Abstract
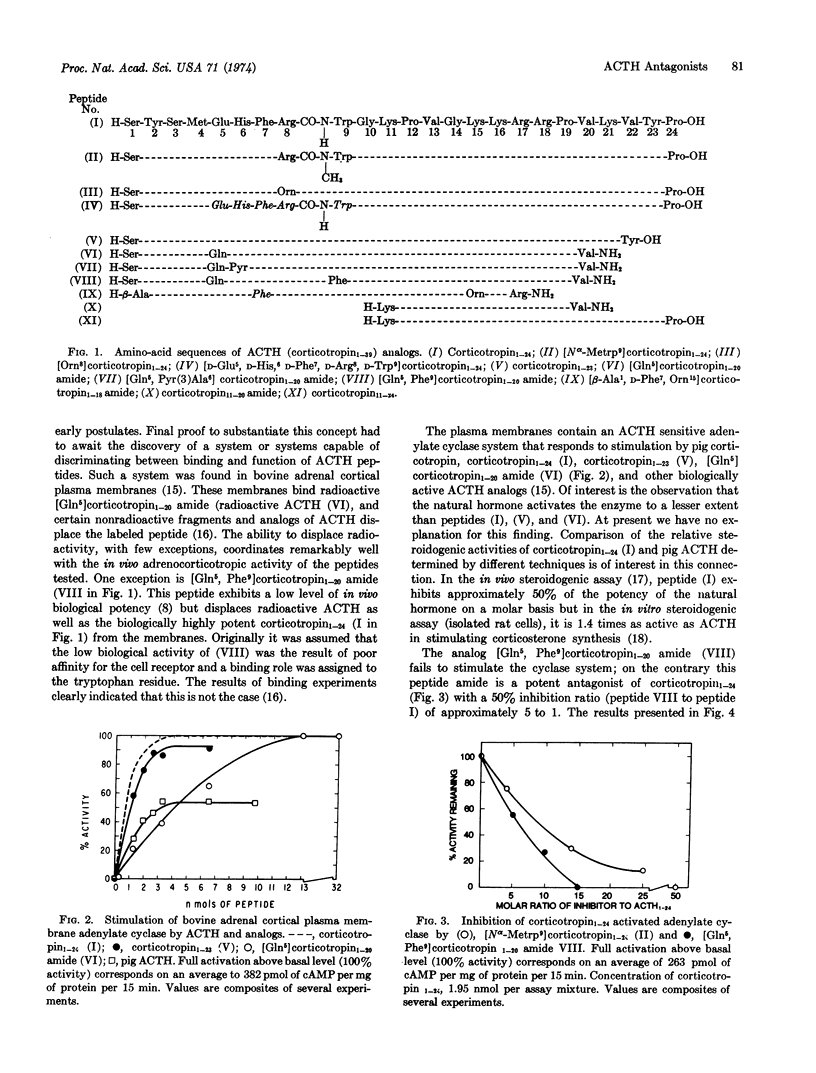
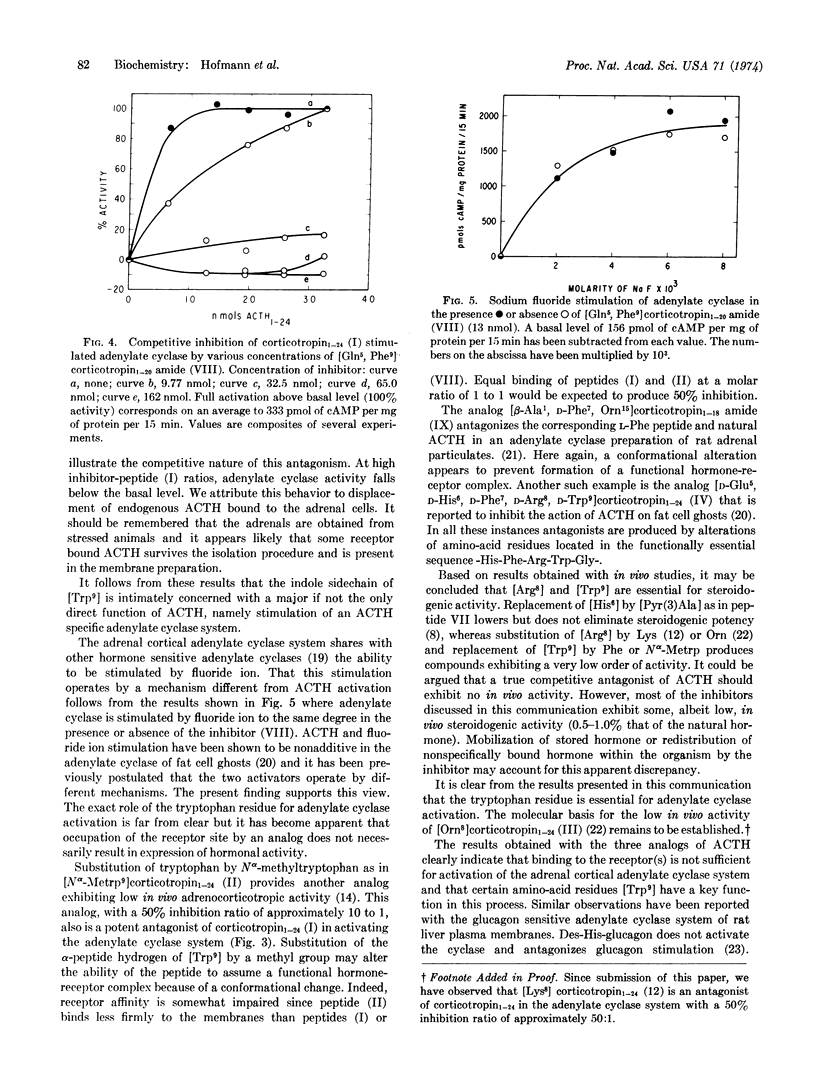
Structural modifications within the active site of the ACTH molecule have produced analogs that inhibit the hormone sensitive adenylate cyclase system of bovine adrenal cortical plasma membranes. It is demonstrated that the tryptophan residue of the ACTH molecule is essential for stimulation of the enzyme. Substitution of tryptophan by phenylalanine or by Nα-methyltryptophan as in [Gln5, Phe9]corticotropin1-20 amide or [Nα-Metrp9]corticotropin1-24 provides ACTH analogs that exhibit high affinity for the ACTH receptor(s) but fail to activate the adenylate cyclase system. It is concluded that affinity for the receptors alone is not sufficient for expression of hormonal activity. The observation that adrenal cortical adenylate cyclase activated by fluoride ion is not inhibited by the antagonists indicates that hormonal and fluoride activation proceed via different mechanisms.
Keywords: ACTH receptor, adenylate cyclase, S-peptide-S-protein system, peptide hormones, adrenal cortical plasma membranes
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- Finn F. M., Hofmann K. Studies on polypeptides. xxxvii. Competitive inhibition in the S-peptide--S-protein system. J Am Chem Soc. 1967 Sep 27;89(20):5298–5300. doi: 10.1021/ja00996a046. [DOI] [PubMed] [Google Scholar]
- Finn F. M., Widnell C. C., Hofmann K. Localization of an adrenocorticotropic hormone receptor on bovine adrenal cortical membranes. J Biol Chem. 1972 Sep 25;247(18):5695–5702. [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- HOFMANN K. Chemistry and function of polypeptide hormones. Annu Rev Biochem. 1962;31:213–246. doi: 10.1146/annurev.bi.31.070162.001241. [DOI] [PubMed] [Google Scholar]
- HOFMANN K. Preliminary observations relating structure and function in some pituitary hormones. Brookhaven Symp Biol. 1960 Nov;13:184–202. [PubMed] [Google Scholar]
- Hofmann K., Andreatta R., Bohn H., Moroder L. Studies on polypeptides. XLV. Structure-function studies in the beta-corticotropin series. J Med Chem. 1970 May;13(3):339–345. doi: 10.1021/jm00297a001. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Wingender W., Finn F. M. Correlation of adrenocorticotropic activity of ACTH analogs with degree of binding to an adrenal cortical particulate preparation. Proc Natl Acad Sci U S A. 1970 Oct;67(2):829–836. doi: 10.1073/pnas.67.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide M., Tanaka A., Nakamura M., Okabayashi T. Stimulation by ACTH analogs of rat adrenal adenyl cyclase activity: correlation with steroidogenic activity. Arch Biochem Biophys. 1972 Mar;149(1):189–196. doi: 10.1016/0003-9861(72)90314-1. [DOI] [PubMed] [Google Scholar]
- Moroder L., Hofmann K. Studies on polypeptides. XLVI. Synthesis of a stably labeled, biologically active adrenocorticotropin fragment. J Med Chem. 1970 Sep;13(5):839–843. doi: 10.1021/jm00299a010. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Choh Hao L. i. Structure-activity relationships of the adrenocorticotropins and melanotropins: the synthetic approach. Adv Enzymol Relat Areas Mol Biol. 1967;29:391–477. doi: 10.1002/9780470122747.ch8. [DOI] [PubMed] [Google Scholar]
- Richards F. M. ON THE ENZYMIC ACTIVITY OF SUBTILISIN-MODIFIED RIBONUCLEASE. Proc Natl Acad Sci U S A. 1958 Feb;44(2):162–166. doi: 10.1073/pnas.44.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Sundby F. The reaction of glucagon with its receptor: evidence for discrete regions of activity and binding in the glucagon molecule. Proc Natl Acad Sci U S A. 1971 May;68(5):909–913. doi: 10.1073/pnas.68.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWYZER R. CHEMISTRY AND METABOLIC ACTION OF NONSTEROID HORMONES. Annu Rev Biochem. 1964;33:259–286. doi: 10.1146/annurev.bi.33.070164.001355. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Schwyzer R., Schiller P., Seelig S., Sayers G. Isolated adrenal cells: Log dose response curves for steroidogenesis induced by ACTH(1-24), ACTH(1-10), ACTH(4-10) and ACTH(5-10). FEBS Lett. 1971 Dec 15;19(3):229–231. doi: 10.1016/0014-5793(71)80520-3. [DOI] [PubMed] [Google Scholar]
- Seelig S., Sayers G., Schwyzer R., Schiller P. Isolated adrenal cells: ACTH(11-24), a competitive antagonist of ACTH(1-39) and ACTH(1-10). FEBS Lett. 1971 Dec 15;19(3):232–234. doi: 10.1016/0014-5793(71)80521-5. [DOI] [PubMed] [Google Scholar]
- Tesser G. I., Maier R., Schenkel-Hulliger L., Barthe P. L., Kamber B., Rittel W. Biolobical activity of corticotrophin peptides with homo-arginine, lysine or ornithine substituted for arginine in position 8. Acta Endocrinol (Copenh) 1973 Sep;74(1):56–66. doi: 10.1530/acta.0.0740056. [DOI] [PubMed] [Google Scholar]
- WESTHEIMER F. H. Mechanisms related to enzyme catalysis. Adv Enzymol Relat Subj Biochem. 1962;24:441–482. doi: 10.1002/9780470124888.ch9. [DOI] [PubMed] [Google Scholar]


