Abstract
Background
The Trypanosoma cruzi satellite DNA (satDNA) OligoC-TesT is a standardised PCR format for diagnosis of Chagas disease. The sensitivity of the test is lower for discrete typing unit (DTU) TcI than for TcII-VI and the test has not been evaluated in chronic Chagas disease patients.
Methodology/Principal Findings
We developed a new prototype of the OligoC-TesT based on kinetoplast DNA (kDNA) detection. We evaluated the satDNA and kDNA OligoC-TesTs in a multi-cohort study with 187 chronic Chagas patients and 88 healthy endemic controls recruited in Argentina, Chile and Spain and 26 diseased non-endemic controls from D.R. Congo and Sudan. All specimens were tested in duplicate. The overall specificity in the controls was 99.1% (95% CI 95.2%–99.8%) for the satDNA OligoC-TesT and 97.4% (95% CI 92.6%–99.1%) for the kDNA OligoC-TesT. The overall sensitivity in the patients was 67.9% (95% CI 60.9%–74.2%) for the satDNA OligoC-TesT and 79.1% (95% CI 72.8%–84.4%) for the kDNA OligoC-Test.
Conclusions/Significance
Specificities of the two T. cruzi OligoC-TesT prototypes are high on non-endemic and endemic controls. Sensitivities are moderate but significantly (p = 0.0004) higher for the kDNA OligoC-TesT compared to the satDNA OligoC-TesT.
Author Summary
Accurate diagnosis of Chagas disease is challenging due to the latent character of the infection and the low parasite load in the blood. Molecular tests such as the polymerase chain reaction (PCR) detect the parasite's DNA and are generally very sensitive and specific. In this study we evaluated two prototypes of a standardized PCR diagnostic kit: the satellite DNA (satDNA) OligoC-TesT and the kinetoplast (kDNA) OligoC-TesT. Sensitivities and specificities of both tests were estimated in a multi-cohort phase II evaluation study with 187 chronic Chagas patients and 88 healthy endemic controls from Argentina, Chile and Spain, and 26 non-endemic controls from D.R. Congo and Sudan with potentially cross-reacting diseases. Specificities in the control persons were high (>97%) and the sensitivity of the kDNA OligoC-TesT (79.1%) was significantly higher than of the satDNA OligoC-Test (67.9%). In a next phase, the kDNA OligoC-TesT should be evaluated in specific niches where standard serological tools have their limitations, e.g. follow-up after treatment and diagnosing newborns and HIV co-infected patients.
Introduction
Trypanosoma (T.) cruzi is a kinetoplastid protozoan parasite and the etiological agent of American trypanosomiasis or Chagas disease. An estimated 8 million people are infected and more than 25 million are at risk of contracting the infection [1]. Chagas disease is endemic in Latin America, but evolved to a global health problem due to migration to other continents [2]. T. cruzi has a broad host range including wild and domestic animals. The parasite is primarily transmitted by infected haematophagous Triatominae bugs. Additional transmission routes include blood transfusion, organ transplantation, congenital infection, accidental infection and consumption of contaminated food causing orally transmitted outbreaks [3]. The initial acute phase lasts 6–8 weeks and clinical symptoms are generally mild and non-specific. Also in the chronic phase, the majority of the infected individuals remain asymptomatic, but up to 30% develop cardiac and digestive complications that can be fatal [4]. T. cruzi is monophyletic, but genetically heterogeneous and divided in six discrete typing units (DTUs): T. cruzi I to VI. The different DTUs have been associated with specific geographical distribution, reservoirs, vectors, virulence, clinical manifestation and susceptibility to drugs [5].
Accurate diagnosis of Chagas disease is challenging due to the latent character of the infection [6]. The parasite load in the blood of acute phase patients is generally high enough to be detected by microscopic analysis of blood smears or buffy coat in microhaematocrit capillaries. However, only 1 to 2% of all infected individuals are actually diagnosed during this phase because of the non-specific clinical manifestations [7]. In chronic patients, the parasite load is often very low and diagnosis is mostly accomplished by serological methods such as the indirect immunofluorescence (IIF), indirect haemagglutination (IHA) and enzyme-linked immunosorbent assays (ELISA). Sensitivity of antibody detection tests is generally high, but false-positive results occur due to cross-reactions with antibodies induced by other microorganisms such as Leishmania spp. or T. rangeli [8]. T. rangeli is closely related to T. cruzi and is non-pathogenic to man but shares the same reservoir animals and vectors [9]. To increase specificity, it is recommended to subject a specimen to at least two different serological assays [7]. For post-treatment monitoring however, the long-term persistence of specific antibodies, even after successful treatment, makes serological tests less informative. In case of congenital infections, serology can only be used after 6 to 8 months of age, because of the presence of maternal antibodies in newborns [7].
The polymerase chain reaction (PCR) has been presented as a promising method for sensitive and specific detection of T. cruzi parasites, especially in newborns [10] and during follow-up after treatment [11]. Real-time PCR offers the possibility to quantify the bloodstream parasite load, which could be of particular interest to follow the response to trypanocidal drugs [12]. DNA targets that have been most widely used in diagnostic PCR's are the T. cruzi minicircle kinetoplast DNA (kDNA) and the 195-bp satellite DNA (satDNA) [13]. Recently, we reported the development of a PCR dipstick assay for standardised detection of T. cruzi DNA in biological samples [14]. This T. cruzi OligoC-TesT (Coris BioConcept, Gembloux, Belgium) targets the satDNA sequence [15]. PCR amplicons are visualised on a lateral flow device that contains internal controls for the PCR reaction and for the chromatographic migration. The phase I evaluation showed high sensitivity and specificity on a diverse panel of biological samples and indicated the potential of the T. cruzi OligoC-TesT as a molecular diagnostic tool for Chagas disease [14]. However, the analytical sensitivity of the T. cruzi OligoC-TesT was 100 to 1000 times lower for TcI compared to the other DTUs, which is probably due to the lower copy number of the satDNA in TcI [16].
A recent multicentre validation study assessed the diagnostic accuracy of 48 different PCR tests, including the T. cruzi OligoC-TesT. The study revealed highly heterogeneous results among the different PCR tests and a sensitivity of 72% (n = 32) and specificity of 60% (n = 10) of the T. cruzi satDNA OligoC-TesT [13].
To increase sensitivity without compromising on T. rangeli cross-reactivity, a new OligoC-TesT prototype targeting a conserved region of the T. cruzi minicircle kDNA was developed. To assess their diagnostic accuracy in different endemic regions, we evaluated both versions of the T. cruzi OligoC-TesTs in a multi-cohort phase II study with 187 Chagas patients and 114 controls.
Methods
Ethics
Ethical clearance for the study was obtained from the ethics committees of the Universidad de Chile, Fundacion Huesped (Argentina) and Hospital Universitario Ramón y Cajal (Madrid, Spain). Written informed consent was obtained from patients and from non-diseased persons. Adult participants provided their own consent and a parent or guardian provided consent for children. All samples were anonymized.
DNA
Purified DNA from T. cruzi Cutia cl1 (TcI), TU18 cl93 (TcII), M5631 cl5 (TcIII), Dog Theis (TcIV), MN cl2 (TcV) and CL Brener (TcVI) was obtained from the DNA reference bank at the London School of Hygiene and Tropical Medicine (LSHTM, UK). DNA from L. chagasi and three T. rangeli isolates LEM2953, P13 and SJMC2 was obtained from the DNA reference bank at the Institute of Tropical Medicine Antwerp (ITM, Belgium). Concentrations were measured using the nanodrop ND-1000 UV-Vis spectrophotometer (NanoDrop Technologies, Wilmington, USA) and the DNA was stored at −20°C. To assess the lower detection limits of the T. cruzi OligoC-TesTs, tenfold serial dilutions of parasite DNA, ranging from 200 pg to 0.002 fg DNA per test reaction, were prepared in water (Accugene, Lonza, Belgium) containing 0.1 mg/ml bovine serum albumin (Promega, Madison, Wis.). Archived DNA from blood of diseased non-endemic control persons was obtained from the biobank at ITM: 15 confirmed visceral leishmaniasis patients from Sudan and 11 confirmed gambiense sleeping sickness patients from D.R. Congo.
Study participants and reference tests
In this prospective study, participants were classified as healthy endemic controls and Chagas disease patients based on the results of the reference tests, as described below. Exclusion criteria for participation in the study were children below 12 years of age, serious illness and not signing the informed consent form.
Healthy endemic controls were recruited from the blood bank of the Clinical Hospital at the University of Chile in 2004 and 2011. Study participants were classified as healthy endemic controls if they had no history of Chagas disease, showed no clinical symptoms and their serum was negative in T. cruzi ELISA and IIF.
Chagas disease patients were recruited in the Santiago Metropolitan Region in Chile in 2009; in Salta province North-West Argentina between 2008 and 2010; and at the Hospital Ramón y Cajal in Madrid, Spain in 2011. The patients recruited in Madrid were immigrants originating from Bolivia. Study participants were classified as Chagas disease patients if positive test results were obtained in ELISA and IHA (Argentina) or ELISA and IIF (Spain and Chile). Specifications of the reference tests are given in table 1.
Table 1. Demographic characteristics of the participants recruited in the study and specifications of blood collections and reference tests.
| Origin | Recruitment period | Participant groups | Number of participants | Age range | Median age | Ratio male∶female | % with Chagas clinical signs | Volume of blood collected (mL) | ELISA | IIF | IHA |
| Chile | 2004 and 2011 | Healthy endemic controls | 88 | Unknown | Unknown | Unknown | 0 | 3.0 | a | b | ND |
| 2009 | Chagas patients | 80 | 23–77 | 50.0 | 0.40 | 68 | 3.0 | a | b | ND | |
| Argentina | 2008 to 2010 | Chagas patients | 73 | 18–77 | 50.0 | 0.78 | 43 | 5.0 | c | ND | d |
| Spain | 2011 | Chagas patients | 34 | 15–51 | 43.0 | 0.48 | 56 | 2.5 | e | f | ND |
Notes: ND: not done; a: ELISA Chagas III, GrupoBios S.A., Chile; b: In house protocol with T. cruzi Tulahuen strain; c: Chagatest ELISA recombinant v. 4.0, Wiener Lab, Argentina; d: Chagatest HAI, Wiener Lab, Argentina; e: In house protocol with T. cruzi Dm28, MC & T strain; f: In house protocol with T. cruzi Dm28,MC & T strain.
Blood collection and DNA extraction
Blood was collected and instantly mixed with an equal volume of guanidium EDTA buffer (GEB; 6M guanidium chloride, 0.2 M EDTA, pH 8.0), stored at 4°C and 1 mL aliquots were shipped to ITM Antwerp, Belgium. Upon receipt, DNA was extracted in duplicate from 200 µL lysed blood using the QIAamp DNA blood mini kit (Qiagen, Hilden, Germany) for the cohort recruited in Chile in 2004 and the High Pure PCR Template Preparation Kit (Roche Applied Sciences, IN, USA) for the other blood specimens. DNA extractions were done according to the manufacturer's instructions but with some modifications for the High Pure PCR Template Preparation Kit. Briefly, 200 µL of the blood/GEB sample were mixed with 600 µL binding buffer, 100 µL proteinase K and 200 µL isopropanol. The mixture was loaded onto a filter tube pre-packed with glass fibers, followed by extensive washing as described in the kit's manual. Finally, the DNA was eluted with 200 µL elution buffer that was preheated at 70°C. DNA was stored at −20°C immediately after extraction.
Index tests
SatDNA OligoC-TesT. The T. cruzi satDNA OligoC-TesT (Coris BioConcept) was performed as previously described by Deborggraeve et al. [14]. The OligoC-TesT kit contains all components needed for performing the PCR and the product analysis by dipstick. Briefly, sequences of the oligonucleotides were: Tc-Sat-F primer 5′CACTCTCTGTCAATGTCTGTTTGCGTG-3′ and Tc-Sat-R primer 5′-GAAATTCCTCCAAGCAGCGGATA-3′; T. cruzi detection probe 5′-TGGACACCAAACAACCC-3′; and internal control (IC) probe 5′-AGGGTCTACTGGGTTACCTG-3′. To the 47.3 µL satDNA Ampli-Mix (Coris BioConcept), 2.5 µL sample DNA and 1 unit Hot Star Taq polymerase (Qiagen) were added and the mixture was subjected to thermal cycling as follows: 94°C for 15 minutes, 40 cycles of 94°C for 20 seconds, 65°C for 20 seconds and 72°C for 20 seconds, and a final extension at 72°C for 1 minute. Forty µL denaturated amplification product were mixed with an equal volume of OligoStrip running buffer preheated at 55°C followed by dipping the T.cruzi satDNA Oligo-Strip into the solution. Test results were read qualitatively after 5 minutes, as previously described [14].
kDNA OligoC-TesT. The T. cruzi kDNA OligoC-Test (Coris BioConcept) is identical to the satDNA OligoC-TesT except for the following specifications. Primers target the conserved region of the T. cruzi minicircle and have the following sequences: TcK-F 5′-GTTTTGGGAGGGGCGTTCAA-3′ and TcK-R 5′-TATATTACACCAACCCCAATCGAACC-3′. The detection probe was 5′-AAATAATGTACGGGGGAGATGCATG-3′ and the sequences of the IC and IC detection probe were identical as for the satDNA OligoC-TesT except for the IC that contained specific primer sites for TcK-F and TcK-R. Five µL sample DNA and 2 units of Hot Diamond Taq DNA polymerase (Eurogentec, Liège, Belgium) were added to 44.6 µL T. cruzi kDNA Ampli-mix (Coris BioConcept) and the reaction mixture was subjected to thermal cycling as follows: 95°C for 5 minutes, 40 cycles of 94°C for 20 seconds, 63°C for 20 seconds and 72°C for 20 seconds, and a final extension at 72°C for 1 minute. Forty µL denaturated amplification product were mixed with an equal volume of OligoStrip running buffer (Coris BioConcept) preheated at 55°C followed by dipping the T.cruzi kDNA Oligo-Strip into the solution. Test results were read after 10 minutes incubation. The executor of the index tests was one of the authors (KDW), a trained molecular biologist who was blinded to the results of the reference tests. The reference tests were performed at the sites of sample collection and the index tests were performed at ITM Antwerp.
Statistical analysis
Sensitivities and specificities of the OligoC-TesTs were calculated from data entered into contingency tables. Differences in sensitivity and specificity between the two tests were estimated by the McNemar test. Repeatabilities of the tests were determined using the Kappa index. All calculations were estimated at a 95% confidence interval (95% CI) using Wilson's score.
Results
Study participants
We recruited 88 healthy endemic controls and 187 Chagas patients between 2009 and 2012. Demographic characteristics of the study participants are presented in table 1. Time between sample collection and conducting the index tests was maximum six months.
Analytical sensitivity and specificity
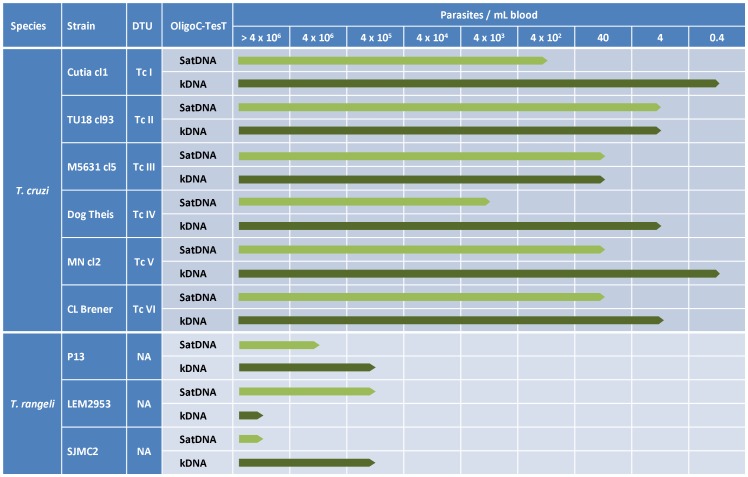
The analytical sensitivity and specificity of the T. cruzi satDNA and kDNA OligoC TesTs were evaluated on tenfold serial dilutions of DNA from reference T. cruzi strains representing the 6 DTUs and from 3 T. rangeli strains. Serial dilutions were prepared in duplicate. Considering that one parasite contains about 100 fg of DNA and taking into account the dilution factors during DNA extraction (800× for the satDNA OligoC TesT and 400× for the kDNA OligoC TesT), the detection limit of each test was expressed as the number of parasites per mL of blood that yielded a positive signal in both repetitions. An overview of the analytical sensitivities and specificities of the two OligoC-TesTs is presented in figure 1. Generally, the OligoC-TesT targeting the kDNA showed a higher analytical sensitivity (lower detection limit: 0.4 to 40 parasites/ml blood) than the OligoC-TesT targeting the satDNA (lower detection limit: 4 to 4000 parasites/ml blood). On the serial dilutions of the T. rangeli DNA the two tests showed lower detection limits of 400,000 parasites per mL of blood or higher. No cross-reaction of the satDNA OligoC-TesT with Leishmania DNA was observed in our proof-of-concept study reported in 2009 [14]. In the current study, we analyzed 5 ng L. chagasi DNA with the kDNA OligoC-TesT and the test remained negative.
Figure 1. Analytical sensitivity and analytical specificity of the T. cruzi satDNA OligoC-TesT and kDNA OligoC-TesT on DNA from 6 T. cruzi and 3 T. rangeli reference strains.
DTU = discrete typing unit, NA = not applicable.
Diagnostic accuracy (table 2)
Table 2. Sensitivities and specificities of the T. cruzi satDNA and kDNA OligoC-TesTs on 187 Chagas patients and 114 endemic and non-endemic controls from Chile, Argentina, Spain, Sudan and D.R. Congo.
| SatDNA OligoC-TesT | kDNA OligoC-TesT | ||||||||||
| Origin | Number of specimens per participant group | Sensitivity % (95% CI) | Specificity % (95% CI) | Sensitivity % (95% CI) | Specificity % (95% CI) | ||||||
| Diseased non-endemic controls | Healthy endemic controls | Chagas disease patients | I | II | I | II | I | II | I | II | |
| Sudan | 15 | 100 (79.6–100) | 100 (79.6–100) | 100 (79.6–100) | 100 (79.6–100) | ||||||
| D.R. Congo | 11 | 100 (74.1–100) | 100 (74.1–100) | 100 (74.1–100) | 100 (74.1–100) | ||||||
| Chile | 88 | 80 | 78.6 (68.6–86.3) | 67.5 (56.6–76.8) | 98.9 (93.8–99.8) | 94.3 (87.4–97.6) | 93.8 (86.2–97.3) | 91.3 (83.0–95.7) | 96.6 (90.5–98.8) | 98.7 (93.8–99.8) | |
| Argentina | 73 | 49.3 (38.2–60.5) | 53.4 (42.1–64.4) | 63.0 (51.6–73.2) | 52.1 (40.8–63.1) | ||||||
| Spaina | 34 | 82.4 (66.5–91.7) | 58.8 (42.2–73.6) | 79.4 (63.2–89.7) | 76.5 (60.0–87.6) | ||||||
Notes: 95% CI = 95% confidence interval; Non-end. = Non-endemic; I = first repetition; II = second repetition; N.D. = not done due to limited number of test kits available;
a All Chagas patients recruited at the Hospital Ramón y Cajal in Madrid were from Bolivian origin.
Sensitivities, specificities and repeatability of the T. cruzi satDNA and kDNA OligoC-TesTs were calculated on the 26 diseased non-endemic controls, 88 healthy endemic controls and 187 Chagas patients.
Both OligoC-TesTs showed high specificity on the diseased non-endemic and healthy endemic controls, ranging from 94.3% to 100%. The overall specificities in these participant groups were 99.1% (95% CI 95.2%–99.8%) for the satDNA OligoC-TesT and 97.4% (95% CI 92.6%–99.1%) for the kDNA OligoC-TesT. No significant difference in specificity was observed between the two tests (p = 0.317).
Considering all Chagas patients recruited in the study, the kDNA OligoC-TesT showed a significantly higher sensitivity (79.1%, 95% CI 72.8%–84.4%) than the satDNA OligoC-TesT (67.9%, 95% CI 60.9%–74.2%) (p = 0.0004). Sensitivities of the OligoC-TesTs on the Chagas patients from Argentina were generally low and ranged from 49.3 to 63.0%. The overall repeatability of the kDNA OligoC-TesT (kappa = 0.85, 95% CI: 0.74–0.96) was higher than of the satDNA OligoC-TesT (kappa = 0.66, 95% CI: 0.54–0.77).
Discussion
Recently, the T. cruzi satDNA OligoC-TesT was developed as a standardised format for molecular detection of T. cruzi satDNA [14]. Here, we report on the development of a second prototype, the T. cruzi kDNA OligoC-TesT, and the phase II evaluation of both tests on human target populations in different endemic regions.
With representative strains of the 6 T. cruzi DTUs, we observed that the T. cruzi kDNA OligoC-TesT, detecting between 0.4 and 40 parasites/mL which corresponds with 0.02 to 2 fg DNA per µL, had a higher (DTU I, IV, V and VI) or equal (DTU II and III) analytical sensitivity than the T. cruzi satDNA OligoC-TesT. These values are comparable to the results obtained in the PCR standardisation study of Schijman et al. [13] wherein the authors reported detection limits between 0.01 and 10 fg/µL for the four best performing PCR's. Both OligoC-TesTs amplified purified T. rangeli DNA, but required between 102 and 107 times more template DNA than for T. cruzi. For the satDNA OligoC-TesT, this is probably due to the number of satDNA repeats being about 1000 times lower in T. rangeli than in T. cruzi [17]. For the kDNA OligoC-TesT, the higher detection limit for T. rangeli, was obtained by one base pair mismatch between the reverse primer and the kDNA sequence of T. rangeli.
The diagnostic specificity of the two OligoC-TesTs was 100% on diseased non-endemic controls (Sudan and D.R. Congo) and between 94% and 99% on healthy endemic controls (Chile). A systematic review of the diagnostic accuracy of PCR for chronic Chagas disease showed that most PCR evaluation studies found similar high specificities [18]. In a next step, prospective evaluation studies with consecutive enrolment of suspected cases should be conducted to have a more accurate estimation of the specificity. When considering all Chagas patients recruited in the study, sensitivities of the OligoC-TesTs were moderate (79.1% for the kDNA OligoC-TesT and 67.9% for the satDNA OligoC-TesT), but comparable with reported sensitivities for other T. cruzi PCR's applied on chronic Chagas patients. In the review cited above, sensitivities ranging from 50 to 90% were observed [18]. Similar sensitivities, from 63% to 69%, were reported for the four best performing molecular diagnostics in the multicentre validation study conducted by Schijman et al. [13]. Particularly during the chronic phase, T. cruzi parasites circulate in very low and variable numbers in the bloodstream [19], [20]. Therefore, although PCR appears to be a sensitive method for the detection of T. cruzi DNA, it may remain negative in patients with very low or intermittent parasitaemia. Recovering DNA from larger blood volumes than the 200 µl used in this study would enhance sensitivity. The sensitivities of the two OligoC-TesTs greatly varied between the different cohorts. We observed a lower sensitivity of the OligoC-TesTs in the patients from Argentina compared to the patients from Chile and Spain. All blood samples in the study were collected, stored and shipped following a standardized procedure. We checked for degradation of the DNA in the Argentinean blood samples using primers targeting the human cytochrome b gene [21]. All samples were positive in this control PCR indicating no DNA degradation (data not shown). Compared to the other cohorts, the Argentinean patients showed a lower percentage of patients with Chagas symptoms which may be related with parasite load. Recently, Moreira et al. estimated the parasite loads in the blood of chronic Chagas patients from Argentina, Brazil and Colombia [22]. The median parasite load in the patients from Argentina was 1.93 parasites per mL of blood which was lower than patients from Colombia but higher than from Brazil. Genetic differences of parasite strains and/or DTUs might influence parasitaemia in man and partially explain the discrepancies of PCR sensitivity between cohorts from different endemic areas. However, no association between DTU and parasitaemia in the blood of patients has been reported yet [23]. In addition, T. cruzi DTUs vary in DNA gene copy number, thus harbouring a variable number of repeats of the PCR targets. Elias et al. described that the satDNA sequences can be 4 to 6 times more abundant in one DTU compared to another [16]. Since DTU typing in the specimens in our study was not performed, no conclusions can be made regarding this aspect. In view of the high sensitivity and PCR dependence of the OligoC-TesTs it is essential to use standard precautions during collection of blood and DNA extractions to avoid cross contamination between samples, and to include sufficient negative controls to detect any such contamination.
The overall repeatability of the kDNA OligoC-TesT was good (kappa = 0.85) and higher than of the satDNA OligoC-TesT (kappa = 0.66). Repeatabilities of the OligoC-Tests are linked to the detection limit, thus sensitivity, which was also higher for the kDNA OligoC-TesT. Therefore, moderate repeatabilities might be caused by the target DNA content in the samples that is situated too close to the lower detection limits of the tests.
In conclusion, the diagnostic accuracies of two standardised PCR formats, the satDNA OligoC-TesT and the kDNA OligoC-TesT, were evaluated in a multi-cohort study on 301 persons from various endemic and non-endemic countries. The specificities of the two tests were high in non-endemic controls as well as healthy endemic controls. The kDNA OligoC-TesT prototype showed a significantly higher sensitivity compared to the satDNA OligoC-TesT. However, it is unlikely that the OligoC-TesTs will play a major role in the diagnosis of chronic Chagas disease because of their low sensitivity compared to standard serological tests. Furthermore, the OligoC-TesTs are restricted to laboratories with PCR facilities, trained personnel and infrastructures that reduce the risk of PCR contamination. Their impact outside the main health centers will thus be limited. In a next phase, the kDNA OligoC-TesT should be evaluated in specific niches where standard serological tools have their limitations, e.g. diagnosing newborns and HIV co-infected patients and follow-up after treatment.
Supporting Information
STARD checklist showing that all essential elements of a diagnostic evaluation study are included in the manuscript.
(PDF)
STARD flowchart describing the design of the study and the flow of the participants.
(PDF)
Acknowledgments
We thank Prof. Dr. Michael Miles and Dr. Martin Llewellyn for providing genomic DNA from T. cruzi and T. rangeli and Dr. Gert Van der Auwera for providing genomic DNA from Leishmania chagasi. We are grateful to Prof. Dr. Michael Miles for critical reading of the manuscript.
Funding Statement
This work was financially supported by the European Commission Seventh Framework Programme, ChagasEpiNet project, grant number 223034. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. World Health Organization (2010) Chagas disease (American trypanosomiasis) fact sheet (revised in June 2010). Wkly Epidemiol Rec 34: 334–336. [PubMed] [Google Scholar]
- 2. Schmunis GA, Yadon ZE (2010) Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115: 14–21. [DOI] [PubMed] [Google Scholar]
- 3. Coura JR, Junqueira ACV, Fernandes O, Valente SVA, Miles MA (2002) Emerging Chagas disease in Amazonian Brazil. Trends Parasitol 18: 171–176. [DOI] [PubMed] [Google Scholar]
- 4. Higuchi ML, Benvenuti LA, Martins Reis M, Metzger M (2003) Pathophysiology of the heart in Chagas' disease: current status and new developments. Cardiovasc Res 60: 96–107. [DOI] [PubMed] [Google Scholar]
- 5. Zingales B, Andrade SG, Briones MRS, et al. (2009) A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 6.Luquetti AO, Schmuñis GA. (2010) Diagnosis of Trypanosoma cruzi Infection. In: Telleria J, Tibayrenc M, editors. American trypanosomiasis: Chagas disease One Hundred Years of Research. Amsterdam: Elsevier. 743–792. [Google Scholar]
- 7. World Health Organization (2002) Control of Chagas disease. WHO Technical Report Series 905: 1–99. [PubMed] [Google Scholar]
- 8. Corredor Arjona A, Alvarez Moreno CA, Agudelo CA, et al. (1999) Prevalence of Trypanosoma cruzi and Leishmania chagasi infection and risk factors in a Colombian indigenous population. Rev Inst Med Trop Sao Paulo 41: 229–234. [DOI] [PubMed] [Google Scholar]
- 9. Cuba CA (1998) Review of the biologic and diagnostic aspects of Trypanosoma (Herpetosoma) rangeli . Rev Soc Bras Med Trop 31: 207–220. [DOI] [PubMed] [Google Scholar]
- 10. Virreira M, Torrico F, Truyens C, et al. (2003) Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am J Trop Med Hyg 68: 574–582. [DOI] [PubMed] [Google Scholar]
- 11. Sánchez G, Coronado X, Zulantay I, et al. (2005) Monitoring the efficacy of specific treatment in chronic Chagas disease by polymerase chain reaction and flow cytometry analysis. Parasite 12: 353–357. [DOI] [PubMed] [Google Scholar]
- 12. Russomando G, de Tomassone MMC, de Guillen I, et al. (1998) Treatment of congenital Chagas' disease diagnosed and followed up by the polymerase chain reaction. Am J Trop Med Hyg 59: 487–491. [DOI] [PubMed] [Google Scholar]
- 13. Schijman AG, Bisio M, Orellana L, et al. (2011) International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis 5: e931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deborggraeve S, Coronado X, Solari A, et al. (2009) T. cruzi OligoC-TesT: a simplified and standardized polymerase chain reaction format for the diagnosis of Chagas disease. PLoS Negl Trop Dis 3: e450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sloof P, Bos JL, Konings AFJM, et al. (1983) Characterization of satellite DNA in Trypanosoma brucei and Trypanosoma cruzi . J Mol Biol 167: 1–21. [DOI] [PubMed] [Google Scholar]
- 16. Elias MCQB, Vargas NS, Zingales B, Schenkman S (2003) Organization of satellite DNA in the genome of Trypanosoma cruzi . Mol Biochem Parasitol 129: 1–9. [DOI] [PubMed] [Google Scholar]
- 17. Brenière SF, Bosseno MF, Barnabé C, Urdaneta-Morales S, Tibayrenc M (1993) Copy number differences in the 195 bp repeated satellite DNA from Trypanosoma cruzi and Trypanosoma rangeli: potential use for epidemiologic surveys. Mem Inst Oswaldo Cruz 88: 163–165. [DOI] [PubMed] [Google Scholar]
- 18. Brasil PE, De Castro L, Hasslocher-Moreno AM, Sangenis LH, Braga JU (2010) ELISA versus PCR for diagnosis of chronic Chagas disease: systematic review and meta-analysis. BMC Infect Dis 10: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castro C, Prata A (2000) Absence of both circadian rhythm and Trypanosoma cruzi periodicity with xenodiagnosis in chronic chagasic individuals. Rev Soc Bras Med Trop 33: 427–430. [DOI] [PubMed] [Google Scholar]
- 20. Galvão LMC, Chiari E, Macedo AM, Luquetti AO, Silva SA, Andrade AL (2003) PCR assay for monitoring Trypanosoma cruzi parasitemia in childhood after specific chemotherapy. J Clin Microbiol 41: 5066–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kocher TD, Thomas WK, Meyer A, et al. (1989) Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci USA 86: 6296–6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreira OC, Ramírez JD, Velázquez E, et al. (2013) Towards the establishment of a consensus real-time qPCR to monitor Trypanosoma cruzi parasitemia in patients with chronic Chagas disease cardiomyopathy: a substudy from the BENEFIT trial. Acta Trop 125: 23–31. [DOI] [PubMed] [Google Scholar]
- 23. Zingales B, Miles MA, Campbell DA, et al. (2012) The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol 12: 240–253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STARD checklist showing that all essential elements of a diagnostic evaluation study are included in the manuscript.
(PDF)
STARD flowchart describing the design of the study and the flow of the participants.
(PDF)