Arch Biochem Biophys. 1968 Sep 10;126(3):912–921. doi: 10.1016/0003-9861(68)90485-2. [DOI] [PubMed] [Google Scholar]


