Abstract
Sheep are commonly used as a model for human spinal orthopaedic research due to their similarity in morphological and biomechanical features. This study aimed to document the volumes of vertebral bodies and compare the generated results as well as morphometry of the sheep lumbar spine to human published data. For this purpose, computed tomography scans were carried out on five adult Merino sheep under general anaesthesia. Transverse 5 mm thick images were acquired from L1 to L6 using a multi-detector-row helical CT scanner. Volume measurements were performed with dedicated software. Four spinal indices and Pavlov's ratio were calculated. Thereafter, the generated data were compared to published literature on humans. The mean vertebral body volume showed an increase towards the caudal vertebrae, but there were no significant differences between the vertebral levels (P>0.05). Compared to humans, sheep vertebral body volumes were 48.6% smaller. The comparison of absolute values between both species revealed that sheep had smaller, longer and narrower vertebral bodies, thinner intervertebral discs, narrower spinal canal, longer transverse processes, shorter dorsal spinous processes and narrower, higher pedicles with more lateral angulations. The comparison of the spinal indices showed a good similarity to human in terms of the vertebral endplates and spinal canal. The results of this study may be helpful for using the sheep as a model for human orthopaedic spinal research if anatomical differences are taken into account.
Keywords: Comparative anatomy, human lumbar spine, spinal index, ovine, vertebral body volume
Human lumbar vertebrae support the weight of the upper body. Moreover, they are the largest vertebrae in the dynamic part of the spinal column. Upright posture and locomotion cause significant loading stress to the vertebral bodies, which in turn predispose the lumbar spinal region to high incidence of spinal disorders such as herniated disc and spinal stenosis [1,2], and thus, new surgical procedures and spinal implants are developed and often tested pre-clinically on cadaver spines before being used in humans.
The human cadaveric spine is the ideal model for biomechanical studies and implant testing whenever anatomy and size are important. However, there are some difficulties in using the human model, such as obtaining it fresh especially from a healthy population and in large quantities in order to obviate the wide scattering effect associated with biological variability [3]. To overcome these obstacles, animal models such as sheep, calves, pigs and dogs have been used as alternatives for human spinal research [4-7].
Sheep are well accepted as appropriate models in orthopaedic research, due to similarities with humans in weight, bone and joint structure and in the bone remodelling processes [8-11]. In addition to their availability, ease of handling and housing, sheep are fully accepted as a research animal model in society [12].
Computed tomography (CT), a sensitive, non-invasive diagnostic and evaluating technique, has been used in morphometric studies to depict the normal volume and shape of the human vertebral canal at various levels [13-16]. Furthermore, via CT morphometric data could be obtained from a live subject, thus preventing the effect of preservation methods.
In humans, the exact volume of the vertebral body is necessary for the evaluation and surgical application related to vertebral body deformities such as vertebroplasty, in which an orthopaedic cement mixture is injected into the empty spaces within weakened vertebrae to strengthen them. Thereby, the vertebral body volume is crucial to decide the amount of the cement that should be injected into the deformed vertebra [15,16]. An animal model to test various compounds for vertebroplasty must be of sufficient size to accommodate realistic volumes of material. However, we have not found any detailed study in the literature describing the volumes of sheep lumbar vertebral bodies.
Morphometry of sheep lumbar vertebrae is essential for designing and interpreting results from studies which contemplate their use. In this study, we aimed to document the volumes of lumbar vertebral bodies and compare the volumes and morphometry of the sheep lumbar vertebrae to published data on humans.
Materials and Methods
This study was approved by the Animal Welfare Commission, Regional Office Leipzig (TVV-No. 03/12). Our study consisted of two parts; a volumetry of sheep lumbar vertebral bodies and a morphometric comparison study between the sheep and human lumbar vertebrae.
Determining the lumbar vertebral bodies' volume
For volumetric study, five female Merino sheep (mean age, 2±0.1 years; mean weight, 62±5.3 kg) without history or clinical signs related to spinal diseases were included.
Each sheep was fasted for 24 hours and deprived of water for 12 hours before being premedicated with 0.1 mg/kg atropine sulphate (Atropinum sulfuricum 0.5 mg Eifelango®, Eifelango, Bad Neuenahr-Ahrweiler, Germany), and a combination of 0.1 mg/kg butorphanol tartrate (Alvegesic®, CP-Pharma GmbH, Burgdorf, Germany) and 0.2 mg/kg midazolam (Midazolam, B. Braun; B.Braun, Melsung, Germany) administered intravenously. Anaesthesia was induced with 3 mg/kg ketamine chlorhydrate (Ursotamin®, Serumwerk Bernburg AG, Bernburg, Germany) intravenously. After endotracheal intubation, anaesthesia was maintained with isoflurane (Isofluran CP®, CP-Pharma GmbH) delivered in oxygen through an endotracheal tube.
The sheep were positioned in sternal recumbency and positioning aid tools were used to obtain a perpendicular position of the spine relative to the x-ray beam of the gantry. Contiguous slices were obtained from the cranial aspect of L1 to the caudal aspect of L6 with a multidetector-row helical CT unit (Philips Medical Systems MX8000 IDT 16, Hamburg, Germany). Technical settings were 120 kV, 200 mA, 0.75 second tube rotation and a pitch of 0.438. The data were reconstructed to a transverse image series with 5 mm slice thickness using a highfrequency image reconstruction algorithm (bone). Window width and level settings were standardised for all measurements (window width, 2000 Hounsfield units; window level, 500 Hounsfield units). Transverse images were reconstructed parallel to the cranial endplate of the vertebral body using multi-planar reconstruction and transferred to a workstation for vertebral volume estimation. The volumes of vertebral bodies were measured using the ImageJ (NIH orgnization, Bethesda, Maryland, USA) and Volumest plugin [17].
Briefly, ImageJ (version 1.47) was downloaded from http://rsbweb.nih.gov/ij/download.html as well as Volumest (http://lepo.it.da.ut.ee/~markkom/volumest/download/) (accession date: 01/04/2013) plug and added to ImageJ software. After opening DICOM images in ImageJ, the scaling of the images is corrected automatically, and volumetric analysis can be continued. On the CT slices the vertebral body was outlined as regions of interest (ROI). To create an ROI, the mouse has to be clicked repeatedly to create line segments. When finished, one has to click in the small box at the starting point, and ImageJ automatically draws the last segment. After outlining ROI in each slice, the Volumest plug calculates the volume of selected ROI using stereology principles (Figure 1).
Figure 1.
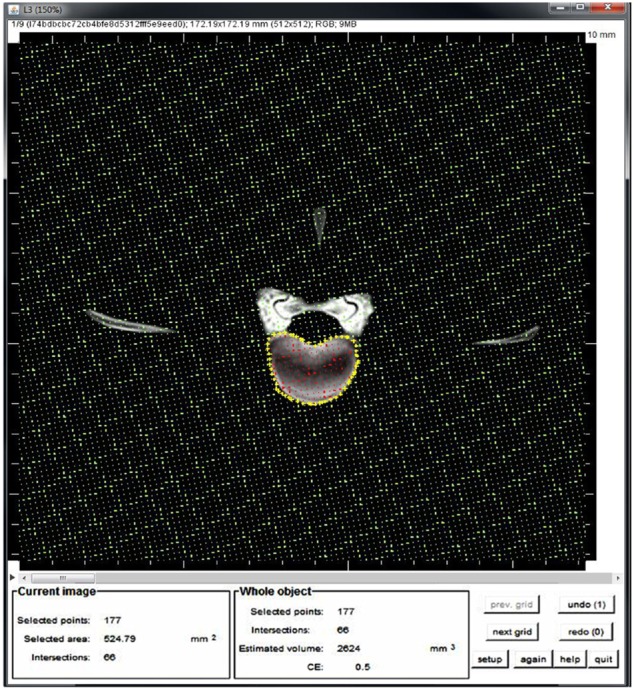
Estimation the vertebral body volume using ImageJ. The vertebral body was outlined (yellow stars), subsequently the Volumest plugin calculates automatically the volume from the area (red stars) and the slice thickness of each image. Finally, these values per image were added up to calculate the volume.
The volume of each vertebra was measured three times by the same observer (MM).
In order to confirm the accuracy of the ImageJ software, a pilot study was performed in which the sheep lumbar spine was carefully cleaned of surrounding soft tissues using suitable dissection tools. Thereafter, continuous CT transverse images were made as described earlier and imported to ImageJ software for measuring the vertebral body volume of each vertebra. Subsequently, the vertebral bodies were prepared as described previously [15], where the vertebral body pedicles, laminas, and transverse processes were sawed off. The exact vertebral body volume was measured using the Archimedean principle, also known as 'fluid displacement technique' in a measuring cylinder. For this purpose, each vertebral body was immersed in a measuring cylinder filled with distilled water at room temperature. The volumes were measured based on the scale in millimetre increments.
Morphometric comparison
For the morphometric comparison part, a total of four spinal indices and Pavlov's ratio were calculated, based on linear and angular dimensions of the sheep lumbar spine which we published recently (Table 1) [18], and compared to literature on humans (Table 2) [16,19-21].
Table 1.
Mean and standard deviation of measured sheep lumbar vertebrae dimensions (mm, °degrees)
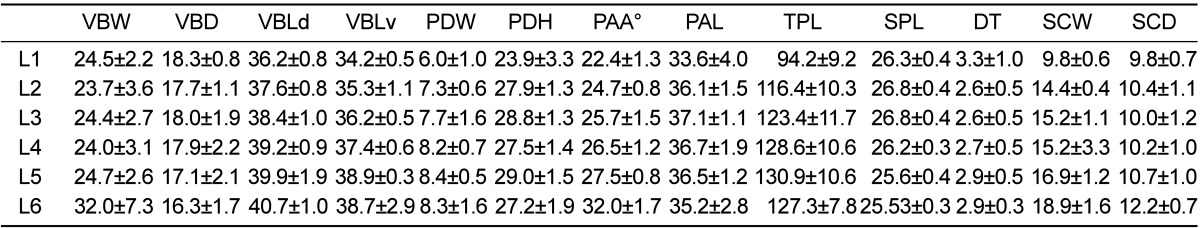
VBW; vertebral body width, WBD; vertebral body depth, VBLd; vertebral body length dorsal, VBLv; vertebral body length ventral, DT; disc thickness. SCW; spinal canal width, SCD; spinal canal depth, SPL; spinal process length, TPL; transverses process length, PDW; pedicle width, PDH; pedicle length, PAL; pedicle axis length, PAA; pedicle axis angle.
Table 2.
Mean and standard deviation of measured human lumbar vertebrae dimensions (mm, °degrees) from literature on humans [19-21]
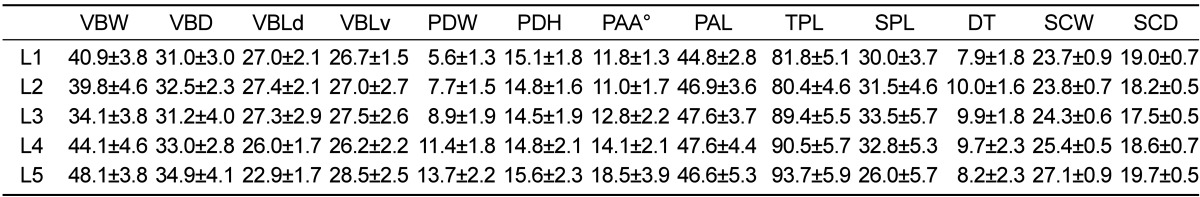
VBW; vertebral body width, WBD; vertebral body depth, VBLd; vertebral body length dorsal, VBLv; vertebral body length ventral, DT; Disc thickness. SCW; spinal canal width, SCD; spinal canal depth, SPL; spinal process length, TPL; transverses process length, PDW; pedicle width, PDH; pedicle length, PAL; pedicle axis length, PAA; pedicle axis angle.
We compared the absolute values of the vertebral dimensions between sheep and humans. Moreover, to increase the comparison reliability between both species, spinal indices and Pavlov's ratio were also compared. The spinal indices include: Concavity index (CI) defined as the ratio between the dorsal and ventral vertebral body length. The endplate index (EI) was calculated as the ratio between the width and depth of the cranial endplate at the transverse plane. The spinal canal index (SCI) was calculated as the ratio between the width and depth of the spinal canal. The pedicle index (PDI) was defined as the ratio between the width and length of the pedicle. Pavlov's ratio was calculated as the ratio between the depth of both the spinal canal depth and vertebral body [22].
Statistical analysis
In the pilot study, the generated volume values of the vertebral bodies using the fluid displacement technique and ImageJ software were compared using the paired Student's t test. One-way analysis of variance was used to determine the volume differences between the vertebral levels. A P value>0.05 was considered statistically significant.
Intraobservor reliability of vertebral body volume was calculated as the difference between three measurements obtained by the first author. The statistical analysis was performed with Microsoft Excel 2010 package (Microsoft Deutschland GmbH, Unterschleissheim, Germany). The volumes of vertebral bodies and spinal indices were compared between human and sheep vertebrae. The parameter was defined comparable if the ratio sheep/human of each individual vertebra showed variation less than 20%.
Results
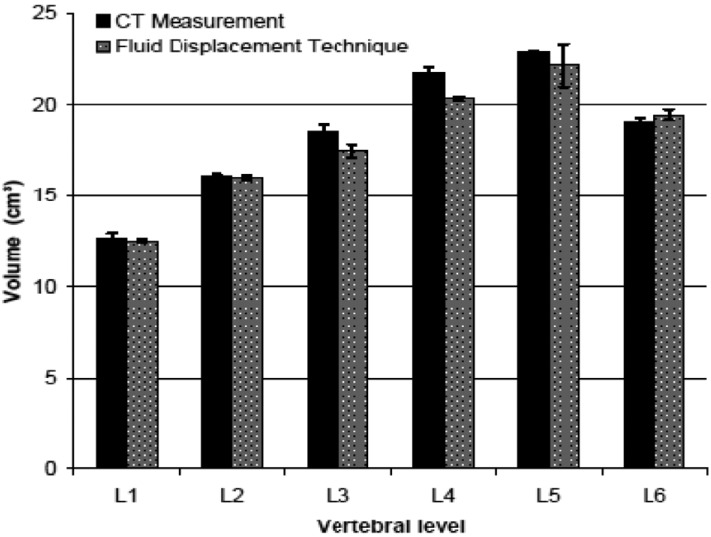
In the pilot study, the Student's t test revealed that the difference between measured volume values using ImageJ software and fluid displacement technique were not statistically different (P<0.05) (Figure 2).
Figure 2.
The means of the vertebral body volumes (cm3) of the sheep using imageJ software compared to real volume of the same vertebrae, which was measured using fluid displacement technique.
In the present study, the volume intraobserver variability was small with the average difference between three measurements for each vertebra being within 1 cm3. There were no statistically significant differences in the volume values between the lumbar vertebrae (P=0.28). The average volume of each vertebral body increased toward caudal vertebral levels (Table 3). Generally, the volume of the lumbar vertebral bodies was fairly constant at about 16 cm3. The comparison of the volumes of lumbar vertebral bodies between sheep and humans revealed that sheep had a smaller volume, as much as 48.6% (volume ranging from 34.4% to 54.4%) than humans. Moreover, the comparison revealed that no vertebral level of sheep lumbar spine was comparable to human.
Table 3.
Mean and standard deviation of sheep and human lumbar vertebral body volume (cm3)
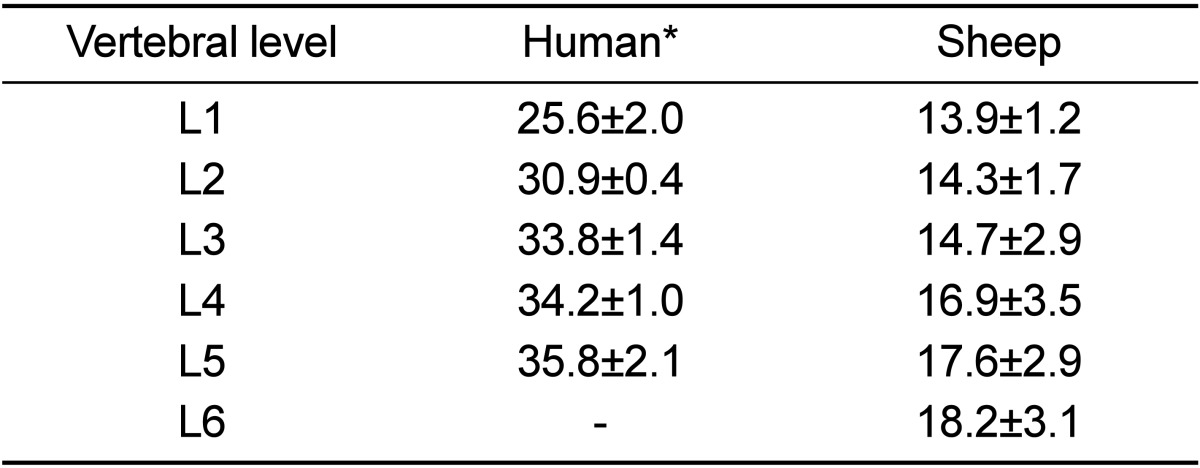
The sheep vertebral body volume was measured using ImageJ software. *Human data were taken from Odaci et al. [16].
Table 4 lists sheep and human spine indices and Pavlov's ratio. The human lumbar vertebral bodies were wider than they were longer, while the sheep lumbar were longer than they were wider especially at level L5. The human vertebrae were wider and deeper than those of sheep, as much as 17.1 mm (40.6%) and 14.7 mm (45.1%), respectively. In contrast, sheep lumbar vertebrae were longer than human ones, as much as 10.5 mm (28.5%). The concavity index of the sheep was greater than humans especially at the caudal lumbar vertebrae and its values at L1, L2 and L5 were comparable with those of humans. The values of the human EI at all vertebral levels were greater than sheep. The endplate indexes of all of the lumbar vertebral levels were comparable with humans (Table 5).
Table 4.
Spine indices of sheep and human lumbar vertebrae
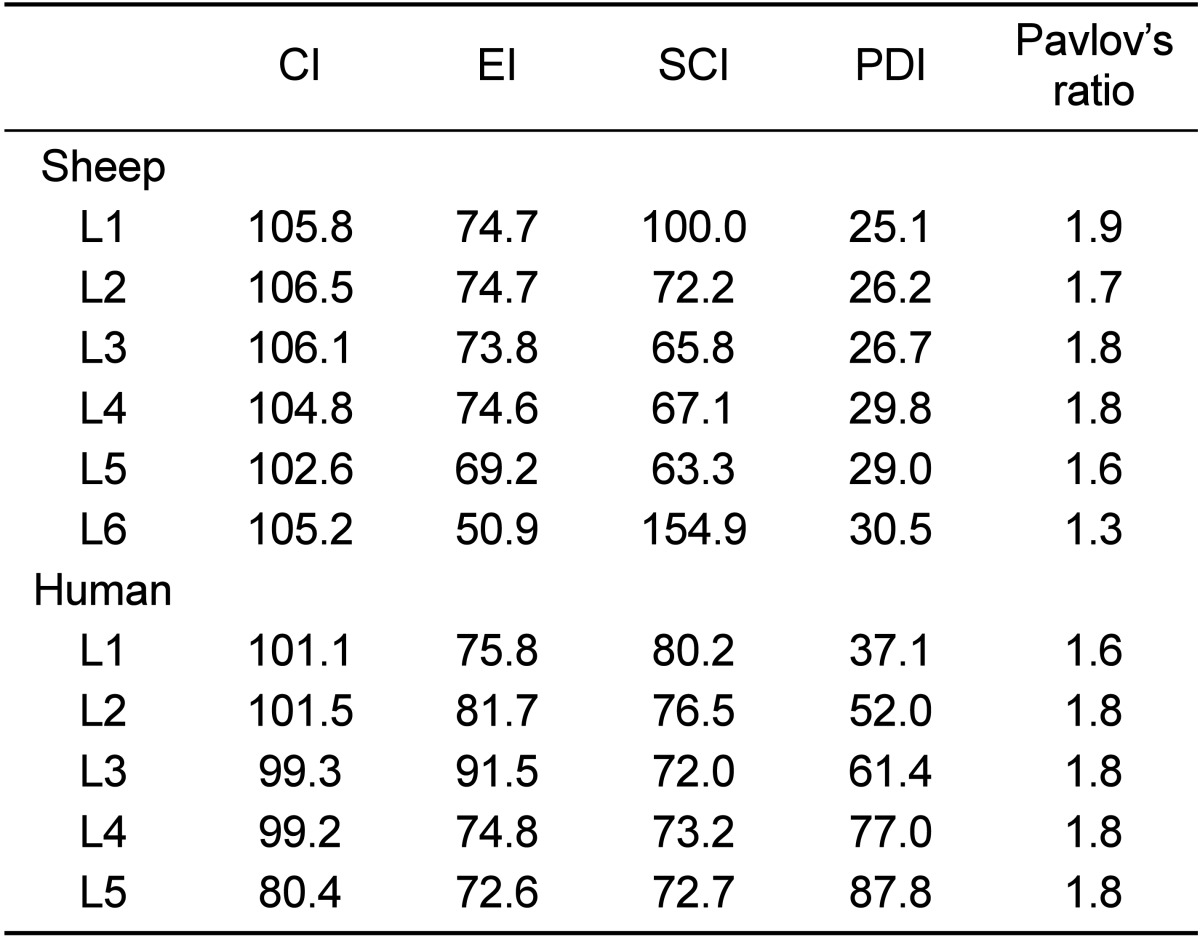
CI (concavity index was calculated as ratio between the dorsal and ventral vertebral body length), EI (Endplate index was calculated as ratio between the width and depth of cranial endplate at the transverse plane), SCI (spinal canal index was calculated as ratio between the width and depth of spinal canal), PDI (pedicle index was defined as the ratio between the width and length of the pedicle. The Pavlov's ratio was calculated as the ratio between the depth of both spinal canal depth and vertebral body.
Table 5.
Comparison of the absolute values of each dimension between humans and sheep
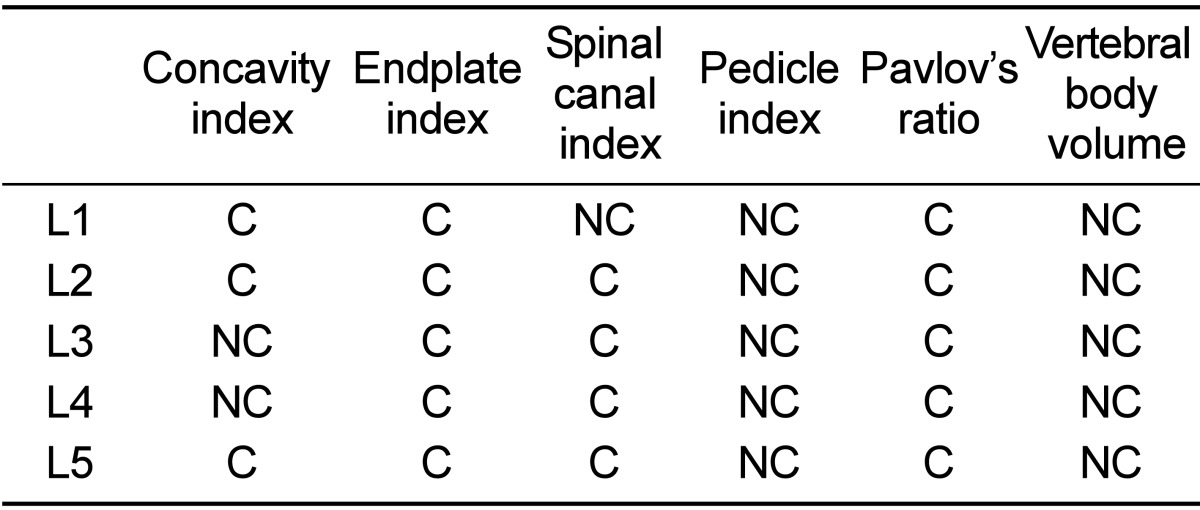
The ratio between human and sheep values of a dimension was calculated for each vertebral level. If the variance of these ratios was less than 20% between the same vertebral level of both species that particular dimension was defined as 'comparable'. C - comparable, NC - not comparable.
In both species, the spinal canal was wider than it was deep and increased in width towards the caudal vertebral level. The human spinal canal was wider and deeper than sheep, as much as 10.6 mm (42.7%) and 8.4 mm (45%) respectively. However, the SCI was comparable between both species, except for L1 (Table 5).
The pedicles in both species were higher than they were wide. Sheep pedicles were higher and had a greater lateral angulation than humans, where the latter had a wider and greater pedicle axis length than sheep. Both findings were most pronounced at the level of the caudal lumbar vertebrae of both species especially at L5. The pedicle index of sheep showed an increasing trend with the ordinal number of lumbar vertebrae. A similar behaviour was observed in humans. The pedicle index values were not comparable at all levels of the lumbar vertebrae (Table 5).
The sheep dorsal spinal process length was smaller than in humans but both of them decreased in a caudal direction. The transverse process length was obviously larger in sheep lumbar vertebrae than in humans and also decreased in a caudal direction. Transverse and dorsal spinous processes have a cranial inclination in sheep.
The human vertebral disc thickness was variable between lumbar vertebral levels. A comparison between the two species revealed that human discs were obviously thicker than sheep. However, the human disc was as much as 69.5% (6.4 mm) thicker than sheep.
The mean Pavlov's ratio value of sheep lumbar vertebrae was 1.7, while the smallest Pavlov's ratio was 1.6 at L5 and the highest was 1.9 at L1. Pavlov's ratio of sheep was greater than in humans. However, Pavlov's ratio of both species was comparable at all vertebral levels (Table 5).
Discussion
The current study aimed to document the normal volume of the sheep lumbar vertebral bodies and to highlight the differences between the sheep and human spine with a view to inform researchers contemplating their use in future studies.
Our results showed that the real volume of the vertebral body, which was measured using fluid displacement technique, agreed well with the volume estimates of ImageJ software. There were no statistical differences detected between them. This indicates that the values of the volume based on ImageJ software are reliable. The interobsarvor variability for each vertebra was less than 1 cm3. The reason for this difference was most likely due to operator error inherent in making a tracing of the irregular region of interest. Operator error could be affected by spatial resolution and eye-hand coordination, both of them expected to be highly variable among different operators [23]. Therefore, the volume in the present study was measured by the same operator. In order to decrease operator error as much as possible, window width and level were kept similar while estimating the volume.
There is an inverse correlation between slice thickness and accuracy required to perform the volume measurement in the case of increasing slice thickness, thereby reducing the number of CT slices that have to be outlined. In the present study, CT images of the vertebrae were reconstructed in the transverse plane with 5 mm thickness. A previous study carried out in human spines using a combination of the Cavalieri principle and CT scans showed that both 3- and 5 mm thickness CT scans proved to be sufficiently accurate for measuring volumes [16]. Moreover, the same study reported that the image planes did not affect the accuracy of the volume measurements.
We found that the mean vertebral body volume showed a gradual increase from the cranial towards the caudal vertebral level, but there were no significant differences between the vertebral levels. This finding could be due to the increasing the vertebral body length towards the caudal lumbar vertebrae (Table 1). The comparison of the volumes of vertebral bodies between humans and sheep showed that sheep vertebrae were smaller, as much as 48.6%, than humans. The cause of this difference is most likely to be the upright posture of humans.
In this study, we derived sheep morphometric data from our previous documentation [18]. The comparative data for human parameters were taken from published literature [16,19-21]. Anatomical comparisons have been made between sheep and human spine using manual measurements in cadaveric spines of three- to four-year-old sheep [24]. However, the previous study subjectively compared the spinal dimensions between both species without taking into account the wide scattering effect associated with methodology.
In contrast to a previous study [24], the current study provides an objective comparison using the spinal indices, which were calculated as the ratio between the vertebral dimensions to rule out the heterogeneity of measuring methods and thus making the comparative results more reliable. The spinal indices were defined as being comparable if the ratio sheep/human of each individual vertebral level showed variation less than 20%, because in humans a vertebra is classified as abnormal if an alteration of more than 20% in vertebral dimensions is detected [25]. Noteworthy, the volume of the sheep lumbar vertebral bodies were documented here for the first time.
The comparison between sheep and humans revealed that humans have larger, wider and shorter vertebral bodies and thicker intervertebral discs. These differences can be attributed to the upright posture of the human spine, putting demands relatively larger and shorter caudal vertebral bodies to balance the higher longitudinal loads. This was also probably the explanation for the larger intervertebral disc thickness observed in the human spine, which was up to three times thicker than the sheep disc in the lumbar region [26]. Biomechanically, the highest range of motion around the X and Y axes has been reported at L2-L3 in sheep and L3-L4 in humans [27]. The vertebral morphometry could support this finding, where the current results showed that the vertebral body width and depth decreased from L1 to L2 and then increased until L5, while in humans the vertebral body width and depth decreased from L1 to L3 and decreased at L4 and L5. In other words, the lumbar spine of both species are composed of two unequal pyramids in coronal plane, which facilitated axial rotation (X axis) and both flexion and extension motion (Y axis) [28].
When comparing pedicle parameters, sheep pedicles are narrower, higher and have greater lateral angulations than humans. Sheep pedicles were not comparable to humans at any lumbar vertebral levels. This means that the pedicle screw designed for humans may not be safely placed in sheep pedicles without modification, the existing screws which are an appropriate length in humans penetrating the ventral vertebral body cortex in sheep. Moreover, the orientation of screw placement should be taken into account to avoid penetrating the lateral cortex of the vertebral body.
The human spinal canal was wider and deeper, and thus covers a greater surface area than in the sheep. We believe this is a kind of adaptation to provide additional support for the upright body weight. The spinal canal area can be evaluated in a variety of ways, such as Pavlov's ratio, which was first proposed as an indicator of the degree of development of the canal narrowing [22]. The spinal canal index and Pavlov's ratio were comparable between humans and sheep.
The spinal processes serve as a lever arm by paraspinal muscles to maintain posture and induce rotation and flexion [29]. In the current study, transverse processes in sheep were longer than in humans. We attribute this to the horizontal position of the sheep spine compared to humans, the sheep paraspinal muscles need a strong support to carry the weight of the abdominal viscera and maintain spinal movements.
One limitation of the present study was the low number of sheep. The number of 5 animals (30 vertebrae) used was the lowest possible to comply with the regulations of 3R but sufficient enough to provide reliable data. Moreover, the animals were of equal age, weight, sex and breed in order to obtain uniformity. However, the repeated sheep dimensions and small variance around the mean indicated that a larger sample was not necessary. Moreover, previous investigators had used similar sample sizes for similar studies [20,24,30].
In conclusion, according to spinal indices results the sheep lumbar spine has good similarity to that of humans in terms of the vertebral endplate regions and spinal canal, suggesting that a sheep spinal model would be appropriate for studying artificial intervertebral discs, implantation of intervertebral fusion, etc. With regard to sheep pedicles, these can be used as a model providing the dimensions of the implant can be adapted to the anatomical dimensions.
References
- 1.Putz RL, Müller-Gerbl M. The vertebral column--a phylogenetic failure? A theory explaining the function and vulnerability of the human spine. Clin Anat. 1996;9(3):205–212. doi: 10.1002/(SICI)1098-2353(1996)9:3<205::AID-CA12>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Mann NH, Brown MD, Enger I. Statistical diagnosis of lumbar spine disorders using computerized patient pain drawings. Comput Biol Med. 1991;21(6):383–397. doi: 10.1016/0010-4825(91)90040-g. [DOI] [PubMed] [Google Scholar]
- 3.Ashman RB, Bechtold JE, Edwards WT, Johnston CE, McAfee PC, Tencer AF. In vitro spinal arthrodesis implant mechanical testing protocols. J Spinal Disord. 1989;2(4):274–281. [PubMed] [Google Scholar]
- 4.Wall EJ, Bylski-Austrow DI, Shelton FS, Crawford AH, Kolata RJ, Baum DS. Endoscopic discectomy increases thoracic spine flexibility as effectively as open discectomy. A mechanical study in a porcine model. Spine. 1998;23(1):9–15. doi: 10.1097/00007632-199801010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Reid JE, Meakin JR, Robins SP, Skakle JM, Hukins DW. Sheep lumbar intervertebral discs as models for human discs. Clin Biomech (Bristol, Avon) 2002;17(4):312–314. doi: 10.1016/s0268-0033(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 6.Gurr KR, McAfee PC, Shih CM. Biomechanical analysis of anterior and posterior instrumentation systems after corpectomy. A calf-spine model. J Bone Joint Surg Am. 1988;70(8):1182–1191. [PubMed] [Google Scholar]
- 7.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28(23):2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 8.Newman E, Turner AS, Wark JD. The potential of sheep for the study of osteopenia: current status and comparison with other animal models. Bone. 1995;16(4):277S–284S. doi: 10.1016/8756-3282(95)00026-a. [DOI] [PubMed] [Google Scholar]
- 9.Nunamaker D. Experimental models of fracture repair. Clin Orthop Relat Res. 1998;355:S56–S65. doi: 10.1097/00003086-199810001-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann G, Graichen F, Rohlmann A. Hip joint forces in sheep. J Biomech. 1999;32(8):769–777. doi: 10.1016/s0021-9290(99)00068-8. [DOI] [PubMed] [Google Scholar]
- 11.Egermann M, Goldhahn J, Schneider E. Animal models for fracture treatment in osteoporosis. Osteoporos Int. 2005;16(Suppl 2):S129–S138. doi: 10.1007/s00198-005-1859-7. [DOI] [PubMed] [Google Scholar]
- 12.Turner AS. Experiences with sheep as an animal model for shoulder surgery: strengths and shortcomings. J Shoulder Elbow Surg. 2007;16(5):S158–S163. doi: 10.1016/j.jse.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Ahlgren BD, Lui W, Herkowitz HN, Panjabi MM, Guiboux JP. Effect of anular repair on the healing strength of the intervertebral disc: a sheep model. Spine. 2000;25(17):2165–2170. doi: 10.1097/00007632-200009010-00004. [DOI] [PubMed] [Google Scholar]
- 14.Moore RJ, Osti OL, Vernon-Roberts B, Fraser RD. Changes in endplate vascularity after an outer anulus tear in the sheep. Spine. 1992;17(8):874–878. doi: 10.1097/00007632-199208000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Limthongkul W, Karaikovic EE, Savage JW, Markovic A. Volumetric analysis of thoracic and lumbar vertebral bodies. Spine J. 2010;10(2):153–158. doi: 10.1016/j.spinee.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Odaci E, Sahin B, Sonmez OF, Kaplan S, Bas O, Bilgic S, Bek Y, Ergür H. Rapid estimation of the vertebral body volume: a combination of the Cavalieri principle and computed tomography images. Eur J Radiol. 2003;48(3):316–326. doi: 10.1016/s0720-048x(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 17.Merzin M. Applying stereological method in radiology: Volume measurement (Bachelor's thesis) Tartu: University of Tartu; 2008. [Google Scholar]
- 18.Mageed M, Berner D, Jülke H, Hohaus C, Brehm W, Gerlach K. Morphometrical dimensions of the sheep thoracolumbar vertebrae as seen on digitised CT images. Lab Anim Res. 2013;29(3):138–147. doi: 10.5625/lar.2013.29.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abuzayed B, Tutunculer B, Kucukyuruk B, Tuzgen S. Anatomic basis of anterior and posterior instrumentation of the spine: morphometric study. Surg Radiol Anat. 2010;32(1):75–85. doi: 10.1007/s00276-009-0545-4. [DOI] [PubMed] [Google Scholar]
- 20.Kumar N, Kukreti S, Ishaque M, Mulholland R. Anatomy of deer spine and its comparison to the human spine. Anat Rec. 2000;260(2):189–203. doi: 10.1002/1097-0185(20001001)260:2<189::AID-AR80>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Wolf A, Shoham M, Michael S, Moshe R. Morphometric study of the human lumbar spine for operation-workspace specifications. Spine. 2001;26(22):2472–2477. doi: 10.1097/00007632-200111150-00015. [DOI] [PubMed] [Google Scholar]
- 22.Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology. 1987;164(3):771–775. doi: 10.1148/radiology.164.3.3615879. [DOI] [PubMed] [Google Scholar]
- 23.Beers GJ, Carter AP, Leiter BE, Tilak SP, Shah RR. Interobserver discrepancies in distance measurements from lumbar spine CT scans. AJR Am J Roentgenol. 1985;144(2):395–398. doi: 10.2214/ajr.144.2.395. [DOI] [PubMed] [Google Scholar]
- 24.Wilke HJ, Kettler A, Wenger KH, Claes LE. Anatomy of the sheep spine and its comparison to the human spine. Anat Rec. 1997;247(4):542–555. doi: 10.1002/(SICI)1097-0185(199704)247:4<542::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 25.Pluijm SM, Tromp AM, Smit JH, Deeg DJ, Lips P. Consequences of vertebral deformities in older men and women. J Bone Miner Res. 2000;15(8):1564–1572. doi: 10.1359/jbmr.2000.15.8.1564. [DOI] [PubMed] [Google Scholar]
- 26.Busscher I, Ploegmakers JJ, Verkerke GJ, Veldhuizen AG. Comparative anatomical dimensions of the complete human and porcine spine. Eur Spine J. 2010;19(7):1104–1114. doi: 10.1007/s00586-010-1326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine. 1997;22(20):2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]
- 28.Masharawi Y, Salame K, Mirovsky Y, Peleg S, Dar G, Steinberg N, Hershkovitz I. Vertebral body shape variation in the thoracic and lumbar spine: characterization of its asymmetry and wedging. Clin Anat. 2008;21(1):46–54. doi: 10.1002/ca.20532. [DOI] [PubMed] [Google Scholar]
- 29.Haussler KK. Anatomy of the thoracolumbar vertebral region. Vet Clin North Am Equine Pract. 1999;15(1):13–26. doi: 10.1016/s0749-0739(17)30161-x. [DOI] [PubMed] [Google Scholar]
- 30.Wilke HJ, Kettler A, Claes LE. Are sheep spines a valid biomechanical model for human spines? Spine (Phila Pa 1976) 1997;22(20):2365–2374. doi: 10.1097/00007632-199710150-00009. [DOI] [PubMed] [Google Scholar]