Abstract
The effects of nattokinase on the in vitro platelet aggregation and in vivo thrombosis were investigated in comparison with aspirin. Rabbit platelet-rich plasma was incubated with nattokinase and aggregation inducers collagen and thrombin, and the platelet aggregation rate was analyzed. Nattokinase significantly inhibited both the collagen- and thrombin-induced platelet aggregations. Nattokinase also reduced thromboxane B2 formation from collagen-activated platelets in a concentration-dependent manner. Rats were orally administered with nattokinase for 1 week, and their carotid arteries were exposed. Arterial thrombosis was induced by applying 35% FeCl3-soaked filter paper for 10 min, and the blood flow was monitored with a laser Doppler probe. Nattokinase delayed the FeCl3-induced arterial occlusion in a dose-dependent manner, doubling the occlusion time at 160 mg/kg. In addition, a high dose (500 mg/kg) of nattokinase fully prevented the occlusion, as achieved with aspirin (30 mg/kg). The results indicate that nattokinase extracted from fermented soybean inhibit platelet aggregation by blocking thromboxane formation, and thereby delay thrombosis following oxidative arterial wall injury. Therefore, it is suggested that nattokinase could be a good candidate without adverse effects for the improvement of blood flow.
Keywords: Platelet aggregation, thromboxane B2, thrombosis, nattokinase
Thrombosis due to embolic artery occlusion is one of the major causes of cardiovascular diseases including stroke (angina). Although platelets are important in the physiological homeostasis, their aggregation plays a crucial role in the thrombus formation [1]. That is, upon arterial injury, adhesive ligands such as collagen and von Willebrand Factors (vWF) as well as soluble agonists including adenosine diphosphate (ADP) and thrombin are exposed or generated. Such coagulating factors facilitate adhesion of platelets to the injured arterial walls, activation, and aggregation [2].
Collagen supports binding of platelets to damaged region of arteries via platelet surface receptors such as glucoprotein VI and integrin α2β1 [3]. Collagen binding induces platelet activation through tyrosine kinase-mediated signal transduction, and then the stimulated platelets adhere to the arterial walls, which is dependent on the release of agonists ADP and prostaglandin H2/thromboxane A2 (TXA2) from platelet granules [4,5]. TXA2, an inducer of vasoconstriction and platelet aggregation, plays a key role in the homeostasis of arteries. Therefore, TXA2 is considered as an etiological mediator in the progress of atherosclerosis and myocardial ischemia [6]. TXA2 is generated from arachidonic acid during oxidation reaction catalyzed by cyclooxygenase (COX) and thromboxane synthase, and then rapidly oxidized to a stable inactive thromboxane B2 (TXB2) [7]. Thus, the blood concentration of TXB2 following blood clotting is a specific marker for the assessment of COX-1 activity [8].
Since transition metals facilitate oxidative radical formation, inducing cellular and tissue injuries as well as endothelial cell damage leading to thrombosis, application of ferric chloride (FeCl3) to arteries has been used as a model to assess the effectiveness of anti-thrombotic blood flow enhancers [9]. Nattokinase, a thrombolytic enzyme rich in 'Natto', an Oriental traditional food, is produced by Bacillus subtilis Natto during fermentation of soybeans [10-12]. It has been reported that nattokinase dissolved thrombi in vitro, and prevented carrageenan-induced rat-tail thrombosis in vivo [13]. In the present study, we demonstrated the anti-thrombosis activity of nattokinase in a FeCl3-induced carotid artery thrombosis model, in addition to its effects on the TXB2 formation and platelet aggregation as action mechanisms.
Bacillus subtilis Natto was cultured in a medium (pH 7.0) composed of 66% soybean powder, 30% corn starch, 3% soybean oil, and 1% calcium carbonate at 35℃ for 24 hours. The culture medium including cells were filtered through 2-µm Celite 505 filter (Celite Co., Lompoc, CA, USA) to remove the solids and vitamin K2, followed by 0.2-µm filtration. The filtrate (final 30%) was mixed with indigestible maltodextrin plus soybean extract (7:3) (Daesang Co., Gunsan, Korea), and then spray-dried. The prepared nattokinase NSK-SD was stored at 4℃ until use, and dissolved in purified water and administered to rats in a volume of 1 mL/kg.
Six-month-old male New Zealand white rabbits (body weight 2.0 kg) and 7-week-old male Sprague-Dawley rats (body weight 200-220 g) were from Daehan-Biolink (Eumseong, Korea), and subjected to the experiment after 1-week acclimation to the laboratory environment. The animals were housed in each cage with free access to feed and water under constant environmental conditions (22±2℃; 40-70% relative humidity; 12-hour light-dark cycle; 150-300 lux brightness). All the animal experiments were conducted according to the Standard Operation Procedures, and approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Korea.
Blood sample was collected from the ear artery of rabbits directly into anti-coagulant citrate dextrose solution containing 0.8% citric acid, 2.2% trisodium citrate, and 2% dextrose. Platelet-rich plasma (PRP) was obtained by centrifugation at 230 g for 10 min. Platelets were sedimented by centrifugation of the PRP at 800 g for 15 min and washed with a HEPES buffer (pH 6.5) [9,14]. The washed platelets were resuspended (4×108 cells/mL) in the HEPES buffer (pH 7.4).
Platelet aggregation was measured with an aggregometer (Chrono-Log Co., Harbertown, CA, USA) according to the turbidimetric method of Born [15] as previous described [14]. In brief, the washed platelet suspension was preincubated with nattokinase (400 µg/mL) or aspirin (200 µg/mL) as a reference control at 37℃ in the aggregometer under stirring at 1,000 rpm. After 3-min preincubation, platelet aggregation was induced by adding collagen (2.5 µg/mL) or thrombin (0.1 U/mL). The extent of aggregation was expressed as a percentage of the vehicle control value stimulated with collagen or thrombin alone.
TXB2 released from platelets was assessed using a kit according to the manufacturer's instructions. In brief, washed rabbit platelets (4×108 cells/mL) were preincubated with nattokinase (1-1,000 µg/mL) or aspirin (100 µM) as a reference control at 37℃ for 3 min in an aggregometer, and aggregation was induced by adding collagen (10 µg/mL). The reaction was stopped by 5 mM indomethacin and 2 mM EGTA, centrifuged at 1,200 rom for 2 min, and analyzed for the concentration of TXB2 by enzyme-linked immunosorbent assay.
Rats (n=8/group) were orally administered with nattokinase (50, 160, or 500 mg/kg) or aspirin (30 mg/kg) for 1 week. Forty min after the final administration, the animals were anesthesized by intramuscular injection of Zoletil® (1 mL/kg). Under constant maintenance of body temperature (36-37℃) using a heating pad, the right carotid artery of rats were exposed, and dissected away from the vagus nerve and surrounding tissues. Aortic blood flow rate was monitored with a laser Doppler flowmeter (AD Instruments, Colorado Springs, CO, USA). At the time point of 1 hour after the final administration, arterial thrombosis was induced by wrapping the artery with a Whatman No. 1 filter paper (3 mm in diameter) saturated with 35% FeCl3 solution near (5 mm anterior to) the flowmeter probe for 10 min. The blood flow was monitored for 90 min. A part of the animals (n=3/group) were sacrificed at the time point of 50 min from the application of FeCl3, and the arteries were cut to observe the thrombus in the artery.
The results are presented as means±standard deviation. The significance of differences of all results was analyzed by one-way analysis of variance followed by the Dunnett's multiple-range test correction. Statistical significance was set a priority at P<0.05.
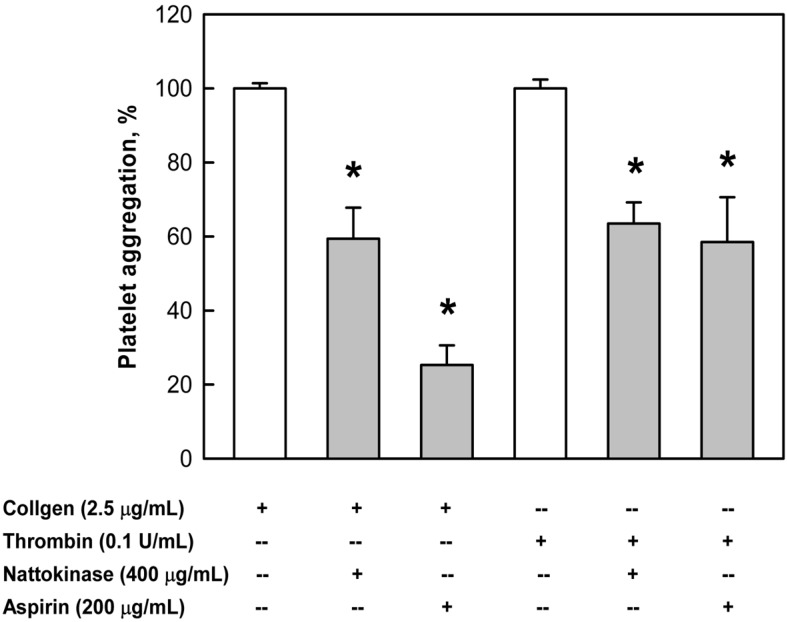
Nattokinase (400 µg/mL) significantly inhibited platelet aggregation induced by collagen (2.5 µg/mL) by approximately 40% (Figure 1), although the effect of nattokinase was inferior to that of aspirin (200 µg/mL). Nattokinase also markedly suppressed the platelet aggregation induced by thrombin (0.1 U/mL), exhibiting an effect comparable to that of aspirin.
Figure 1.
Inhibition by nattokinase (400 µg/mL) or aspirin (200 µg/mL) of platelet aggregation induced by collagen (2.5 µg/mL) or thrombin (0.1 U/mL). *Significantly different from vehicle controls (collagen or thrombin alone).
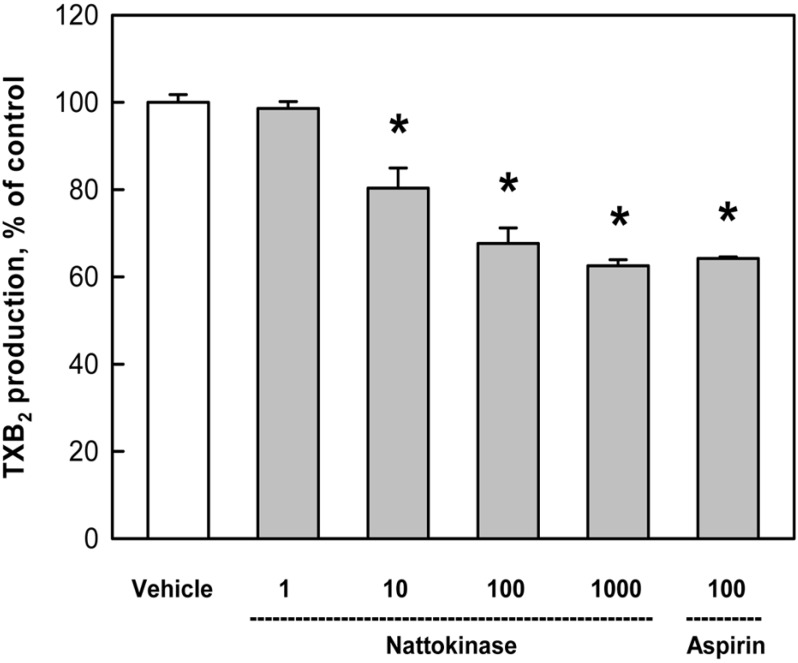
During collagen-induced platelet aggregation, TXB2 formation was inhibited by nattokinase in a concentration-dependent manner; by 20%, 32.4%, and 37.5% at 10, 100, 1,000 µg/mL, respectively (Figure 2). Notably, the effect of a high dose (1,000 µg/mL) was similar to that (35.8%) of aspirin (100 µM).
Figure 2.
Inhibition by nattokinase (1-1,000 µg/mL) or aspirin (100 µM) of thromboxane B2 production from rabbit platelets induced by collagen (2.5 µg/mL). *Significantly different from vehicle control.
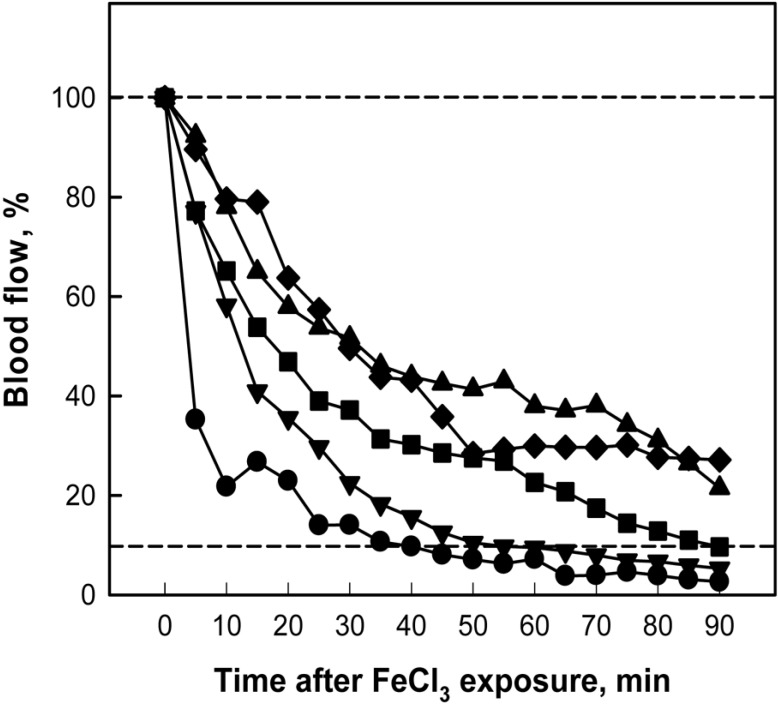
Ten-min application of 35% FeCl3 to the external surface of carotid artery caused gradual decrease in the blood flow that near-fully ceased in 40 min (Figure 3). However, 1-week oral treatment with nattokinase inhibited the thrombus formation in a dose-dependent manner, in which the blood flow was maintained longer than 90 min in the rats treated with a high dose (500 mg/kg) of nattokinse or with aspirin (30 mg/kg).
Figure 3.
Time-course of carotid arterial blood flow following FeCl3 application outside the arterial wall. Nattokinase and aspirin were orally administered for 1 week prior to FeCl3 exposure. Lower dot line indicates a practical cessation of blood flow. ●, vehicle; ▾, nattokinase 50 mg/kg; ▪, nattokinase 160 mg/kg; ♦, nattokinase 500 mg/kg; ▴, aspirin 30 mg/kg.
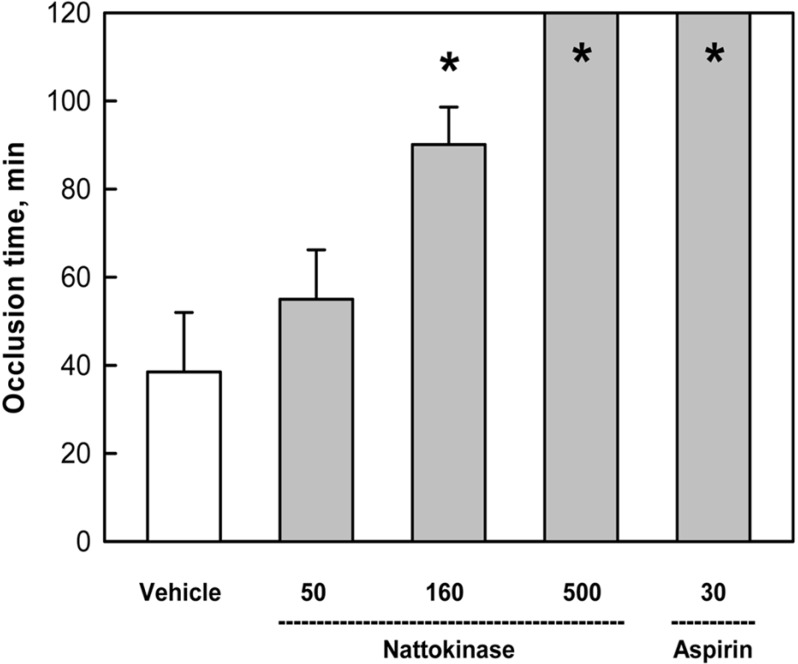
The mean occlusion time in the vehicle control group was calculated to be 38.5 min, based on the time point when the blood flow dropped to 10% (practical cessation) of initial flow rate (Figure 4). In comparison, treatment with 50 and 160 mg/kg of nattokinase extended the occlusion time to 55.0 and 90.1 min, respectively. In addition, a high dose (500mg/kg) of nattokinase prolonged the occlusion time longer than 120 min, as achieved with aspirin (30 mg/kg).
Figure 4.
Time to occlusion of carotid arteries after application of FeCl3 to outside the arterial wall. Nattokinase (50-500 mg/kg) and aspirin (30 mg/kg) were orally administered for 1 week prior to FeCl3 exposure. *Significantly different from vehicle control.
As dissected 50 min after the application of FeCl3, the arteries were entirely plugged with thrombi in the vehicle control group (Figure 5). However, in the animals treated with nattokinase or aspirin, the thrombi were small and loose, without fully obstructing the arterial lumens.
Figure 5.
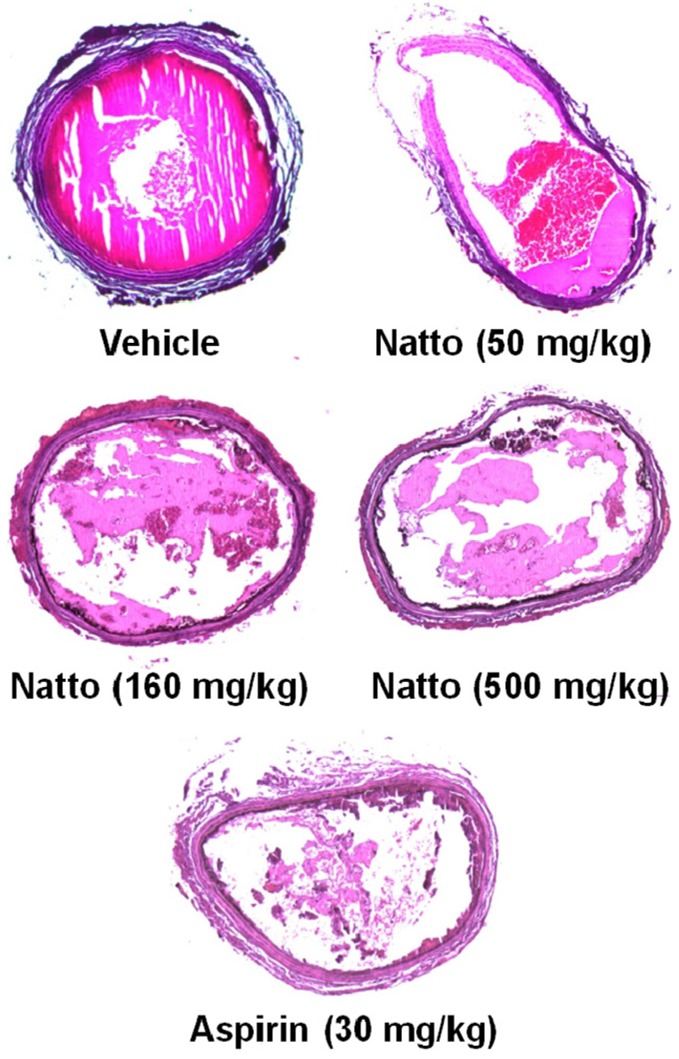
Representative findings of arterial thrombi produced by FeCl3 application outside the arterial wall. Nattokinase (50-500 mg/kg) and aspirin (30 mg/kg) were orally administered for 1 week prior to FeCl3 exposure.
Oral administration of nattokinase from Bacillus subtilis Natto substantially inhibited both the collagen- and thrombin-induced platelet aggregations. The results mean that nattokinase not only inhibits blood clotting triggered by thrombin, but also blocks TXA2-mediated adhesion of platelets to the injured vessel walls as shown in the collagen-induced TXB2 formation [3-5]. Therefore, it is inferred that the effects of nattokinase are similar to those of aspirin, a well-known blood flow enhancer exerting its effect via both mechanisms.
FeCl3 brings about oxidative endothelial injury and exposure of subendothelial extracellular matrix. Then platelets interact with collagen and vWF in the matrix via their respective platelet membrane receptors, leading to platelet adhesion. Activated platelets undergo calcium mobilization and the release of ADP and TXA2 to further accelerate recruitment and aggregation of platelets for thrombus formation [16]. Based on the in vitro results, in vivo anti-thrombotic efficacy of nattokinase was anticipated. Indeed, oral administration of nattokinse delayed the occlusion time in a FeCl3-induced artery thrombosis model. Notably, the effects of crude nattokinase at high doses were comparable to those of aspirin (30 mg/kg), a purified drug, in which nattokinase doubled the occlusion time and especially fully prevented occlusion at 160 and 500 mg/kg, respectively.
It was also reported that subcutaneous injection of nattokinase substantially reduced the tail vein infarction in a carrageenan-induced tail thrombosis model [13]. The result indicates that nattokinase also attenuates inflammation-induced vascular thrombosis, in addition to the effects in the oxidative injury-mediated thrombosis in the present study. It is of interest to note that oral administration of nattokinase enhanced fibrinolytic activity and the production of tissue-type plasminogen activator in the plasma [11,17]. The findings represent possibilities of both the prevention and treatment of ischemic diseases via inhibition of thrombus formation and facilitation of fibrinolysis, since nattokinase dissolves the fibrin clot [18,19]. However, it is unclear whether a part of nattokinase proteins is absorbed in the gastrointestinal tracts without digestion or ingredients other than nattokinase activating intrinsic fibrinolytic machinery exist in the compound.
It is well known that non-steroidal anti-inflammatory drugs including aspirin can induce gastric ulcers and bleeding at high doses [20]. Thus, there is a need for an effective improvement of blood flow without risk of adverse effects, and natural products should fulfill this requirement. In the present study, the nattokinase extracted from fermented soybeans displayed excellent anti-platelet aggregation and anti-thrombotic activities in vitro and in vivo. Although additional exact action mechanisms remain to be clarified, it is suggested that nattokinase could be a good health functional food for the improvement of blood flow.
Acknowledgments
This work This work was supported by Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0094034).
References
- 1.Majid A, Delanty N, Kantor J. Antiplatelet agents for secondary prevention of ischemic stroke. Ann Pharmacother. 2001;35(10):1241–1247. doi: 10.1345/aph.10381. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003;1(7):1602–1612. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 3.Farndale RW, Sixma JJ, Barnes MJ, de Groot PG. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. 2004;2(4):561–573. doi: 10.1111/j.1538-7836.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 4.Konno C, Oshima Y, Hikino H. Morusinol, isoprenoid flavone from Morus root barks. Planta Med. 1977;32(2):118–124. doi: 10.1055/s-0028-1097569. [DOI] [PubMed] [Google Scholar]
- 5.Cowan DH. Platelet adherence to collagen: role of prostaglandin-thromboxane synthesis. Br J Haematol. 1981;49(3):425–434. doi: 10.1111/j.1365-2141.1981.tb07245.x. [DOI] [PubMed] [Google Scholar]
- 6.Dogné JM, Hanson J, de Leval X, Pratico D, Pace-Asciak CR, Drion P, Pirotte B, Ruan KH. From the design to the clinical application of thromboxane modulators. Curr Pharm Des. 2006;12(8):903–923. doi: 10.2174/138161206776055921. [DOI] [PubMed] [Google Scholar]
- 7.Arita H, Nakano T, Hanasaki K. Thromboxane A2: its generation and role in platelet activation. Prog Lipid Res. 1989;28(4):273–301. doi: 10.1016/0163-7827(89)90002-7. [DOI] [PubMed] [Google Scholar]
- 8.Gilmer JF, Murphy MA, Shannon JA, Breen CG, Ryder SA, Clancy JM. Single oral dose study of two isosorbide-based aspirin prodrugs in the dog. J Pharm Pharmacol. 2003;55(10):1351–1357. doi: 10.1211/0022357022007. [DOI] [PubMed] [Google Scholar]
- 9.Lee JJ, Yang H, Yoo YM, Hong SS, Lee D, Lee HJ, Lee HJ, Myung CS, Choi KC, Jeung EB. Morusinol extracted from Morus alba inhibits arterial thrombosis and modulates platelet activation for the treatment of cardiovascular disease. J Atheroscler Thromb. 2012;19(6):516–522. doi: 10.5551/jat.10058. [DOI] [PubMed] [Google Scholar]
- 10.Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43(10):1110–1111. doi: 10.1007/BF01956052. [DOI] [PubMed] [Google Scholar]
- 11.Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 1990;84(3):139–143. doi: 10.1159/000205051. [DOI] [PubMed] [Google Scholar]
- 12.Ko JH, Yan JP, Zhu L, Qi YP. Identification of two novel fibrinolytic enzymes from Bacillus subtilis QK02. Comp Biochem Physiol C Toxicol Pharmacol. 2004;137(1):65–74. doi: 10.1016/j.cca.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya S, Hagimori M, Ogasawara M, Arakawa M. In vivo evaluation method of the effect of nattokinase on carrageenan-induced tail thrombosis in a rat model. Acta Haematol. 2010;124(4):218–224. doi: 10.1159/000321518. [DOI] [PubMed] [Google Scholar]
- 14.Lee JJ, Jin YR, Yu JY, Munkhtsetseg T, Park ES, Lim Y, Kim TJ, Pyo MY, Hong JT, Yoo HS, Kim Y, Yun YP. Antithrombotic and antiplatelet activities of fenofibrate, a lipid-lowering drug. Atherosclerosis. 2009;206(2):375–382. doi: 10.1016/j.atherosclerosis.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Born GV, Cross MJ. The aggregation of blood platelets. J Physiol. 1963;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115(12):3355–3362. doi: 10.1172/JCI26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita M, Hong K, Ito Y, Fujii R, Kariya K, Nishimuro S. Thrombolytic effect of nattokinase on a chemically induced thrombosis model in rat. Biol Pharm Bull. 1995;18(10):1387–1391. doi: 10.1248/bpb.18.1387. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki Y, Kondo K, Matsumoto Y, Zhao BQ, Otsuguro K, Maeda T, Tsukamoto Y, Urano T, Umemura K. Dietary supplementation of fermented soybean, natto, suppresses intimal thickening and modulates the lysis of mural thrombi after endothelial injury in rat femoral artery. Life Sci. 2003;73(10):1289–1298. doi: 10.1016/s0024-3205(03)00426-0. [DOI] [PubMed] [Google Scholar]
- 19.Urano T, Ihara H, Umemura K, Suzuki Y, Oike M, Akita S, Tsukamoto Y, Suzuki I, Takada A. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis Cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem. 2001;276(27):24690–24696. doi: 10.1074/jbc.M101751200. [DOI] [PubMed] [Google Scholar]
- 20.Rao ChV, Ojha SK, Radhakrishnan K, Govindarajan R, Rastogi S, Mehrotra S, Pushpangadan P. Antiulcer activity of Utleria salicifolia rhizome extract. J Ethnopharmacol. 2004;91(2-3):243–249. doi: 10.1016/j.jep.2003.12.020. [DOI] [PubMed] [Google Scholar]