Abstract
Catalase is an iron porphyrin enzyme, which serves as an efficient scavenger of reactive oxygen species (ROS) to avoid oxidative damage. In sugarcane, the enzymatic activity of catalase in a variety (Yacheng05–179) resistant to the smut pathogen Sporisorium scitamineum was always higher than that of the susceptible variety (Liucheng03–182), suggesting that catalase activity may have a positive correlation with smut resistance in sugarcane. To understand the function of catalase at the molecular level, a cDNA sequence of ScCAT1 (GenBank Accession No. KF664183), was isolated from sugarcane infected by S. scitamineum. ScCAT1 was predicted to encode 492 amino acid residues, and its deduced amino acid sequence shared a high degree of homology with other plant catalases. Enhanced growth of ScCAT1 in recombinant Escherichia coli Rosetta cells under the stresses of CuCl2, CdCl2 and NaCl indicated its high tolerance. Q-PCR results showed that ScCAT1 was expressed at relatively high levels in the bud, whereas expression was moderate in stem epidermis and stem pith. Different kinds of stresses, including S. scitamineum challenge, plant hormones (SA, MeJA and ABA) treatments, oxidative (H2O2) stress, heavy metal (CuCl2) and hyper-osmotic (PEG and NaCl) stresses, triggered a significant induction of ScCAT1. The ScCAT1 protein appeared to localize in plasma membrane and cytoplasm. Furthermore, histochemical assays using DAB and trypan blue staining, as well as conductivity measurement, indicated that ScCAT1 may confer the sugarcane immunity. In conclusion, the positive response of ScCAT1 to biotic and abiotic stresses suggests that ScCAT1 is involved in protection of sugarcane against reactive oxidant-related environmental stimuli.
Introduction
Sugarcane smut, a prevalent and worldwide disease of sugarcane, is caused by the basidiomycete Sporisorium scitamineum (S. scitamineum). The characteristic symptom of this disease is the emergence of black whips after three months of exposure to infection with smut [1]. The tainted buds may either produce symptoms, or exist as a latent infection and produce black whips in the following season [2]. The enormous quantity of teliospores as well as the quick spread within the sugarcane-producing area makes it almost impossible to completely eliminate this disease. Smut usually results in poor cane growth with profuse tillering, spindly shoots, and narrow leaves, therefore causing considerable loss in yield and sugar content [3]. The release of smut resistant sugarcane varieties, correct quarantine and integrated field management are three main pathways for the control this disease [1]. It is reported that the rates and patterns of colonization of S. scitamineum differ in resistant and susceptible sugarcane tissues [4]. Solas et al. found that buds of the resistant sugarcane cultivar were not subjected to intracellular penetration by S. scitamineum compared to that of the susceptible cultivar [5]. Susceptible cultivars produce a large number of sori which develop earlier than that in resistant cultivars [6]. Therefore, breeding for smut resistant sugarcane varieties has proved to be the most effective method [7].
Due to the complicated genetic background (a polyploid-aneuploid genome) and pressures of breeding selection (the interaction among sugarcane, smut pathogen and environmental factors), many years and multipoint resistance evaluation tests are needed to obtain relatively high smut resistant sugarcane variety [8]. Alternatively, genetic modification, with directional improvement and molecular assisted breeding technology linked to a target trait, is an alternative way to obtain a resistant variety more quickly and efficiently [9]. By introducing disease-resistance genes to improve gene expression, or by silencing disease-susceptible genes to increase resistance, genetic engineering has made it practical to generate smut resistant sugarcane cultivars [10].
Catalase (E.C.1.11.1.6; H2O2:H2O2 oxidoreductase; CAT) is an iron porphyrin enzyme, mostly localized in peroxisomes [11]. It serves as an efficient scavenger of reactive oxygen species (ROS). The main function of catalase is to remove excessive H2O2 (hydrogen peroxide) during developmental process or biotic/abiotic stress, to avoid oxidative damage [12]. Plant catalases are composed of a multi-gene family and have been reported in many plant species [13]. There are three members identified in Arabidopsis thaliana [14], Nicotiana tabacum and Zea mays [15], [16], two in Hordeum vulgare [17], one in Solanum lycopersicum [18]. In the catalase gene family, different members encode distinct catalase proteins that exhibit different patterns of subcellular localization and expression regulation [19].
The expression of various plant catalase genes is regulated temporally and spatially and responds to developmental and environmental oxidative stimuli [11], [13], [20], [21]. In Panax ginseng, PgCat1 gene was expressed at different levels in leaves, stems, roots of P. ginseng seedlings and was induced by different stresses including heavy metals, osmotic agents, plant hormones and high light irradiances [11]. Kwon and An cloned a Capsicum annuum catalase cDNA, and northern hybridization showed its transcript was more abundant in stems than in leaves and roots, and more in the early stages than that in the mature stage of fruit development [19]. They also found that aluminum, sodium chloride (NaCl) and light treatment could induce its transcript. Previous research also revealed that the expression of three different maize catalase genes was regulated differentially in response to developmental phase or the fungal toxin cercosporin [22], abscisic acid (ABA) and salicylic acid (SA) [16], [22]. Wang et al. found increased transcription of a catalase gene (MmeCAT) in resistant clam Meretrix meretrix which indicated that MmeCAT could most probably benefit the immune system of clams to defend against pathogen infection [23]. The positive response of catalase genes to various stimuli suggested that catalase may help to protect the plant against reactive oxidant related environmental stresses. It is therefore interesting to determine the role of sugarcane catalases and their encoding genes in response to biotic and abiotic stresses.
To date, a partial cDNA sequence (GenBank Accession No. CF572408.1) similar to catalase has been cloned from Saccharum hybrid cultivar Q117 [24], while its function remained unclear. In the present research, we analyzed the differences of sugarcane catalase enzyme activity between Yacheng05–179 (resistant) and Liucheng03–182 (susceptible) inoculated with S. scitamineum. A novel full-length catalase gene ScCAT1 (GenBank Accession No. KF664183) from sugarcane bud infected by S. scitamineum pathogen was cloned and characterized. Its response in Escherichia coli (E. coli) Rosetta strains, subcellular localization and expression patterns in sugarcane tissues under various biotic/abiotic stresses were reported. Agrobacterium-mediated transient expression of this gene in Nicotiana benthamiana (N. benthamiana) was used to functionally test ScCAT1.
Materials and Methods
Plant Materials and Treatments
Smut whips were collected in the most popular cultivar “ROC”22 in the Key Laboratory of Sugarcane Biology and Genetic Breeding, Ministry of Agriculture (Fuzhou, China), and stored at 4°C. Sugarcane varieties of Yacheng05–179 (smut resistant) and Liucheng03–182 (smut susceptible) were cultivated in the Key Laboratory of Sugarcane Biology and Genetic Breeding, Ministry of Agriculture (Fuzhou, China). All of the treatments were repeated independently three times.
For tissue distribution studies, six healthy 10 month old plants were selected. For each plant, the youngest fully expanded leaf viz +1 leaf with a visible dewlap (the collar between the leaf blade and sheath), all the buds, stem epidermis and the stem pith were taken for RNA extraction.
During biotic treatments, two-bud sets of both sugarcane genotypes, Yacheng05–179 and Liucheng03–182, were inoculated with 0.5 µL suspension containing 5×106 spores·mL−1 in 0.01% (v/v) Tween-20, while controls were mock inoculated with 0.01% (v/v) Tween-20 in sterile distilled water instead of spores [25], [26]. All the inoculated sets were grown at 28°C in condition of 12 h light/12 h dark. Five buds from each of both genotypes were collected at each of the time point of 0 h, 6 h, 12 h, 24 h, 48 h, 72 h and 96 h. Samples were frozen in liquid nitrogen, and stored at −80°C.
During abiotic treatments, uniform four-month-old sugarcane tissue cultured plantlets of Yacheng05–179 were grown in water for one week and then transferred to the following seven different treatments in conical tubes at 28°C with 16 h light/8 h dark. The plantlets were treated with 5 mM SA solution, 25 µM MeJA (methyl jasmonate) in 0.1% (v/v) ethanol and 0.05% (v/v) Tween-20, 100 µM ABA, and 25% PEG (polyethylene glycol), and the plantlets were set to different periods of time (0 h, 6 h, 12 h and 24 h), respectively. In addition, plantlets were separately treated with 250 mM NaCl and 100 µM CuCl2 (copper chloride) for 0 h, 12 h, 24 h and 48 h [27], [28] . For H2O2 stress, the leaves were sprayed with 10 mM H2O2, and the sampling time points were 0 h, 6 h, 12 h and 24 h, respectively. After treatments, three sugarcane plantlets per time point were collected and immediately fixed in liquid nitrogen, and then kept at −80°C until used for analysis.
Enzyme Extraction and Activity Assay
To analyze quantitative change in catalase activity in Yacheng05–179 and Liucheng03–182 after inoculation with smut pathogen, 0 h, 6 h, 12 h, 24 h, 48 h, 72 h and 96 h buds were sampled as above. Controls were mock inoculated with sterile distilled water. The frozen buds of 0.5 g were homogenized in a mortar and pestle with 3.0 mL of ice-cold phosphoric acid buffer (pH7.8) and a small amount of quartz sand. The supernatant was centrifuged at 4,000 ×g for 15 min at 4°C. The supernatant was used as a crude enzyme solution. After incubation at 25°C (for blank control, incubated in a boiling water for 10 min), 0.2 mL supernatant was mixed with 1.5 mL phosphoric acid buffer (pH7.8) added with polyvinylpyrrolidone (PVP) and 0.3 mL 0.1 mol/L H2O2 in 10 mL tube, which initiated the reaction. The decrease in absorbance was recorded by Lambda 35 UV WinLab software (Perkin Elmer, China) followed by the decomposition of H2O2 at 240 nm, and measured for a total of 3 min [29]. One unit of enzyme activity (U) was defined as A240 reduced 0.1 enzyme quantity per min per g. The enzyme activity was calculated as follows:
Among them, AS0 means the absorbance of the blank control, AS1 and AS2 stand for the absorbance of the samples, VT means the total volume of the crude enzyme solution (mL), V1 represents the volume of the detected crude enzyme solution (mL), FW means the fresh weight of sample (g) and t means the time from adding H2O2 to the last time (min). The activity of catalase was calculated by the activity level of inoculation minus the level of the mock at each corresponding time point.
RNA Extraction
Total RNA of Yacheng05–179 and Liucheng03–182 was extracted with Trizol reagent (Invitrogen, China) according to the manufacturer’s protocol. The quality of the RNA was monitored by measuring the absorbance at 260 nm, 280 nm (NanoVue plus, GE, USA), and the 28 S and 18 S were examinated by electrophoresis. DNase I (Promega, China) was used to remove DNA contamination. The first-strand cDNA synthesis was completed by the Prime-Script™ RT Reagent Kit (TaKaLa, China).
Isolation of Sugarcane Catalase Gene
Eighty-six sugarcane expressed sequence tags (ESTs), which share homology to the mRNA sequence of the sugarcane catalase gene (GenBank Accession No. CF572408.1), were obtained from sugarcane sequence database (cultivated sugarcanes (taxid:286192); wild sugarcane (taxid:62335); sugarcane (taxid:128810); sugarcane (taxid:4547)) in GenBank. The CAP3 sequence assembly program (http://pbil.univ-lyon1.fr/cap3.php) was used to construct a putative cDNA sequence of sugarcane catalase gene (ScCAT1). Then the cDNA of ScCAT1 was amplified with primers designed on the basis of this assembled sequence.
Amplification of ScCAT1 gene was performed with primers ScCAT1-cDNAF and ScCAT1-cDNAR (Table 1) on first-strand cDNA template of Yacheng05–179 post 48 h S. scitamineum inoculation in a Mastercycler (Eppendorf, Hamburg, Germany). The reaction was performed at 94°C for 5 min, and then subjected to 35 cycles of 94°C for 30 s, 57°C for 45 s and 72°C for 1 min, followed by a final extension at 72°C for 10 min. The expected length of the amplified fragments was 1,658 bp. PCR products were gel-purified, cloned into the pMD18-T vector (TaKaLa, China) and sequenced (Shenggong, China).
Table 1. Primers used in this study.
| Primer | Sequence | Strategy |
| ScCAT1-cDNAF | GCGGCTTCCTACTCCTCGTCCTT | RT-PCR |
| ScCAT1-cDNAR | CGCCTGCTTTCTTCCTTGTCAATC | RT-PCR |
| ScCAT1-SublocF | TGCTCTAGAATGGATCCGTACAAGCAC | Subcellular localization vector construction |
| ScCAT1-SublocR | GGACTAGTCATGTTTGGCTTCAGGTTCAG | Subcellular localization vector construction |
| ScCAT1-32aF | CGGAATTCATGGATCCGTACAAGCAC | prokaryotic expression vector construction |
| ScCAT1-32aR | CCCTCGAGTTACATGTTTGGCTTCAGGT | prokaryotic expression vector construction |
| GAPDH-QF | CACGGCCACTGGAAGCA | Q-PCR |
| GAPDH-QR | TCCTCAGGGTTCCTGATGCC | Q-PCR |
| ScCAT1-QF | CTCTGCTCCTCCAATCCC | Q-PCR |
| ScCAT1-QR | GAGTGACCTCAAAGAAACCCT | Q-PCR |
| ScCAT1-1301F | GCTCTAGAATGGATCCGTACAAGCACCG | Over expression vector construction |
| ScCAT1-1301R | TCCCCCGGGTTACATGTTTGGCTTCA | Over expression vector construction |
Protein Structural Analysis and Phylogenetic Tree Construction
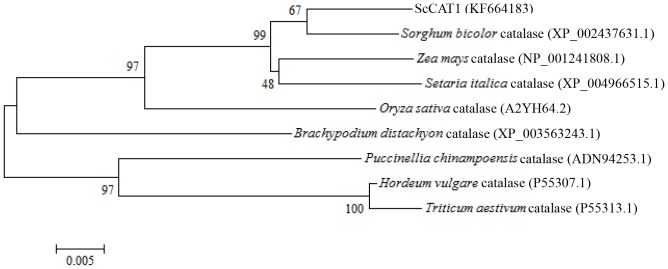
Sequence data were analyzed by ORF (open reading frame) Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html), ProtParam (http://web.expasy.org/protparam/), SignalP 4.0 Server (http://www.cbs.dtu.dk/services/SignalP/), TargetP 1.1 server (http://www.cbs.dtu.dk/services/TargetP/), SMART (http://smart.embl-heidelberg.de/), PSORT Prediction (http://psort.hgc.jp/form.html). After blast comparison, the amino acid sequence of ScCAT1 was aligned with published plant catalases, including Sorghum bicolor catalase (XP_002437631.1), Z. mays catalase (NP_001241808.1), Oryza sativa catalase (A2YH64.2), Brachypodium distachyon catalase (XP_003563243.1), Puccinellia chinampoensis catalase (ADN94253.1), H. vulgare catalase (P55307.1), Triticum aestivum catalase (P55313.1) and Setaria italica catalase (XP_004966515.1). Multiple alignment of the amino acid sequences was carried out using the Clustal W software. The phylogenetic tree was constructed following the neighbor-joining (NJ) method (1,000 bootstrap replicates) by using the MEGA 5.05 software [13].
Agrobacterium-mediated Transient Expression and Subcellular Localization Assay
For the studying of subcellular location constructs of pCAMBIA 2300-GFP were generated, ScCAT1 gene was PCR amplified from pMD18-T-ScCAT1 using primers ScCAT1-SublocF and ScCAT1-SublocR (Xba I and Spe I sites) as indicated in Table 1. The fragment was inserted into the vector of pCAMBIA 2300-GFP to construct the fusion protein expression vector of 35S::ScGluA1::GFP (Fig. S1). The recombinant plasmid, was verified by PCR, double digestion and sequencing followed by transfection of the competent cells of Agrobacterium tumefaciens strain EHA105.
The assay for Agrobacterium-mediated transformation referred to the method as previously described [30]. Agrobacterium strain EHA105 carrying the indicated construct was grown overnight in LB liquid medium containing 35 µg·mL−1 rifampicin and 50 µg·mL−1 kanamycin. The suspension at OD600 = 0.8 (containing 200 µM acetosyringone) was infiltrated into 4–5 weeks old N. benthamiana leaves and cultured at 24°C for 2 days (16 h light/8 h darkness). The subcellular localization of the fusion protein was visualized using fluorescence microscopy (Axio Scope A1, Germany).
Expression in Escherichia coli Rosetta Cells
To study the function of ScCAT1 in prokaryotes, the ScCAT1 ORF was amplified by PCR from the identified cDNA clone using the primers ScCAT1-32aF and ScCAT1-32aR (Table 1) followed by 94°C for 4 min; 94°C for 30 s, 58°C for 30 s, 72°C for 1.5 min, 35 cycles; and 72°C for 10 min. The ScCAT1 ORF with EcoR I and Xho I sites was subcloned into pET 32a (+) vector with EcoRI-XhoI sites in the E. coli Rosetta strains to generate the putative recombinant (pET 32a-ScCAT1). The desired recombinant plasmid was identified by PCR amplification, double digestion and sequencing. The prokaryotic expression product was induced in 1.0 mM isopropyl β-D-thiogalactoside (IPTG) for 8 h at 37°C and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Meanwhile, LB medium with blank E. coli Rosetta strains (blank) or Rosetta+pET 32a (control) was each induced in IPTG for 8 h and also analyzed by SDS-PAGE [26], [31].
During the response of E. coli cells to various abiotic stresses, the growth of E. coli Rosetta strains transformed with pET 32a and pET 32a-ScCAT1 was analyzed using spot assay in different treatments of CuCl2, CdCl2 or NaCl. When OD600 of the LB medium (plus 170 µg·mL−1 chloramphenicol and 80 µg·mL−1 ampicillin) with E. coli cells reached 0.6, IPTG was added to a final concentration of 1.0 mM, and then continued growth for 12 h at 37°C. Thereafter, the cultures were diluted to 0.6 (OD600), and then to two levels (10−3 and 10−4). Ten microlitres from each dilutions was spotted on LB plates (plus 170 µg·mL−1 chloramphenicol and 80 µg·mL−1 ampicillin) containing CuCl2 (250, 500 and 750 µM), CdCl2 (250, 500 and 750 µM) or NaCl (250, 500 and 750 mM) [26], [31]. All these plates were cultured in 37°C overnight and photographed.
The effect of 750 µM CuCl2, 750 µM CdCl2 and 250 mM NaCl on the growth of E. coli strains with pET 32a-ScCAT1 or pET 32a was studied in LB medium followed with Su et al. [26]. As above, when cells were grown as earlier described and then diluted to 0.6 (OD600), 400 µL of cells were transferred into 10 mL of LB medium containing 170 µg·mL−1 chloramphenicol and 80 µg·mL−1 ampicillin, 750 µM CuCl2 or 750 µM CdCl2 or 250 mM NaCl [32]. Cultures were shaken at 200 rpm at 37°C and growth of the cells was measured at every 2 h by Lambda 35 UV WinLab software (Perkin Elmer, USA).
Real-time Quantitative PCR Analysis
The each time point of 0 h, 6 h, 12 h, 48 h and 72 h during Yacheng05–179-smut incompatible interaction and Liucheng03–182-smut compatible interaction, as well as mock plants inoculated with sterile distilled water at each corresponding time point, were used to analyze the expression patterns of the ScCAT1. The relative expression of the target gene under certain biotic stress was calculated by the expression level of the inoculated sample minus the level of the mock at each corresponding time point. For tissue-specific expression of ScCAT1, the leaf, bud, stem epidermis and stem pith of sugarcane variety Yacheng05–179 were used as experimental materials. The expression of ScCAT1 under the stresses of SA, MeJA, ABA, H2O2, PEG, CuCl2 and NaCl were also performed by real-time quantitative PCR (Q-PCR).
The method of Q-PCR followed the instruction of the SYBR Green Master (ROX) (Roche, China) on a 7500 Q-PCR system (Applied Biosystems, USA). The GAPDH gene (GAPDH-QF/GAPDH-QR) (Table 1) was chosen as the internal control of the Q-PCR [28]. According to the sequence of ScCAT1, a pair of specific primers ScCAT1QF/ScCAT1-QR was designed using the Primer Premier 5.0 software. Q-PCR was carried out with FastStart Universal SYBR Green Master (ROX) in a 20 µL volume containing 10 µL FastStart Universal SYBR Green PCR Master (ROX), 0.5 µM of each primer and 2.0 µL template (100 × diluted cDNA). PCR with distilled water as template was performed as control. The Q-PCR reaction condition was held at 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. When the reaction was complete, the melting curve was analyzed. Each Q-PCR was repeated three times. The 2−△△Ct method was adopted to analyze the Q-PCR results [33].
Histochemical Assay
For analysis of defense response caused by ScCAT1 over-expression, primers of ScCAT1-1301F/ScCAT1-1301R in Table 1 (Xba I -Sma I sites) were used to construct binary vector expressing ScCAT1 (pCAMBIA 1301-ScCAT1). Agrobacterium strain EHA105 containing recombinant vector and pCAMBIA 1301 vector alone were grown overnight in LB liquid medium (plus 35 µg·mL−1 rifampicin and 50 µg·mL−1 kanamycin) at 28°C. Then cultures were pelleted and resuspended in MS liquid medium (plus 200 µM acetosyringone) at OD600 = 0.8 and infiltrated into N. benthamiana leaves at eight-leaf stage [34]. Plants were incubated at 24°C for 1–2 days (16 h light/8 h darkness), which were employed to following different tests.
DAB (3,3′-diaminobenzidinesolution) staining. Agroinfiltrated leaves were incubated in 1.0 mg·mL−1 DAB-HCl solution in the dark overnight. Then the leaves were destained by boiling in 95% ethanol for 5 min. The bronzing color of the leaves for H2O2 detection which generated in leaves after treatments was photographed [35].
Trypan blue staining. The infiltrated leaves were boiled for 5 min in lactophenol-ethanol trypan blue solution (10 mL glycerol, 10 mL lactic acid, 10 g phenol, 10 mg trypan blue, 30 mL absolute ethanol and 10 mL distilled water). Then the leaves were destained in 2.5 g·mL−1 choloral hydrate in distilled water and the blue color indicates the cell death [30].
Measurement of ion conductivity. It was performed as previously described with some modifications [36]. Six leaf discs (11 mm in diameter per leaf) were cut and washed in distilled water and then incubated in 20 mL of distilled water and shaken slowly at room temperature for 60 min. After that, electrolyte leakage was measured using a conductivity meter (SevenEasy, METTLER TOLEDO, Switzerland).
Results
Enzyme Activity of Catalase
To analyze the correlation between catalase activity and smut resistance, the changes in enzyme activity in smut challenged Yacheng05–179 (resistant) and Liucheng03–182 (susceptible) cultivars were studied and different patterns of enzyme activity change were found. As shown in Fig. 1, activity of catalase in Yacheng05–179 increased at 6 h (118.19 U) and reached the peak value of 254.14 U at 24 h compared to its mock. It should be noted that the catalase activity in the resistant sugarcane variety (Yacheng05–179) was always higher than that of the mock at all the sampling time points, but the tendency was an increased at 12 h and 120 h, decreased at 6 h and 24 h and almost unchanged at 0 h, 48 h, 72 h in the susceptible one (Liucheng03–182). In addition, when compared with the mock, the catalase activity was much higher in the resistant variety (from 40.00 U to 254.14 U) than that in the susceptible one (from −76.94 U to 52.97 U) at all the time points. These results suggest that there are positive correlations between catalase activity and smut resistance in sugarcane.
Figure 1. The catalase activity in smut resistant (Yacheng05–179) and smut susceptible (Liucheng03–182) sugarcane varieties inoculated with Sporisorium scitamineum.
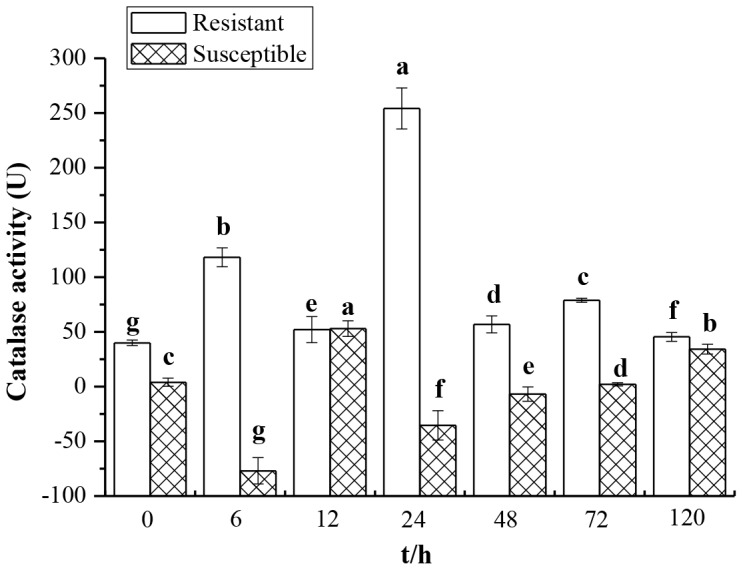
All data points (deduction its mock) are means±SE (n = 3).
Cloning and Sequence Analysis of ScCAT1
To study the sugarcane catalase at the molecular level, a 1,658 bp full-length catalase gene ScCAT1 (GenBank Accession No. KF664183) was cloned using RT-PCR method combined with in silico cloning technique. There were 1,479 nucleotides in its ORF (Fig. S2). ScCAT1 encoded a predicted polypeptide of 492 amino acids with no signal peptide. The predicted protein had a molecular mass of 56.81 kDa with a pI of 6.72. A search at the NCBI for conserved protein domains indicated that ScCAT1 belonged to a member of catalase-like superfamily. The catalytic active site and heme binding motifs of ScCAT1 were detected by Motif Scan Online program. 17 amino acids at the position of 54–70 (FDRERIPERVVHARGAS) were reported to be a catalase active site signature, and the heme-ligand signature was detected at the position of 344–352 (RIFSYADTQ). These data suggested clearly that sugarcane ScCAT1 encoded a putative peroxisomal catalase. Furthermore, it also predicted that ScCAT1 contains no transmembrane helix domain, implying that ScCAT1 is not a membrane located or secretory protein.
A GenBank Blastp comparison showed that ScCAT1 exhibited high identity with other plant catalases, including S. bicolor catalase (XP_002437631.1) (97.97% identity), Z. mays catalase (NP_001241808.1) (97.56% identity), O. sativa catalase (A2YH64.2) (94.92% identity), B. distachyon catalase (XP_003563243.1) (93.29% identity), P. chinampoensis catalase (ADN94253.1) (92.07% identity), H. vulgare catalase (P55307.1) (91.67% identity), T. aestivum catalase (P55313.1) (91.26% identity) and S. italica catalase (XP_004966515.1) (87.02% identity). Phylogenetic analysis (Fig. 2) revealed that ScCAT1 was closely related to S. bicolor catalase (XP_002437631.1), Z. mays catalase (NP_001241808.1) and S. italica catalase (XP_004966515.1), with the homologies of 97.97%, 97.56% and 87.02%, respectively.
Figure 2. Phylogenetic trees based on catalase amino acid sequences, showing the phylogenetic relationships between ScCAT1 (KF664183) and the catalases from other plant species.
Neighbor-joining method was used.
Subcellular Localization of ScCAT1
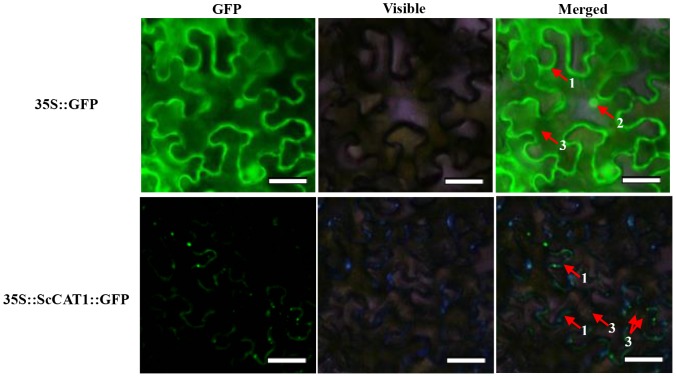
To further understand the function of ScCAT1 gene, its subcellular localization was conducted. ScCAT1 was recombined into plant expression vector pCAMBIA 2300 between the sites of the 35S promoter and GFP (Figs. S1 and S3), and its location was characterized by transient expression of the target gene and GFP in N. benthamiana leaves with Agrobacterium-mediated transformation. After 2 days of cultivation, the infiltrated leaves were harvested and the reporter protein GFP was observed under a fluorescence microscope. The results revealed that 35S::ScCAT1::GFP was located in plasma membrane and cytoplasm (Fig. 3). In contrast, GFP was shown in the nucleus, cytoplasm and plasma membrane cells transiently transfected with 35S::GFP.
Figure 3. Subcellular localizations of ScCAT1 and empty vector in Nicotiana benthamiana leaves 48 h after infiltration.
The epidermal cells were used for taking images of green fluorescence, visible light and merged light. Read arrows 1, 2 and 3 indicated plasma membrane, nucleus and cytoplasm, respectively. Bar = 50 µm.
Expressions of ScCAT1 in E. coli
As reported before, different stresses, such as copper (Cu), cadmium (Cd), high temperature, wounding, ethylene (ET), H2O2, SA, jasmonic acid (JA), ABA and other inducers, could trigger an induction of plant catalases [11], [13], [21], [37], [38]. To study the function of ScCAT1 in response to different kinds of adverse environments in vivo, pET 32a-ScCAT1 (Fig. S4A) was transformed into E. coli Rosetta cell. The recombinant protein of 62 kDa was specifically induced and accumulated approximately after 8 h IPTG induction on the SDS-PAGE (Fig. S4B).
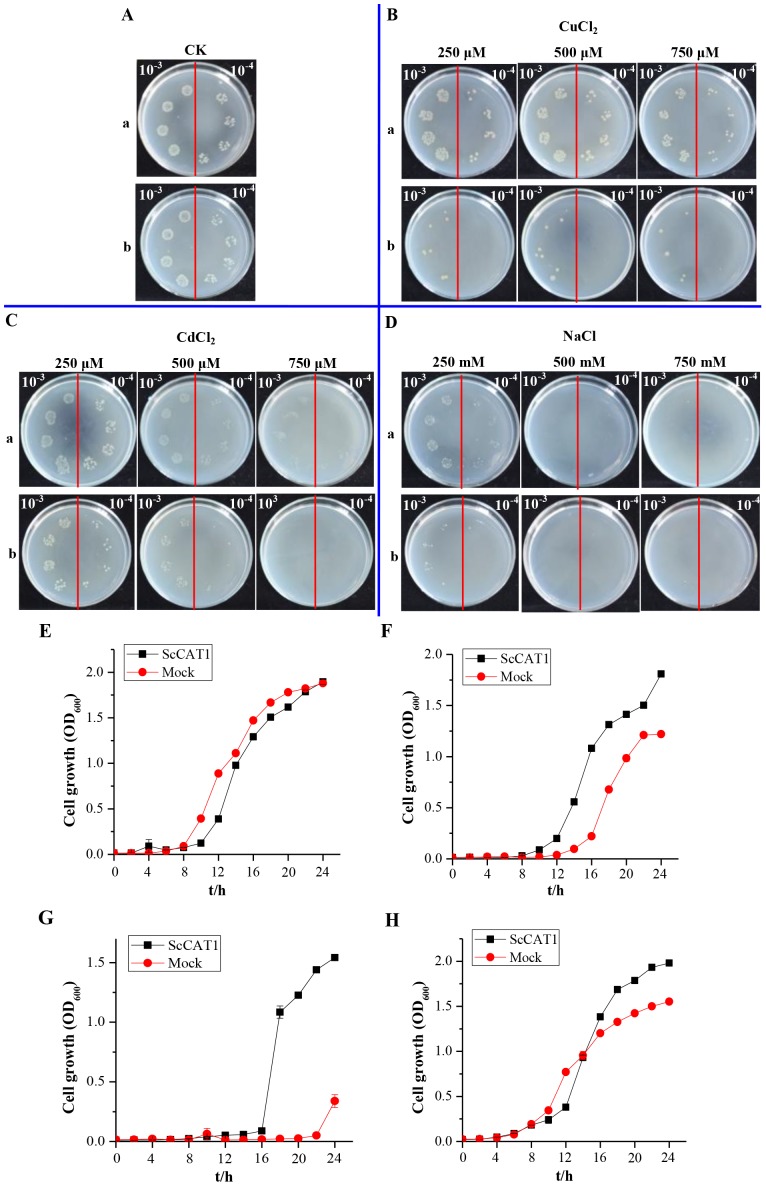
The growth of gene-expressed cells (Rosetta+pET 32a-ScCAT1) and mock (Rosetta+pET 32a) was analyzed on LB plates with different supplements (Figs. 4A, B, C, and D). After one day culture, Rosetta+pET 32a-ScCAT1 showed an increased number of colonies as compared to the control cells on LB plates containing CuCl2, CdCl2 and NaCl. The growth was also analyzed in the LB liquid medium containing 750 µM CuCl2, 750 µM CdCl2 and 250 mM NaCl (Figs. 4E, F, G and H). All the Rosetta+pET 32a-ScCAT1 cells showed faster growth as compared to that of the mock which revealed that ScCAT1 had an effect on increasing the tolerance to CuCl2, CdCl2 and NaCl. These results demonstrat that the recombinant protein of ScCAT1 enhanced growth ability of prokaryotic E. coli Rosetta strains in stress conditions.
Figure 4. Spot assays of Rosetta+pET 32a-ScCAT1 (a) and Rosetta+pET 32a (mock) (b) on LB plates with CuCl2, CdCl2 and NaCl (A–D).
And liquid culture assay in LB liquid medium with 750 µM CuCl2, 750 µM CdCl2 and 250 mM NaCl (E–H). IPTG (isopropyl β-D-thiogalactoside) was added to the cultures of Rosetta+pET 32a-ScCAT1 and Rosetta+pET 32a to induce the expression of recombinant protein. The cultures were adjusted to OD600 = 0.6. Ten microliters from 10−3 (left side of red line on plate) to 10−4 (right side of red line on plate) dilutions were spotted onto LB basal (A) plates or with CuCl2 (250, 500 and 750 µM) (B), CdCl2 (250, 500 and 750 µM) (C), NaCl (250, 500 and 750 mM) (D). For studying the growth analysis of ScCAT1, Rosetta+pET 32a-ScCAT1 and Rosetta+pET 32a were grown in LB liquid medium with LB basal medium (E) or with 750 µM CuCl2 (F), 750 µM CdCl2 (G), and 250 mM NaCl (H). All data points are means±SE (n = 3). CuCl2: copper chloride; CdCl2: cadmium chloride; NaCl: sodium chloride.
Tissue-specific Expression Analysis of ScCAT1
The relative expression of ScCAT1 was detected in four kinds of sugarcane tissues, including leaf, bud, stem epidermis and stem pith. As showed in Fig. 5, the bud exhibited the highest mRNA expression, while the mRNA expression of stem epidermis and stem pith was at a moderate level. The leaf showed a relatively low level in comparison with the other three kinds of tissues.
Figure 5. Tissue-specific expression analysis of the ScCAT1 in sugarcane variety Yacheng05–179.
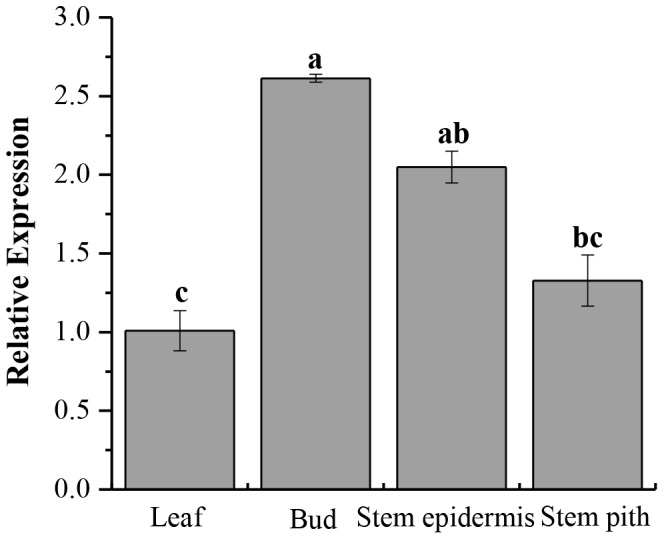
Data are normalized to the GAPDH expression level. All data points are means±SE (n = 3).
ScCAT1 Expression in Response to Different Stress Treatments
Smut challenged sugarcane (Yacheng05–179 and Liucheng03–182) buds were detected by Q-PCR for examination whether the expression of ScCAT1 was induced or inhibited (Fig. 6). In order to eliminate the influence of wounding, the relative expression of the target gene was calculated by the expression level of the inoculated sample minus the level of the mock at each corresponding time point. As indicated in Fig. 6, after the inoculation of smut pathogen, the mRNA expression of ScCAT1 in resistant variety Yacheng05–179 was higher than that in susceptible variety Liucheng03–182. During the sugarcane-smut incompatible interaction, the transcript of ScCAT1 in Yacheng05–179 began was elevated as early as 6 h post-inoculation (6 hpi), while that of ScCAT1 in Liucheng03–182 appeared delayed (12 hpi). Furthermore, the transcript of ScCAT1 in Yacheng05–179 and Liucheng03–182 reached the maximum at 48 h, but the expression in incompatible interaction was 1.55 times that of the compatible one, and then decreased in both. During the whole process of interaction, the transcript of ScCAT1 in the incompatible cultivar almost always higher than that of the compatible, except at 12 h. These data reveal that the up-regulation of ScCAT1 expression was most probably associated with smut resistance in sugarcane.
Figure 6. Q-PCR analysis of the ScCAT1 expression patterns in biosystem of sugarcane-smut (Sporisorium scitamineum) interaction.
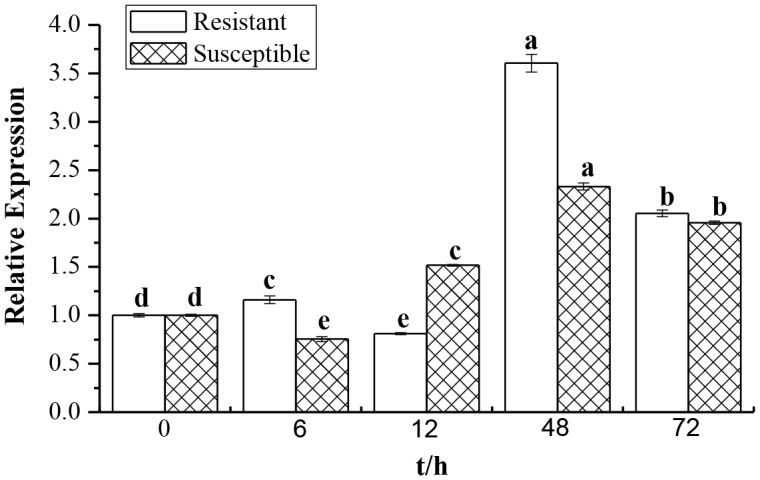
Data was normalized to the GAPDH expression level. All data points (deduction its mock) are means±SE (n = 3). Resistant: Yacheng05–179 variety; Susceptible: Liucheng03–182 variety.
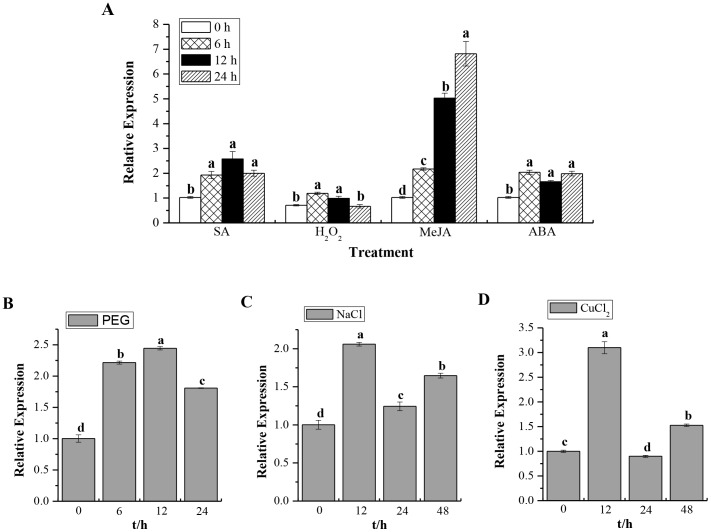
Expression of ScCAT1 in response to various abiotic stimuli in Yacheng05–179 plantlets was checked after treatment with 5 mM SA, 10 mM H2O2, 25 µM MeJA, 100 µM ABA, 25% PEG, 250 mM NaCl and 100 µM CuCl2, and the results shown in Fig. 7. Interestingly, ScCAT1 showed a positive response to exogenous stresses, including plant hormones stresses of SA, MeJA and ABA, oxidative stress of H2O2, hyper-osmotic stresses of PEG and NaCl, as well as mental stress of CuCl2. ScCAT1 transcription was always up-regulated and the expression level usually increased steadily from 0 h to 24 h or 48 h after post-treatment with these seven exogenous inducers. These results suggest that ScCAT1 may be a positive responsive component of abiotic stress in sugarcane.
Figure 7. Q-PCR analysis of the ScCAT1 expression patterns in Yacheng05–179 plantlets with abiotic elicitors.
Data are normalized to the GAPDH expression level. (A) The relative expression of ScCAT1 under the stresses of 5 mM SA, 10 mM H2O2, 25 µM MeJA and 100 µM ABA. (B) The relative expression of ScCAT1 under 25% PEG stress. (C) The relative expression of ScCAT1 under 250 mM NaCl stress. (D) The relative expression of ScCAT1 under 100 µM CuCl2 stress. All data points are means±SE (n = 3). SA: salicylic acid; H2O2: hydrogen peroxide; MeJA: methyl jasmonate; ABA: abscisic acid; PEG: polyethylene glycol; NaCl: sodium chloride; CuCl2: copper chloride.
Transient Over-expression of ScCAT1 in N. benthamiana Leaves Induces Hypersensitive Reaction Response
To test whether ScCAT1 can induce HR and immunity in plant, ScCAT1 was transient over-expressed in N. benthamiana leaves by infiltration with Agrobacterium EHA105 carrying pCAMBIA 1301 (mock) and pCAMBIA 1301-ScCAT1. The results showed that at the time point of 48 h after infiltration, a typical HR symptom, darker DAB staining and enhanced electrolyte leakage, was found in the leaves expressing the target gene (Figs. 8A and C). Furthermore, injected leaves 5 d after agroinfiltrated by 35S::ScCAT1 presented yellow symptoms (Fig. 8B). What is more, cell death measured by qualitative trypan blue staining showed a darker color than that in mock (Fig. 8B). These results indicate the involvement of ScCAT1 in cell death responses.
Figure 8. The effect of transient over-expression of ScCAT1 on immunity induction in Nicotiana Benthamiana leaves.
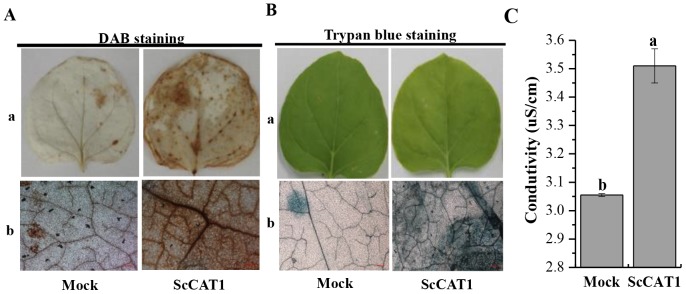
(A) DAB staining with N. benthamiana leaves 48 h after 35S::ScCAT1-containing Agrobacterium strain infiltration to assess the H2O2 production; a: images taken by SONY camera; b: images taken by microscope. (B) Cell death measured by trypan blue staining of transient expression leaves 5 d after agroinfiltration; a: phenotypes of N. Benthamiana 5 d after infiltration taken by SONY camera; b: images of trypan blue staining taken by microscope. (C) Conductivity measurement of N. Benthamiana leaves infiltrated with 35S::ScCAT1-containing Agrobacterium strain after 48 h. Mock: Agrobacterium strain carrying 35S::00. Bar = 10 µm. All data points are means±SE (n = 3).
Discussion
Fungal disease is a major concern worldwide for sugarcane production and most other crops. During plant-pathogen interactions, many antifungal components have been identified [39]. Peroxidase (POD) activity increased in resistant sugarcane varieties (and not in susceptible) implies that it may be related to smut resistance [40]. Our previous report showed that β-1,3-glucanase activity in the resistant variety increased faster and lasted longer than that of the susceptible one after challenged by S. scitamineum, which showed a positive relationship between the activity of the sugarcane β-1,3-glucanase and smut resistance [26]. Plant catalase, one of the scavenger enzymes, has been also shown to be involved in plant defense and development [41]. Wang et al. found that catalase could be induced by pathogen infection in resistant clam Meretrix meretrix [23]. As showed in Fig. 1, the activity of catalase increased in the resistant genotype (Yacheng05–179) after challenged by S. scitamineum in comparison to the susceptible cultivar (Liucheng03–182). There appears to be a positive correlation between catalase activity and sugarcane smut resistance. This observation should be repeated in different resistant genotypes.
The capacity of a plant to scavenge H2O2 may result from increased activities of scavenger enzymes or up-regulated expression of genes increasing of the levels of the corresponding proteins [42]. Multiple catalase isozymes in plants have been observed. Previous research demonstrated that there were at least six catalase isozymes existing in A. thaliana encoded by a multi-gene family including three genes (cat1, cat2 and cat3) [14]. In Z. mays, three catalase isoenzymes encoded by three different structural genes were observed [38]. A sweet potato catalase SPCAT1 was cloned from mature leaves treated with ethephon and found that it could alleviate ethephon-mediated leaf senescence and H2O2 elevation [13]. Until now, there has been no report on sugarcane catalase genes involved in the sugarcane-smut interaction. In this study, we isolated and characterized a full-length sugarcane catalase gene ScCAT1 which encoded a polypeptide of 492 amino acids and had high identities with several other plant catalases. Using the method of Agrobacterium-mediated transformation in N. benthamiana leaves, 35S::ScCAT1::GFP was located in plasma membrane and cytoplasm in cells (Fig. 3) which is consistent with a previous report that catalase mostly localized in peroxisomes, glyoxysome and cytoplasm [11], [43].
Recent publications have reported that E. coli cells can be enhanced or inhibited under stress expressing recombinant proteins [27], [31], [32], [44]. Some of the protective mechanisms were similar in both eukaryotes and prokaryotes under stress stimuli [45]. Gupta et al. studied an A-2 type DREB transcription factor from extreme halophyte Salicornia brachiata and found it conferred abiotic stress tolerance in E. coli cells under NaCl, PEG and mannitol treatments, which may be due to the stress regulated function by this transcription factor [32]. Guo et al. tested a sugarcane dirigent protein gene ScDir and a metallothionein gene ScMT2-1-3 in the E. coli system, which indicated that they offered different tolerance against PEG, NaCl and mental stresses [27], [31]. Chaurasia et al. studied that phytochelatin synthase gene PCS, when expressed in E. coli, provided better protection against the stresses of heat, salt, carbofuron, cadmium, copper and UV [44]. In the present study, the ScCAT1 recombinant protein expressed in E. coli Rosetta cells leads to a better growth under the stresses CuCl2, CdCl2 and NaCl. In eukaryote, the previous studies found that the increased tolerance to stress maybe due to the activity and expression of scavenging enzymes which increased in plants placed in different conditions [12]. It has been proposed that catalase, one of the antioxidant enzymes, can be modulated and controlled in response to excessive iron stress, due to alterations in the electron transport chain and damages to the thylakoidal membranes [46]. Therefore, it is plausible to predict that the ScCAT1 encoded by ScCAT1 gene cloned in this study could be helpful for the tolerance/stresses of sugarcane to CuCl2, CdCl2 and NaCl.
The plant faces variable environmental stresses like soil salinity, temperature, drought and cold, and may often present a series of physiological and biochemical changes which are a highly complex and disturb plant growth and yield. To examine the accumulation of sugarcane catalase gene in different developmental processes and environmental conditions, the expression of ScCAT1 gene in sugarcane was analyzed by Q-PCR method (Figs. 5, 6 and 7). Results indicated that while expressed at moderate levels in stem epidermis and stem pith, ScCAT1 was expressed at a relatively high level in the bud (Fig. 5). Similar to other species of Ustilago, the S. scitamineum is a parasite of young meristematic tissues and gains entry into the host, exclusively through the bud scales [47]. From above, high expression of ScCAT1 in sugarcane bud may help to defend against the smut pathogen. In our study, the target transcript of ScCAT1 was found to be higher in the incompatible interaction than that in the compatible one during sugarcane-S. scitamineum interaction (Fig. 6). After the smut pathogen challenge in Yacheng05–179, the expression of ScCAT1 increased at 6 h and reached the maximum level at 48 h (1.5 times that in Liucheng03–182). As previous reported, the phenomenon of smut hypha entry into the sugarcane bud meristem occurs between 6 h and 36 h after the teliospore deposition [48]. It should be also noted that ScCAT1 expression decreased gradually after 48 h, but the expression level still maintained at a higher level than that at 0 h, and the gene expression pattern of ScCAT1 was coincident with the activity change of catalase in this study. So we assume that ScCAT1 may have a protective effect on smut penetration in sugarcane.
Q-PCR analysis of the expression of ScCAT1 in response to hydrogen peroxide and plant hormones showed that from 0 h to 24 h its levels increased under the stresses of 10 mM H2O2, 5 mM SA, 25 µM MeJA and 100 µM ABA (Fig. 7A). In Panax ginseng, PgCat1 transcript accumulated during 1–12 h of 10 mM H2O2 treatment [11]. Maize Cat1 gene transcript increased in developing embryos by the treatments of 1.5 mM SA, 50 mM H2O2, 100 µM JA and 1 mM ABA [38], [49]. In the present study, for hyper-osmotic stress, ScCAT1 mRNA levels increased until 12 h then slightly decreased at 24 h and induced at 48 h under 250 mM NaCl treatment. ScCAT1 transcript was also stimulated till 24 h after 25% PEG stress (Figs. 7B and C). 500 mM NaCl stress induced the expression of Cat1 in Avicennia marina seedlings till 12 h then subsequently decreased [50]. In Panax ginseng, PgCat1 transcripts accumulated till 24 h then decreased till 72 h after 100 mM NaCl treatment [11]. Plants suffering from NaCl stress not only because of increased osmolarity but also oxidative stress caused by ionic character [51]. In our study, the ScCAT1 transcript increased 1.5 fold until 48 h under the stress of 100 µM CuCl2. The maximum expression was observed to be 3.0 fold at 12 h after treatment. Previous study revealed that copper toxicity caused ultra structural damage which resulting in the increasing production of ROS [46]. The Prunus cerasifera Cat1 gene expression and enzyme activity were high for 10 days under 100 mM copper stress [52]. These results lead us to conclude that ScCAT1 may be a positive responsive component of abiotic stresses in sugarcane.
N. benthamiana has been widely employed in functional characterization of the target genes by over-expression [30]. Cell death presented at the infected site is the most efficient strategy to restrict pathogen growth and development [30]. The induction of R gene expression, ion fluxes, stimulation of ROS and defense-related hormones, are the common response of cell death [53], [54]. Here, DAB staining showed deep brown in the presence of H2O2 in N. benthamiana leaves after 48 h infiltration and resulted in an increase of electrolyte leakage (Figs. 8A and C). Trypan blue staining exhibited a darker color post 5 d injection than that in mock (Fig. 8B). Previous studies have shown that there is a close relationship between HR and H2O2 accumulation [55]. It can be deduced from this study that H2O2 accumulation by transient over-expression of ScCAT1 may confer the HR cell death in sugarcane.
In conclusion, after inoculation with S. scitamineum, sugarcane catalase was found to significantly increase in the resistant variety and maintain at much higher level than that of the susceptible one which suggested a positive correlation between the activity of the catalase and the smut resistance in sugarcane. ScCAT1 was isolated from sugarcane buds and the recombinant protein resulted in a better growth of E. coli Rosetta cells under certain stresses. The expression of ScCAT1 was up-regulated by smut infection and by different stresses such as plant hormones (SA, MeJA and ABA) treatments, oxidative (H2O2) stress, heavy metal (CuCl2) and hyper-osmotic (PEG and NaCl) stresses. ScCAT1 was located in plasma membrane and cytoplasm in cells. Histochemical assays indicated that ScCAT1 acted positively in sugarcane immunity. From these observations, we can conclude that ScCAT1 should be a positive responsive component of biotic and abiotic stresses in sugarcane.
Supporting Information
Construction of subcellular localization vector 35S :: ScCAT1 :: GFP .
(TIF)
Nucleotide acid sequences and deduced amino acid sequences of ScCAT1 obtained by RT-PCR. The deduced amino acid sequences were shown in one-letter code under the cDNA sequences. The underlines showed the catalase active site signature (FARERIPERVVHARGAS) and the heme-ligand signature (RVFAYADTQ) of ScCAT1.
(TIF)
The enzyme digestion to identify the insert-integrated subcellular localization expression vector 35S :: ScCAT1 :: GFP . 1, 15,000+2,000 bp DNA marker; 2, 35S::GFP/Xba I; 3, ScCAT1 ORF PCR product; 4, 35S::ScCAT1::GFP/Xba I; 5, 35S::ScCAT1::GFP/Xba I+Spe I; 6, 100 bp ladder DNA marker.
(TIF)
The enzyme digesting identification of insert-integrated prokaryotic expression vector pET 32a- ScCAT1 (A) and corresponding protein expressions in Escherichia coli Rosetta strains (B). (A) 1, 100 bp ladder DNA marker; 2, pET 32a/EcoR I; 3, ScCAT1 ORF PCR product; 4, pET 32a-ScCAT1/EcoR I; 5, pET 32a-ScCAT1/EcoR I+Xho I; 6, 15,000+2,000 bp DNA Marker. (B) 1, Protein marker; 2, blank without induction; 3, blank induction for 8 h; 4, control without induction; 5, control induction for 8 h; 6, pET 32a-ScCAT1 without induction; 7 and 8, pET 32a-ScCAT1 induction for 4 h and 8 h, respectively. The induced protein was shown by arrow.
(TIF)
Funding Statement
This research was supported by National Natural Science Foundation of China (No.31101196), the earmarked fund for the Modern Agriculture Technology of China (CARS-20), Research Funds for Distinguished Young Scientists in Fujian Agriculture and Forestry University (xjq201202), National High Technology Research and Development Program of China (863 Program) Project (2013AA102604). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sundar AR, Barnabas EL, Malathi P, Viswanathan R (2012) A mini-review on smut disease of sugarcane caused by Sporisorium scitamineum. In: Mworia J, editor. Botany. Croatia: InTech. 109–128.
- 2.Agnihotri VP (1990) Diseases of sugarcane and sugarbeet Oxford & IBH Publishing Co Pvt Ltd. New Delhi: 72–103.
- 3. Hoy JW, Hollier CA, Fontenot DB, Grelen LB (1986) Incidence of sugarcane smut in Louisiana and its effects on yield. Plant Dis 70: 59–60. [Google Scholar]
- 4. Singh N, Somai BM, Pillay D (2004) Smut disease assessment by PCR and microscopy in inoculated tissue cultured sugarcane cultivars. Plant Sci 167: 987–994. [Google Scholar]
- 5. Solas MT, Pinon D, Vicent C, Legaz ME (1999) Ultrastructural aspects of sugarcane bud infection by Ustilago scitaminea teliospores. Sugar Cane 2: 14–18. [Google Scholar]
- 6. Waller JM (1970) Sugarcane smut (Ustilago scitaminea) in Kenya: II. Infection and resistance. T British Mycol Soc 54: 405–414. [Google Scholar]
- 7. Xu LP, Chen RK, Chen PH (2004) Analysis on infection index of smut caused by Ustilago scitaminea in sugarcane segregated population. Chin J Trop Crop 25: 33–36. [Google Scholar]
- 8. Lin YQ, Chen RK, Gong DM (1996) Analysis of quantitative inheritance for smut resistance in sugarcane. J Fujian Agr U China 25: 271–275. [Google Scholar]
- 9. Dussle CM, Quint M, Melchinger AE, Xu ML, Lubberstedt T (2003) Saturation of two chromosome regions conferring resistance to SCMV with SSR and AFLP markers by targeted BSA. Theor Appl Genet 106: 485–493. [DOI] [PubMed] [Google Scholar]
- 10. Lakshmanan P, Geijskes RJ, Aitken KS, Grof CLP, Bonnett GD, et al. (2005) Sugarcane biotechnology: the challenges and opportunities. In Vitro Cell Dev-Pl 41: 345–363. [Google Scholar]
- 11. Purev M, Kim YJ, Kim MK, Pulla RK, Yang DC (2010) Isolation of a novel catalase (Cat1) gene from Panax ginseng and analysis of the response of this gene to various stresses. Plant Physiol Bioch 48: 451–460. [DOI] [PubMed] [Google Scholar]
- 12. Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van-Breusegem F, et al. (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61: 4197–4220. [DOI] [PubMed] [Google Scholar]
- 13. Chen HJ, Wu SD, Huang GJ, Shen CY, Afiyanti M, et al. (2012) Expression of a cloned sweet potato catalase SPCAT1 alleviates ethephon-mediated leaf senescence and H2O2 elevation. J Plant Physiol 169: 86–97. [DOI] [PubMed] [Google Scholar]
- 14. Frugoli JA, Zhong HH, Nuccio ML, McCourt P, McPeek MA, et al. (1996) Catalase is encoded by a multigene family in Arabidopsis thaliana (L.) Heynh. Plant physiol 112: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willekens H, Villarroel R, Van-Montagu M, Inze D, Van-Camp W (1994) Molecular identification of catalases from Nicotiana plumbaginifolia (L.). FEBS Lett 352: 79–83. [DOI] [PubMed] [Google Scholar]
- 16. Guan L, Scandalios JG (1995) Developmentally related responses of maize catalase genes to salicylic acid. P Natl A Sci 92: 5930–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skadsen RW, Schulze-Lefert P, Herbst JM (1995) Molecular cloning, characterization and expression analysis of two catalase isozyme genes in barley. Plant Mol Biol 29: 1005–1014. [DOI] [PubMed] [Google Scholar]
- 18. Drory A, Woodson WR (1992) Molecular cloning and nucleotide sequence of a cDNA encoding catalase from tomato. Plant Physiol 100: 1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kwon SI, An CS (2001) Molecular cloning, characterization and expression analysis of a catalase cDNA from hot pepper (Capsicum annuum L.). Plant Sci 160: 961–969. [DOI] [PubMed] [Google Scholar]
- 20. Du YY, Wang PC, Chen J, Song CP (2008) Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana . J Integr Plant Biol 50: 1318–1326. [DOI] [PubMed] [Google Scholar]
- 21. Guan ZQ, Chai TY, Zhang YX, Xu J, Wei W (2009) Enhancement of Cd tolerance in transgenic tobacco plants overexpressing a Cd-induced catalase cDNA. Chemosphere 76: 623–630. [DOI] [PubMed] [Google Scholar]
- 22. Williamson JD, Scandalios JG (1992) Differential response of maize catalases and superoxide dismutases to the photoactivated fungal toxin cercosporin. The Plant J 2: 351–358. [DOI] [PubMed] [Google Scholar]
- 23. Wang C, Yue X, Lu X, Liu BZ (2013) The role of catalase in the immune response to oxidative stress and pathogen challenge in the clam Meretrix meretrix . Fish Shellfish Immun 34: 91–99. [DOI] [PubMed] [Google Scholar]
- 24. Casu RE, Dimmock CM, Chapman SC, Grof CP, McIntyre CL, et al. (2004) Identification of differentially expressed transcripts from maturing stem of sugarcane by in silico analysis of stem expressed sequence tags and gene expression profiling. Plant Mol Biol 54: 503–517. [DOI] [PubMed] [Google Scholar]
- 25. Moosawi-Jorf SA, Mahin BI (2007) In vitro detection of yeast-like and mycelial colonies of Ustilago scitaminea in tissue-cultured plantlets of sugarcane using polymerase chain reaction. J Appl Sci 7: 3768–3773. [Google Scholar]
- 26. Su YC, Xu LP, Xue BT, Wu QB, Guo JL, et al. (2013) Molecular cloning and characterization of two pathogenesis-related β-1,3-glucanase genes ScGluA1 and ScGluD1 from sugarcane infected by Sporisorium scitamineum. . Plant Cell Rep 32: 1503–1519. [DOI] [PubMed] [Google Scholar]
- 27.Guo JL, Xu LP, Su YC, Wang HB, Gao SW, et al. (2013) ScMT2-1-3, a metallothionein gene of sugarcane, plays an important role in the regulation of heavy metal tolerance/accumulation. BioMed Res Int: doi.10.1155/2013/904769. [DOI] [PMC free article] [PubMed]
- 28. Que YX, Xu LP, Xu JS, Zhang JS, Zhang MQ, et al. (2009) Selection of control genes in real-time qPCR analysis of gene expression in sugarcane. Chin J Trop Crop 30: 274–278. [Google Scholar]
- 29.Hao JJ, Kang ZL, Yu Y (2001) Plant physiology experiment technology. In: Hao JJ, editor. China: Liaoning Sci Technol Press. 53–55.
- 30. Hwang IS, Hwang BK (2012) Requirement of the cytosolic interaction between pathogenesis-related protein10 and leucine-rich repeat protein1 for cell death and defense signaling in pepper. Plant Cell 24: 1675–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo JL, Xu LP, Fang JP, Su YC, Fu HY, et al. (2012) A novel dirigent protein gene with highly stem-specific expression from sugarcane, response to drought, salt and oxidative stresses. Plant Cell Rep 31: 1801–1812. [DOI] [PubMed] [Google Scholar]
- 32. Gupta K, Agarwal PK, Reddy MK, Jha B (2010) SbDREB2A, an A-2 type DREB transcription factor from extreme halophyte Salicornia brachiata confers abiotic stress tolerance in Escherichia coli . Plant Cell Rep 29: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△Ct method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 34. Choi HW, Kim YJ, Hwang BK (2011) The hypersensitive induced reaction and leucine-rich repeat proteins regulate plant cell death associated with disease and plant immunity. Mol Plant Microbe In 24: 68–78. [DOI] [PubMed] [Google Scholar]
- 35. Huckelhoven R, Fodor J, Trujillo M, Kogel KH (2000) Barley Mla and Rar mutants compromised in the hypersensitive cell death response against Blumeria graminis f. sp. hordei are modified in their ability to accumulate reactive oxygen intermediates at sites of fungal invasion. Planta 212: 16–24. [DOI] [PubMed] [Google Scholar]
- 36. Hwang IS, Hwang BK (2011) The pepper mannose-binding lectin gene CaMBL1 is required to regulate cell death and defense responses to microbial pathogens. Plant Physiol 155: 447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scandalios JG, Acevedo A, Ruzsa S (2000) Catalase gene expression in response to chronic high temperature stress in maize. Plant Sci 156: 103–110. [DOI] [PubMed] [Google Scholar]
- 38. Guan LM, Zhao J, Scandalios JG (2000) Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. The Plant J 22: 87–95. [DOI] [PubMed] [Google Scholar]
- 39. Takken FLW, Joosten MHAJ (2000) Plant resistance genes: their structure, function and evolution. Eur J Plant Pathol 106: 699–713. [Google Scholar]
- 40. Xu LP, Wang JN, Chen RK (1994) Biochemical reaction of sugarcane to smut and its relation to resistance. Sugarcane 1: 13–16. [Google Scholar]
- 41. Wan LL, Zha WJ, Cheng XY, Liu C, Lv L, et al. (2011) A rice β-1,3-glucanase gene Osg1 is required for callose degradation in pollen development. Planta 233: 309–323. [DOI] [PubMed] [Google Scholar]
- 42. Rezaee F, Ghanati F, Behmanesh M (2013) Antioxidant activity and expression of catalase gene of (Eustoma grandiflorum L.) in response to boron and aluminum. S Afr J Bot 84: 13–18. [Google Scholar]
- 43. Song XX, Zhao FY (2007) Research progress on catalase in plants. J Anhui Agric Sci 35: 9824–9827. [Google Scholar]
- 44. Chaurasia N, Mishra Y, Ai LC (2008) Cloning expression and analysis of phytochelatin synthase (pcs) gene from Anabaena sp. PCC 7120 offering multiple stress tolerance in Escherichia coli . Biochem Bioph Res Co 376: 225–230. [DOI] [PubMed] [Google Scholar]
- 45. Liu Y, Zheng Y (2005) PM2, a group 3 LEA protein from soybean, and its 22-mer repeating region confer salt tolerance in Escherichia coli . Biochem Bioph Res Co 331: 325–332. [DOI] [PubMed] [Google Scholar]
- 46. Sandmann G, Boger P (1980) Copper-mediated lipid peroxidation processes in photosynthetic membranes. Plant Physiol 66: 797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fawcett GL (1942) Circular, Estacion Experimental Agricola. In: Fawcett GL, editor. Tucuman. 114.
- 48. Alexander KC, Ramakrishnan K (1980) Infection of the bud, establishment in the host and production of whips in sugarcane smut (Ustilago scitaminea) of sugarcane. Proc Int Soc Sugar Cane Technol 17: 1452–1455. [Google Scholar]
- 49. Guan LM, Scandalios JG (2000) Hydrogen peroxide-mediated catalase gene expression in response to wounding. Free Radical Bio Med 28: 1182–1190. [DOI] [PubMed] [Google Scholar]
- 50. Jithesh MN, Prashanth SR, Sivaprakash KR, Parida A (2006) Monitoring expression profiles of antioxidant genes to salinity, iron, oxidative, light and hyperosmotic stresses in the highly salt tolerant grey mangrove, Avicennia marina (Forsk.) Vierh. by mRNA analysis. Plant Cell Rep 25: 865–876. [DOI] [PubMed] [Google Scholar]
- 51. Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25: 239–250. [DOI] [PubMed] [Google Scholar]
- 52. Lombardi L, Sebastiani L (2005) Copper toxicity in Prunus cerasifera: growth and antioxidant enzymes responses of in vitro grown plants. Plant Sci 168: 797–802. [Google Scholar]
- 53. Melech-Bonfil S, Sessa G (2010) Tomato MAPKKKε is a positive regulator of cell-death signaling networks associated with plant immunity. The Plant J 64: 379–391. [DOI] [PubMed] [Google Scholar]
- 54. Li Y, Tessaro MJ, Li X, Zhang Y (2010) Regulation of the expression of plant resistance gene SNC1 by a protein with a conserved BAT2 domain. Plant physiol 153: 1425–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Construction of subcellular localization vector 35S :: ScCAT1 :: GFP .
(TIF)
Nucleotide acid sequences and deduced amino acid sequences of ScCAT1 obtained by RT-PCR. The deduced amino acid sequences were shown in one-letter code under the cDNA sequences. The underlines showed the catalase active site signature (FARERIPERVVHARGAS) and the heme-ligand signature (RVFAYADTQ) of ScCAT1.
(TIF)
The enzyme digestion to identify the insert-integrated subcellular localization expression vector 35S :: ScCAT1 :: GFP . 1, 15,000+2,000 bp DNA marker; 2, 35S::GFP/Xba I; 3, ScCAT1 ORF PCR product; 4, 35S::ScCAT1::GFP/Xba I; 5, 35S::ScCAT1::GFP/Xba I+Spe I; 6, 100 bp ladder DNA marker.
(TIF)
The enzyme digesting identification of insert-integrated prokaryotic expression vector pET 32a- ScCAT1 (A) and corresponding protein expressions in Escherichia coli Rosetta strains (B). (A) 1, 100 bp ladder DNA marker; 2, pET 32a/EcoR I; 3, ScCAT1 ORF PCR product; 4, pET 32a-ScCAT1/EcoR I; 5, pET 32a-ScCAT1/EcoR I+Xho I; 6, 15,000+2,000 bp DNA Marker. (B) 1, Protein marker; 2, blank without induction; 3, blank induction for 8 h; 4, control without induction; 5, control induction for 8 h; 6, pET 32a-ScCAT1 without induction; 7 and 8, pET 32a-ScCAT1 induction for 4 h and 8 h, respectively. The induced protein was shown by arrow.
(TIF)