Abstract
The decline of circulating testosterone levels in aging men is associated with adverse health effects. During studies of probiotic bacteria and obesity, we discovered that male mice routinely consuming purified lactic acid bacteria originally isolated from human milk had larger testicles and increased serum testosterone levels compared to their age-matched controls. Further investigation using microscopy-assisted histomorphometry of testicular tissue showed that mice consuming Lactobacillus reuteri in their drinking water had significantly increased seminiferous tubule cross-sectional profiles and increased spermatogenesis and Leydig cell numbers per testis when compared with matched diet counterparts This showed that criteria of gonadal aging were reduced after routinely consuming a purified microbe such as L. reuteri. We tested whether these features typical of sustained reproductive fitness may be due to anti-inflammatory properties of L. reuteri, and found that testicular mass and other indicators typical of old age were similarly restored to youthful levels using systemic administration of antibodies blocking pro-inflammatory cytokine interleukin-17A. This indicated that uncontrolled host inflammatory responses contributed to the testicular atrophy phenotype in aged mice. Reduced circulating testosterone levels have been implicated in many adverse effects; dietary L. reuteri or other probiotic supplementation may provide a viable natural approach to prevention of male hypogonadism, absent the controversy and side-effects of traditional therapies, and yield practical options for management of disorders typically associated with normal aging. These novel findings suggest a potential high impact for microbe therapy in public health by imparting hormonal and gonad features of reproductive fitness typical of much younger healthy individuals.
Introduction
The main cellular source of testosterone in male mammals is the Leydig cell, which resides in clusters within the testicular interstitium. Adult Leydig cell androgen production is regulated by luteinizing hormone-mediated signals, originating from the hypothalamus-pituitary gland axis [1]. In males, an inevitable reduction of circulating testosterone occurs with increasing age [2]–[4]. The mechanisms governing this phenomenon remain largely unknown. Studies in both humans and rodents, however, suggest that low testosterone is due to age-related lesions in testes rather than irregular luteinizing hormone metabolism [1], [5]–[7].
The reduction of testosterone has been implicated in many adverse effects of aging in men, including reduced spermatogenesis, libido and sexual function, increased body fat, decreased muscle and bone mass, low energy levels, fatigue, poor physical performance, depressed mood, and impaired cognitive dysfunction [8]–[11]. Whether low testosterone can be considered the cause of a defined clinical entity in elderly men or not has been a matter of debate [12]. Additionally, different names have been used to describe this condition (male menopause, andropause, partial androgen deficiency of the aging male and late-onset hypogonadism) and different criteria have been used to define it, hence the discrepancies of epidemiological studies during reporting on its prevalence [12]–[13]. Nonetheless, there is an unquestionable consensus in that age-related progressive morphological and functional alterations of Leydig cells lead to low testosterone levels that, in turn, reduce reproductive fitness and affect the quality of life of the aging male [1], [4]–[5], [9]–[10], [12]–[13].
Various dietary factors and diet-induced obesity have been shown to increase the risk for late onset male hypogonadism and low testosterone production in both humans and mice. Testosterone deficiency and metabolic diseases such as obesity appear to inter-digitate in complex cause-and-effect relationships [14]–[17].
Recently we have found that the dietary supplementation of aged mice with the probiotic bacterium Lactobacillus reuteri makes them appear to be younger than their matched untreated sibling mice, at least in part by inducing beneficial integumentary effects that manifest as luxuriant hair [18] and inhibition of diet-induced obesity [19]. Interestingly, both these effects were linked with a CD4+ regulatory-T cell and Il-10-associated systematic down-regulation of the pro-inflammatory cytokine Il-17 [18]–[19]. These results indicate that gut microbiota induce modulation of local gastrointestinal immunity resulting in systemic effects on the immune system which activate metabolic pathways that restore tissue homeostasis and overall health. Indeed, in another study we discovered that aged mice eating L. reuteri show accelerated healing of skin wounds, which depends upon the suppressive arm of the immune system and the up-regulation of the pituitary gland neuropeptide hormone oxytocin (manuscript submitted for publication). During all these studies we consistently observed that young and aged mice consuming purified L. reuteri organisms had particularly large testes and a dominant male behavior.
Our previous studies and observations, taken together, led us to hypothesize that dietary L. reuteri may act to prevent age- and obesity-related testicular atrophy in mice. The results of the present study confirmed this hypothesis. The testes of probiotic-fed aged mice were rescued from both seminiferous tubule atrophy and interstitial Leydig cell area reduction typical of the normal aging process. Preservation of testicular architecture despite advanced age or high-fat diet coincided with remarkably high levels of circulating testosterone. The beneficial effects of probiotic consumption were recapitulated by the depletion of the pro-inflammatory cytokine Il-17.
Given the aggravated controversy about the benefits and side-effect risks of testosterone replacement therapy [9]–[10], [13], [20], dietary L. reuteri may provide an alternative natural approach to the prevention of late-onset male hypogonadism.
Results
Lactobacillus reuteri Consumption Increases Testicular Weight of Mice Fed with Normal Diet
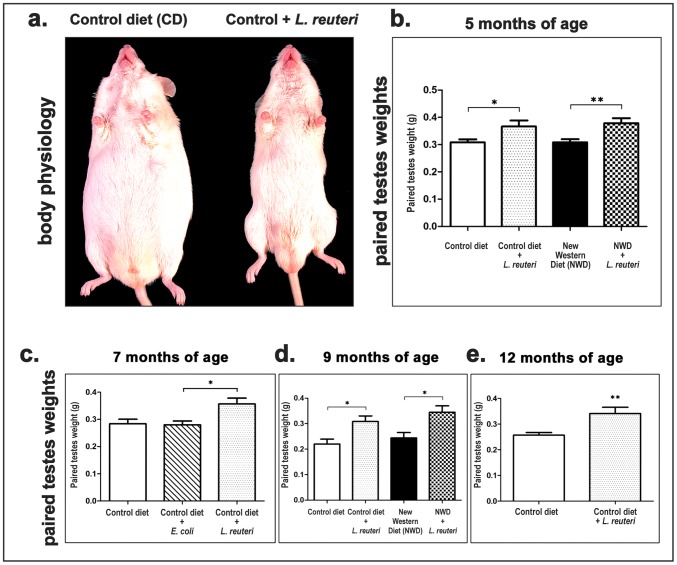
We observed unusually large testes and social dominance behaviors in male mouse models while feeding probiotic yogurt or purified lactic acid bacteria during obesity and cancer studies (Figure 1a). To test specific effects of probiotics on testes, outbred Swiss mice on normal (control) diet were given Lactobacillus reuteri ATCC 6475 using a dosage of 3.5×105 organisms/mouse/day starting at 2 months of age and their testes were weighed at 3, 5, 7 and 10 months later. At each time-point the weight of testes from mice consuming probiotics was significantly higher than that of control mice. Specifically, at five months (CD vs CD+LR, P = 0.0408) (Fig. 1b), seven months (CD vs CD+LR, P = 0.0328) (Fig. 1c), nine months (CD vs CD+LR, P = 0.0178) (Fig. 1e), and 12 months of age (CD vs CD+LR, P = 0.0062) (Fig. 1e) the statistical significance of testes weight difference between probiotic-treated and non-treated groups of mice progressively increased with age. When the testes weight values were adjusted for body weight (testis weight/body weight ratio) the L. reuteri effect was more pronounced in all different age time-points examined (Fig. S1). While feeding of L. reuteri consistently increased the gonadal weights, consumption of a non-pathogenic strain of Escherichia coli (E. coli) K12 organisms did not affect testicular weight (CD+E.coli vs CD+LR, P = 0.026) (Fig. 1c) or the testicular/body weight ratio when compared at age 7 months, confirming the specificity of the probiotic organism effect (Fig. S1).
Figure 1. Consumption of probiotic microbes increases the weight of mouse testes.
a. Gross appearance of outbred Swiss male mice with oral Lactobacillus reuteri treatment at 12 months of age. Testes of L. reuteri-fed mice are larger compared to control mice. b–e. Results of statistical analyses of testes weight at different time-points after starting treatment, including the (b) 5, (c) 7, (d) 9 and (e) 12 months of age time-points. L. reuteri consistently increased testicular weight in both control- and new western-diet-fed mice compared to age- and diet-matched controls. Dietary supplementation with L. reuteri, but not with E coli K12, increased testicular weight in control-diet-fed mice at age 7 months when compared to age- and diet-matched controls (c). Numbers on the y-axis of bar graphs correspond to the mean±SEM of testes weight or the testicular weight; *p<0.05, **p<0.001.
L. reuteri Increases Testicular Weight of Mice on High-fat Diet
Since obesity has been reported to have an adverse effect on testicular function [14]–[17], we next sought to examine whether consumption of L. reuteri could increase testicular weight of obese mice consuming a commercially available New Westernized type of diet (NWD) with high fat and low fiber with substandard levels of B vitamins and Vitamin D mimicking human ‘fast food’ diets. We found that probiotic-fed mice had significantly increased testicular weight compared to age- and diet-matched controls at five months (NWD vs NWD+LR, P = 0.0096) (Fig. 1b) and nine months (NWD vs NWD+LR, P = 0.0491) of age (Fig. 1d). Similar to what we have recently reported, these mice with dietary L. reuteri supplements were rescued from diet-induced obesity and had normal body weight and lean physique [19]. Therefore, when testicular weight values adjusted for body weight (instead of absolute testes weight values) were analyzed, the significance of statistical differences was increased at both five month (NWD vs NWD+LR, P = 0.0007) and nine month time points (NWD vs NWD+LR, P = 0.0159) (Fig. S1).
Testes from L. reuteri-treated Mice have Increased Seminiferous Tubule Cross-sectional Profile Areas
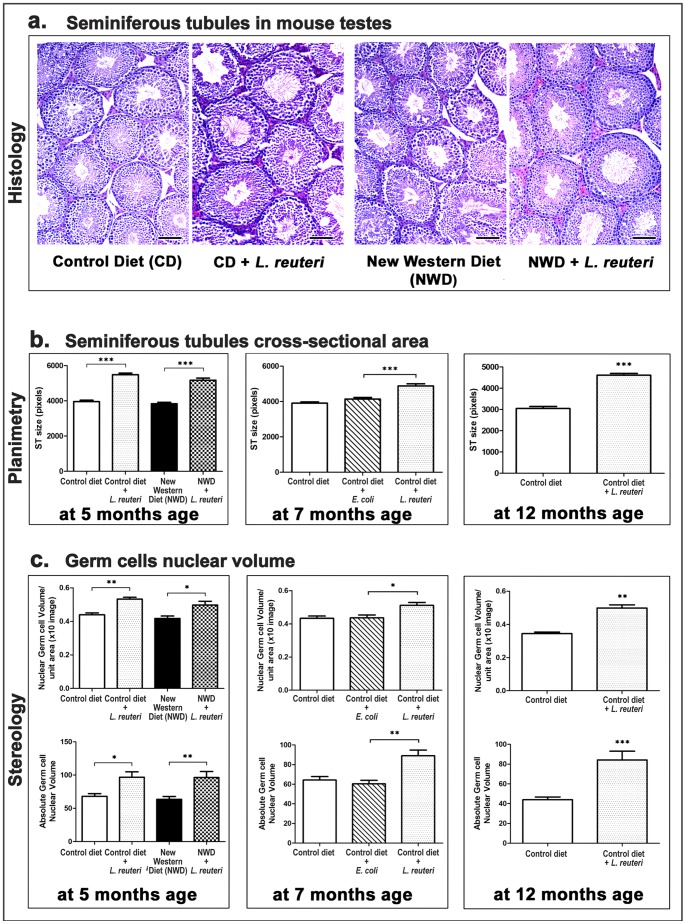
In order to explain the probiotic-associated increase in paired testicular mass, we applied microscopy–assisted histomorphometry and assessed critical parameters of testes architecture. We found that L. reuteri consumption did not affect the total number of seminiferous tubule (ST) profiles counted in histological sections of testes. At 5 months of age there was no difference in ST counts of both control and NWD-fed groups of mice. Similar results were obtained from the testes of mice at seven months and 12 months (data not shown). Despite the comparable numbers of ST profiles, we determined that testes from L. reuteri-treated mice had increased ST cross-sectioned profiles (Fig. 2a). To quantify this result we measured the area size of circular ST profiles in experimental groups. The statistical analysis of results confirmed our observations. At 5 months of age circular ST profiles from L. reuteri-fed mouse testes were significantly larger than those of their diet-matched counterparts (CD vs CD+LR, P<0.0001; NWD vs NWD+LR, P<0.0001). Similar pronounced statistical differences were found at seven months (CD vs CD+LR, P<0.0001; CD+E.coli vs CD+LR, P<0.0001) and 12 months of age (CD vs CD+LR, P<0.0001) (Fig. 2b). To determine whether increased cross-sectional area of ST profiles in L. reuteri-treated mice was due to increased germ cells, we further measured germ cell nuclear volumes using point-counting, stereology-based morphometry. We found that the nuclear volume of germ cells per unit size area (×10 image) was significantly higher in mice consuming L. reuteri at 5 months (CD vs CD+LR, P = 0.0026; NWD vs NWD+LR, P = 0.04), at 7 months (CD vs CD+LR, P = 0.0103; CD+E.coli vs CD+LR, P = 0.018) and at 12 months of age (CD vs CD+LR, P = 0.0021) (Fig. 2c). Consequently, the absolute nuclear volume of germ cells per testis in those experimental groups was also significantly higher at 5 (CD vs CD+LR, P = 0.0126; NWD vs NWD+LR, P = 0.007), 7 (CD vs CD+LR, P = 0.0026; CD+E.coli vs CD+LR, P = 0.0026) and 12 months of age (CD vs CD+LR, P = 0.0006) (Fig. 2c). When these results were expressed as the relative percentage of nuclear volume of germ cells per testis and analyzed, we found percentage values that were comparable between experimental groups (data not shown). This result suggests that the increase of testes weight due to L. reuteri consumption was proportional to the increase of the testicular germ cell population.
Figure 2. Dietary L. reuteri increases the cross-sectional profile area of seminiferous tubules and their germ cell population.
a. Representative histology of seminiferous tubules cross-sectioned profiles of Swiss mice at the age of 5 months. Increased testicular weight due to L. reuteri consumption in both control- and New Western diet (NWD)-fed mice co-existed with a histologically discernible increase of seminiferous tubule (ST) cross-sectional area. Hematoxylin and eosin. Bars = 100 µm. b. Histomorphometrical assessment of circular ST cross-sectioned profiles size in different time points. The effect of L. reuteri remains highly significant with the progression of age. Numbers on the y axis of bar graphs correspond to the mean±SEM of ST area size; *** p<0.0001. c. Stereological morphometric assessment shows that L. reuteri-fed mouse testes have significantly increased nuclear volumes of germ cells. Numbers on the y axis of bar graphs correspond to the mean±SEM of “Nuclear volume of germ cells per unit area” or the “absolute nuclear volume of germ cells”; *p<0.05, **p<0.001, ***p<0.0001.
Testes from L. reuteri-treated Mice have More Conspicuous Leydig Cell Areas
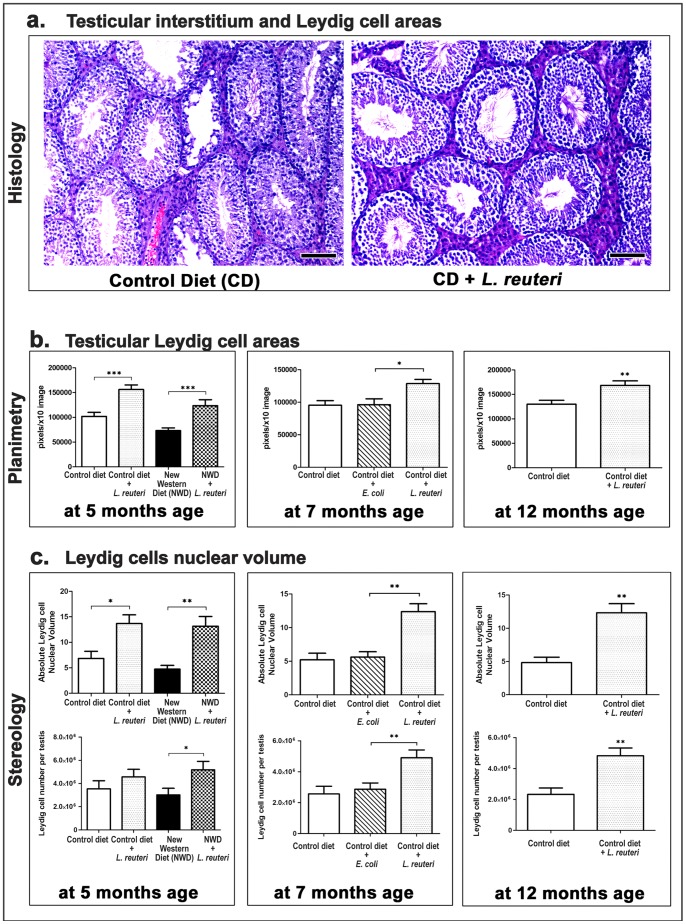
Our histopathological examination revealed that the L. reuteri effect on mouse testicular tissue was not limited to ST's but also extended to the interstitium. Specifically, the probiotic organism induced prominent Leydig cell accumulations in the interstitial tissue between the ST's (Fig. 3a). To quantify this observation further we morphometrically assessed Leydig cell areas in the different treatment groups. At 5 months of age there were significant differences in Leydig cell area counts of both control (CD vs CD+LR, P<0.0001) and NWD-fed groups of mice (NWD vs NWD+LR, P = 0.0002). The probiotic-associated increase of interstitial Leydig cell areas was sustained with advancing age at 7 (CD vs CD+LR, P = 0.0025; CD+E.coli vs CD+LR, P = 0.0251) and 12 months (CD vs CD+LR, P = 0.0012) (Fig. 3b).
Figure 3. L. reuteri consumption increases the size of interstitial Leydig cell areas and Leydig cell numbers.
a. Representative histology of Swiss mice testes at the age of 12 months. Leydig cell areas in the testicular interstitium of probiotic-fed mice are of increased size compared to the corresponding areas of control mice. Hematoxylin and eosin. Bars = 100 µm b. Histomorphometrical counts of Leydig cell areas at different time-points revealed the statistical significance of the L. reuteri effect. Numbers on the y-axis of bar graphs correspond to the mean±SEM of Leydig area size. c. Point-counting stereology counts reveal that L. reuteri-fed mouse testes have significantly increased nuclear volumes and an increased number of Leydig cells per testis. Numbers on the y axis of bar graphs correspond to the mean±SEM of “Nuclear volume of germ cells per unit area” or the “absolute nuclear volume of germ cells”; *p<0.05, **p<0.001, ***p<0.0001.
Using the point-counting method, we found that the nuclear volumes of Leydig cells per unit size area (×10 image) was significantly higher in mice eating the probiotic organism at 5 months (CD vs CD+LR, P = 0.0136; NWD vs NWD+LR, P = 0.0055), at 7 months (CD vs CD+LR, P = 0.0031; CD+E.coli vs CD+LR, P = 0.0036) and at 12 months of age (CD vs CD+LR, P = 0.0036) (Fig. S2a). As expected, the absolute nuclear volume of Leydig cells per testis in those experimental groups was also significantly higher at 5 (CD vs CD+LR, P = 0.0175; NWD vs NWD+LR, P = 0.0012), 7 (CD vs CD+LR, P = 0.0023; CD+E.coli vs CD+LR, P = 0.0023) and 12 months of age (CD vs CD+LR, P = 0.0026) (Fig. S2b). The relative percentages of nuclear volumes of Leydig cells per testis were significantly higher in the L. reuteri-fed mice at the age of 5 months (CD vs CD+LR, P = 0.0111; NWD vs NWD+LR, P = 0.0111), at the age of 7 months (CD vs CD+LR, P = 0.0006; CD+E.coli vs CD+LR, P = 0.0041) and at the age of 12 months (CD vs CD+LR, P = 0.0126) (Fig. S2a). Therefore, the increase of the testicular Leydig cell population was disproportionally higher compared to the increase of testes weight in the mice consuming L. reuteri, which is highly suggestive of a particularly robust effect of the probiotic organism on Leydig cells.
In order to provide further evidence for this effect, we morphometrically examined the nuclear diameter of Leydig cells in the different experimental groups of mice. The size of Leydig cell nuclei was consistently higher in the groups of mice fed with L. reuteri at 5 months (CD vs CD+LR, P<0.0001; NWD vs NWD+LR, P<0.0001), at 7 months (CD vs CD+LR, P<0.0001; CD+E.coli vs CD+LR, P<0.0001) and at 12 months of age (CD vs CD+LR, P = 0.0009) (Fig. S2c).
By using the Floderus equation and the nuclear volumes of Leydig cells per unit size area, the Leydig cell number contained in size unit areas was calculated. Having determined the mean nuclear diameter of Leydig cells contained in testes of known volume (weight), we were next able to assess the mean nuclear volume and, finally, the total Leydig cell number contained in each testis. The analysis of these data showed that the number of Leydig cells per testis was higher in L. reuteri-treated mice at 5 months (CD vs CD+LR, P = 0.0963; NWD vs NWD+LR, P = 0.0262), at 7 months (CD vs CD+LR, P = 0.0041; CD+E.coli vs CD+LR, P = 0.0111) and at 12 months of age (CD vs CD+LR, P = 0.007) (Fig. 3c). Taken together, these results show that the more conspicuous Leydig cell areas characterizing the testes of L. reuteri-fed mice are due to increased numbers of Leydig cells which, in addition, bear nuclei of increased size.
To further elaborate on this result we assessed immunohistochemically proliferation and apoptosis in the testes of 5 and 12 months-old mice. Apoptotic (Caspase3+) Leydig cells were practically undetectable at both time-points. Small numbers of proliferating (Ki-67+) cells, however, did exist in the testicular interstitium of 5 months-old mice. Ki-67+ cells had a large, round, centrally located nucleus and abundant vacuolated cytoplasm (Fig. 4a). Based on their typical morphology and the presumptive absence of foamy macrophages (which in granulation tissue might resemble vacuolated Leydig cells) these proliferating cells were identified as Leydig cells. Morphometric counts of ki-67+ Leydig cells in the different treatment groups revealed significant differences in proliferating Leydig cell counts of both control (CD vs CD+LR, P = 0.0024) and NWD-fed groups of mice (NWD vs NWD+LR, P = 0.0049) (Fig. 4a).
Figure 4. L. reuteri increases Leydig cell proliferation and serum testosterone levels.
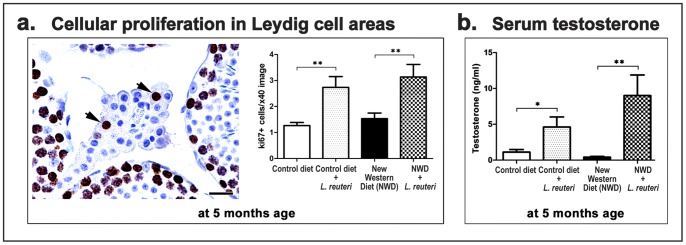
a. At the age of 5 months a small number of proliferating (Ki-67+) Leydig cells did exist in the testicular interstitium (arrows). Proliferating Leydig cells were significantly more in probiotic-fed mice. DAB chromogen, Hematoxylin counterstain. Bars = 25 µm. The y-axis of the bar graph stands for the mean±SEM of Ki-67+ Leydig cells. b. Serum testosterone levels are significantly higher when consuming L. reuteri, as compared to their diet-matched controls. Numbers on the y-axis corresponds to the mean±SEM of serum testosterone level in ng/ml.; *p<0.05, **p<0.001.
L. reuteri Induces Elevation of Serum Testosterone
Given that the Leydig cell is the main cellular source of testosterone, we next examined the effect of the probiotic organism in testosterone serum levels of mice at the age of 5 months. We found that mice eating L. reuteri had profoundly increased levels of circulating testosterone regardless of the type of diet they consumed (CD mice vs CD+LR, P = 0.0266) and NWD-fed groups of mice (NWD vs NWD+LR, P = 0.0037) (Fig. 4b).
L. reuteri Consumption Benefits Post-testicular Sperm Attributes
Testes histomorphometry and serum androgen concentration data were both suggestive of a probiotic-associated up-regulation of spermatogenesis in mice. To directly test this, we assessed sperm concentration and activity in sperm gathered form epididymites of mice at the age of 12 months. Sperm from L. reuteri-treated mice had significantly higher concentration (CD vs CD+LR, P = 0.0286) and activity (CD vs CD+LR, P = 0,0286) compared to non-treated age-matched control mice (Fig. S3).
L. reuteri Counteracts Age-associated Testicular Atrophy
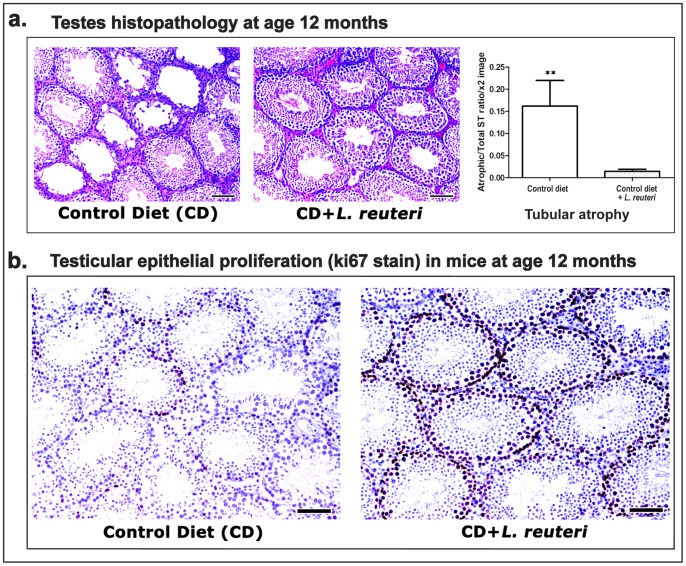
Aged-related histopathological changes of spermatogenic epithelium have been described in both rodents and humans. In the present study, we found that mice eating control chow exhibited age-related changes in the testes at approximately the age of 7 months. These changes included ST epithelial vacuolation, segmental reduction of germ cells in ST profiles and the presence of small numbers of ST profiles lined only with Sertoli cells (end-stage atrophic tubules). At the age of 12 months the same lesions appeared with increased frequency and distribution (Fig. 5a). In addition, there were small number of tubules with atypical residual bodies and occasional ST's showing features of mineralization. In order to quantify the effect of L. reuteri in age-related atrophy of ST, we morphometrically measured the atrophic ST/Total ST ratio in the testes of mice at 12 months of age. We found that the atrophic tubule percentage was significantly less in mice fed with the probiotic organism compared to control mice (CD vs CD+LR, P = 0.0043) (Fig. 5a). Caspase-3-specific immunohistochemistry showed that germ cell apoptosis was unremarkable in both L. reuteri-treated and non-treated mice. Proliferating ki-67+ ST epithelial cells, however, were evidently increased in the mice consuming the probiotic organism (Fig. 5b).
Figure 5. Dietary L. reuteri rescues mice from age-associated testicular atrophy.
a. Representative histology of Swiss mouse testes at the age of 12 months. The aged control mouse testes demonstrated atrophic seminiferous tubules. By contrast, L. reuteri-fed mice had uniformly normal ST epithelium with rare atrophic tubules. Hematoxylin and eosin. Bars = 100 µm. The y-axis in the bar graph stands for the mean±SEM of the atrophic/total ST ratio counted in each experimental group;**p<0.001. b. Ki-67-specific immunohistochemistry in the testes of 12-months-old mice. A side-by-side comparison of representative images from control and L. reuteri-fed mouse testes reveals that the probiotic organism counteracts the age-associated decrease of epithelial proliferation observed in control mice. DAB chromogen, Hematoxylin counterstain. Bars = 100 µm.
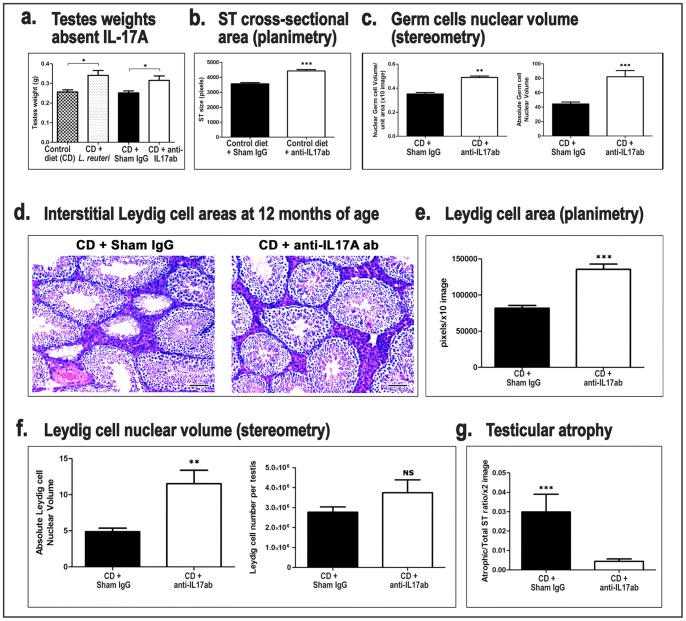
Blocking Il-17 Recapitulates the L. reuteri Effect on Mouse Testes
In our previous studies we found that dietary probiotics counteract obesity [19] and age-related integumentary pathology [18] at least in part by down-regulating systemic pro-inflammatory IL-17A-dependent signaling. To test whether beneficial effects of L. reuteri within testes also associate with this anti-inflammatory mechanism, we examined the effect of Il-17 depletion in the testes of 12-month-old mice. We found that blocking pro-inflammatory Il-17 signaling entirely recapitulates the beneficial effects of probiotics (Fig. 6). The weight of testes of Il-17-depleted mice was significantly higher than that of sham IgG-treated control mice (CD+anti-ShamIgG vs CD+anti-IL17, P = 0.0230) (Fig. 6a). The testis weight/body weight analysis had a similar outcomes (Fig. S1). As with feeding of L. reuteri, the anti-IL17 treatment had no effect in total ST number (data not shown), but it did affect significantly the cross-sectional profile size of ST (CD+anti-ShamIgG vs CD+anti-IL17, P<0.0001) (Fig. 6b). The point-counting stereometry method showed that nuclear volume of germ cells per unit size area (CD+anti-ShamIgG vs CD+anti-IL17, P = 0.0021) and the absolute nuclear volume of germ cells per testis (CD+anti-ShamIgG vs CD+anti-IL17, P = 0.0006) were also increased with IL-17 neutralization (Fig. 6c). As with probiotic dietary supplementation, IL-17 depletion did not affect the relative percentages of nuclear volumes of germ cells per testis (data not shown).
Figure 6. Systemic depletion of IL-17 recapitulates reproductive fitness attributes of L. reuteri.
Compared to sham IgG-treated control mice, 12 month-old mice depleted of IL-17A had a. increased paired testicular weights; b. increased cross-sectional ST areas; c. increased germ cell nuclear volumes; d and e. increased interstitial Leydig cell areas. Hematoxylin and eosin. Bars = 100 µm; f. increased Leydig cell nuclear volumes and total number per testis and g. decreased ST atrophy. *p<0.05, **p<0.001, ***p<0.0001.
The Leydig cell area size also increased (Fig. 6d) with depleting mice from IL-17 (CD+anti-ShamIgG vs CD+anti-IL17, P<0.0001) (Fig. 6e) in a manner similar to L. reuteri treatment in age-matched Swiss male mice. This result was further confirmed by stereological Leydig cell nuclear volume measurements. According to these, the nuclear volume of Leydig cells per unit size area (CD+anti-ShamIgG vs CD+anti-IL17, P = 0.003) (Fig. S2a), the absolute nuclear volume of Leydig cells per testis (CD+anti-ShamIgG vs CD+anti-IL17, P = 0.0021) (Fig. 6f) and the relative percentages of nuclear volumes of Leydig cells per testis (CD+anti-ShamIgG vs CD+anti-IL17, P = 0.0213) (Fig. S2b) were all significantly increased after depleting IL-17. Likewise, the nuclear diameter of Leydig cells was increased with this anti-inflammatory treatment (CD+anti-ShamIgG vs CD+anti-IL17, P<0.0001) (Fig. S2c). The total number of Leydig cells per testis was also increased. This difference, however, did not reach statistical significance (CD+anti-ShamIgG vs CD+anti-IL17, P = 0.3706) (Fig. 6f).
Finally, the age-related atrophy exhibited per total ST percentage was ameliorated (CD+anti-ShamIgG vs CD+anti-IL17, P<0.0001) (Fig. 6g). These findings showed that systemic reduction in inflammation in the form of IL-17A was sufficient to mimic the testicular benefits of oral L. reuteri supplementation.
Discussion
While conducting diet studies involving probiotic microbe Lactobacillus reuteri we discovered that aging male animals had larger testes compared to their age-matched controls [18]–[19]. This led us to hypothesize that dietary L. reuteri supplementation may act to prevent age- and diet-related testicular atrophy in mice. Knowing that age-related hypogonadism has been linked with functional alterations of Leydig cells and low testosterone levels that reduce reproductive fitness and quality of life [1], [4]–[5], [9]–[10], [12]–[13], we examined testes of probiotic microbe-fed mice and found that they had less testicular atrophy coinciding with higher levels of circulating testosterone compared to their age-matched controls. Similar testicular health benefits were produced using systemic depletion of the pro-inflammatory cytokine Il-17 alone, implicating a chronic inflammatory pathway in hypogonadism. Based on these observations, we propose a model whereby probiotic bacteria modulate gastrointestinal immunity resulting in systemic effects on the immune system that activate metabolic pathways that restore tissue homeostasis and overall health.
One specific aspect of this paradigm is reciprocal activities of pro-inflammatory Th-17 and anti-inflammatory Treg cells [21]. Along mucosal surfaces, anti-inflammatory cytokine Interleukin (IL)-10 facilitates immune tolerance [22] and recruitment of CD4+ Treg cells to skew host immunity away from pro-inflammatory IL-17. Feeding of L. reuteri organisms was previously shown to up-regulate IL-10 levels and reduce levels of IL-17 [19] serving to lower systemic inflammation. Conversely, insufficient levels of IL-10 may increase the risk for autoimmunity, obesity, and other inflammatory disease syndromes [23]–[27]. Indeed, many health disorders associated with Westernized life are believed due to insufficient IL-10 and improper host immune calibration resulting in uncontrollable inflammation [28]. Westernized diets are also low in vitamin D, a nutrient that when present normally works together with IL-10 to protect against inflammatory disorders [29]–[31] and even some types of cancer [32]. Physiological feedback loops apparently exist between microbes, host hormones, and immunity. The hormone testosterone has been shown to act directly through androgen receptors on CD4+ cells to increase IL-10 expression [33]. Leydig cells are the main cellular source of testosterone in male mammals that naturally decline during aging [2]–[4]. Although adult Leydig cell androgen production is regulated by LH-mediated signals originating from the hypothalamus-pituitary gland axis [1], studies in both humans and rodents suggest that hypogonadism is due to age-related lesions in testes rather than irregular LH metabolism [1], [5]–[7]. Further studies of LH and the hypothalamus-pituitary axis are underway to investigate this possibility.
We investigated spermatogenesis and other features associated with reproductive fitness [8]–[11], seeing significant retention of testicular heath criteria after feeding of L. reuteri. We postulate that probiotic gut microbes function symbiotically with their mammalian hosts to impart immune homeostasis to maintain systemic and testicular health [34]–[35] despite suboptimal dietary conditions. Dietary factors and diet-induced obesity were previously shown to increase risk for age-associated male hypogonadism, reduced spermatogenesis, and low testosterone production in both humans and mice [2]–[4], [8]–[11], [14]–[17], phenotypic features that in this study were inhibited by oral probiotic therapy absent milk sugars, extra protein, or vitamin D supplied in yogurt. Similar beneficial effects of probiotic microbes on testosterone levels and sperm indices were reported in male mice that had been simultaneously supplemented with selenium [36]. Although neither of these studies analyze testicles of human males, probiotic yogurt did produce leaner physiques in mice [19] matching findings of a large epidemiological survey of human subjects when eating yogurt [37] [38]–[43]. Additional studies using human subjects are needed to assess translational potential. A proposed health-protective role for L. reuteri in mammalian host metabolism offers mutually beneficial gut symbiont-host relationship reflecting co-evolutionary relationships [44].
From an evolutionary perspective, we assert that lactic acid bacteria may have co-evolved with mammals exploiting testosterone to optimize mental, physical, and reproductive fitness. Higher serum testosterone levels compared to controls in our separate studies correlated with not only leaner physique but also increased muscle mass and higher activity levels in mice (data not shown). Benefits of this microbial synergy may extend beyond individual fitness to reproductive success, impacting a natural selection process favoring evolutionary success for the microbe and mammalian host.
Our data suggest that the L. reuteri-associated prevention of age- and diet-related testicular atrophy correlates with increased numbers and size of Leydig cells. At the earliest time-point examined in the present study (age = 5months), we found no histological evidence of age-related testicular atrophy in mice. This matches the results of a previous study, which describes that the initial changes of testicular atrophy begin to occur in mice from the age of 6 moths onwards [7] and indicates that the trophic effect of L. reuteri on Leydig cells is a key event which precedes and prevents age-related changes in the testes of mice. This effect is reminiscent of earlier studies describing Leydig cell hyperplasia and/or hypertrophy in the mouse and the rat testis that were achievable by the administration of gonadotropins, including human chorionic gonadotropin, FSH and LH [45]–[49].
The similarity of the effects of hypopheseal hormones and L. reuteri administration on Leydig cells warrant further investigation, especially in the light of recent evidence for the effects of edible Lactobaccili in the hypothalamus and pituitary gland [50]–[51]. In human males as well as in Brown Norway rats, a strain that is often used to study the effects of aging in Leydig cells, the age-associated decrease of serum testosterone does not co-exist with decreased plasma luteinizing hormone (LH) concentrations. This indicates that sub-normal testosterone levels are due to testicular dysfunction rather than a disruption of the hypothalamus-pituitary axis. The primary gonadal deficit was found to result from compromised Leydig cell steroidogenesis, but not from their loss [1], [4]–[6]. Studies in other strains of rats as well as recent studies in humans, however, suggest that LH and the hypothalamic-pituitary level are important in male hypogonadism [1], [4]–[6], [52]. These discrepancies make it difficult to determine whether the L. reuteri-induced effects found in mice in the present study could be directly relevant to aging human males. The restoration of testosterone levels, if proven true as an L. reuteri-induced effect in aged humans, would directly benefit their health regardless to whether the level of the L. reuteri intervention in the hypothalamus-pituitary-testis axis [9], [11], [16], [20].
Taken together, our data indicate that probiotic organisms may offer practical options for management of disorders frequently associated with normal aging. Reduced circulating testosterone levels have been implicated in many adverse effects including reduced spermatogenesis, libido and sexual function, increased body fat, decreased muscle and bone mass, low energy levels, fatigue, poor physical performance, depressed mood, and impaired cognitive dysfunction [8]–[11]. Ultimately, dietary L. reuteri or other probiotic supplementation may provide an alternative natural approach to prevention of male hypogonadism, absent the controversy and side-effect risks of testosterone replacement therapy [9]–[10], [13], [20]. Such microbial immune system re-programming may ultimately target other diseases linked to low testosterone including increased body fat, decreased muscle and bone mass, weak physical and mental performance, and depressed mood [8]–[11] for more healthful longevity.
Experimental Procedures
Animals
Genetically outbred CD-1 mice (Charles River; Wilmington, MA) were housed and handled in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facilities with diets, experimental methods, and housing as specifically approved by the Institutional Animal Care and Use Committee. The MIT CAC (IACUC) specifically approved the studies as well as the housing and handling of these animals. Mice were bred in-house or purchased directly from the vendor to achieve experimental groups. Mice were housed in groups of four animals per cage, except for those animals specifically used in breeding experiments.
The experimental design was to expose mice to diets starting at age = eight weeks, and then continue the treatment until euthanasia using carbon dioxide: 1) for 12 weeks to achieve the cohort collected at five months of age; 2) for 20 weeks to achieve the cohort collected at seven months of age; 3) for 28 weeks to achieve the cohort collected at nine months of age; and 4) for 40 weeks to achieve the cohort collected at 12 months of age. Each experiment included 5–10 animals per group with at least one replicate for each experiment. Necropsy procedures were performed in mid-afternoon to standardize collections relative to circadian rhythms. After humane euthanasia with CO2 overdose, 1 ml of blood was collected using cardiac puncture method in unconscious animals. Body weights of whole mice or their isolated paired testes were recorded (Scout pro sp202; Ohaus Corporation, Pine Brook NJ) for comparisons between treatment groups.
Special Diets for Animals
Mice of 6–8 wks were placed on experimental diets: control AIN-76A (Harlan-Teklad Madison WI), and a Westernized diet with high fat and low fiber with substandard levels of Vitamin D (TD.96096; Harlan-Teklad) starting at 8 weeks of age until euthanasia at time points of five months of age, seven months of age, nine months of age, or 12 months of age. Separate groups of animals received a purified preparation of an anti-inflammatory strain of Lactobacillus reuteri ATCC PTA 6475 cultivated as described elsewhere [53] using a dosage of 3.5×105 organisms/mouse/day, or a sham E coli K12 3.5×105 organisms/mouse/day in drinking water. Drinking water was replaced twice weekly.
Systemic Depletion of Interleukin-17A using Anti-cytokine Antibodies
Mice at age 6 months or older were treated with intraperitoneal injection of anti-IL17A antibody (clone 17F3; BioXcel, Lebanon, NH) at 500 µg per mouse three times weekly for at least four weeks. Mice were then euthanized and compared with age-matched mice that received isotype-matched sham IgG antibody alone.
Measurements of Serum Testosterone
Terminal cardiac blood collections were performed upon euthanasia in mid-afternoon to normalize for Circadian rhythm effect on testosterone. RadioImmunoAssay (RIA) was performed using Coat-A-Count Total Testosterone RIA kit (Siemens Diagnostics CA) as performed by AniLytics Inc (Gaithersburg MD).
Quantification of Sperm Quality
After humane euthanasia using C02, both testes were isolated to remove combined epididymal and vas tissues from a particular male mouse. Tissue was immediately placed into a plastic Petri dish and immersed in 1 ml FHM solution (Specialty medium, Millipore). Under dissecting microscope, sperm were mobilized from each epididymis and vas deferens using microdissection with a 16 G needle. The dish was placed to a CO2 incubator at 37°C for 10 minutes to allow for complete sperm mobilization. Sperm were then suspended 1∶10 with FHM to evaluate sperm concentration using a hemocytometer (Fisher). Sperm concentration/ml was calculated using (Dilution factor)×(count number in 5 squares)×(0.05×106). Sperm activity was assessed as number of actively moving sperm/total number of sperm [54]–[56].
Histopathology and Immunohistochemistry
For histologic evaluation, testes were fixed in neutral-buffered formalin, cut transversely in half, embedded in paraffin and cut at 4 µm or at 10 µm for stereology. Serial sections were stained with hematoxylin and eosin and immunohistochemistry (IHC). Rabbit monoclonal anti-Ki-67 (Cell Marque, Rocklin, CA) or polyclonal anti-cleaved caspase-3 (Cell Signaling, Beverly, MA) antibodies were used for IHC. Heat-induced antigen retrieval was performed with CC1 epitope retrieval solution (Ventana Medical Systems, Inc., Tucson, AZ) for ki-67 or with citrate buffer pH6 for caspase-3.
Quantitative histomorphometry was done using planimetry-based morphometry for the assessment of seminiferous tubules (ST) cross-sectional areas, the Leydig cell areas and nuclear diameters and for total ST profiles and atrophic/total ST profile measurements. A stereology-based point-counting morphometry method [57] was applied for determining germ and Leydig cell nuclear volumes. Six to eight histological sections from each mouse testes were randomly selected after step-sectioning and used for the morphometric counts. Images taken at ×2 magnification were used for ST cross-sectional areas and for total ST and atrophic/total ST measurements. ×10 magnification images were used to measure Leydig area size and for germ and Leydig cell nuclear volume stereology, ×40 for ki67+ Leydig cell counts and ×60 for determining Leydig cell nuclear diameters. Images were analyzed using the Image J image processing and analysis program (NIH, Bethesda, MD). For ST cross-sectional areas, for total ST and atrophic/total ST and for ki67+ Leydig cell counts, depending on the experimental group size, 1–3 images were randomly selected from each mouse to achieve a total number of 25 images per group. Total ST profiles, atrophic ST profiles and ki-67+ Leydig cells were counted in each image. For ST cross-sectional area planimetry, the largest 4–5 perfectly circular in shape ST profiles found in each image were subscribed and each ones' area was automatically measured in pixels using the ImageJ “measure” command. A total number of at least 100 ST cross-section area values was achieved per group. For Leydig cell area planimetry, the 3 images containing the largest proportion of Leydig cell areas from each mouse were used. Depending on the group size 1–3 images were randomly selected from each mouse to achieve a total number of 25 images to work with per group. The multiple Leydig cell areas in each image were subscribed, excluding vessels or “empty” spaces, and measured. The total Leydig cell area per image was recorded. For Leydig cell nuclear diameter measurements, 5 images from randomly selected areas of each mouse testis were captured using the ×60 high power magnification lens. Depending on the experimental group size, the diameter of 10–20 circular Leydig cell nuclear profiles were measured to achieve 200 nuclear size values per treatment group. For germ and Leydig cell point-counting, seven mice were randomly selected from each experimental group. Ten ×10 images were randomly captured from each mouse testis. Specifically, from each one of the 6–8 histological sections available for that assessment, the first 1 to 2 fields found to contain target cells after a random movement of the microscope stage with a closed field diaphragm were captured (simple random sampling)”. A grid with 130 points was super-imposed in each image using the “grid” plug-in of the ImageJ software. The number of points co-localizing with germ or Leydig cell nuclei was counted in each image. The mean count of nuclei-positive points found in the 10 images was determined for each mouse. The ratio of the mean “nuclei-positive points”/130, determined the “Nuclear volume of cells per unit area” (×10 figure) for a given mouse. The product of “Nuclear volume per unit area” of a mouse testis multiplied by the testis weight (“fresh” testis weight) determined the “absolute nuclear volume of cells” in each mouse testis. The quotient “Nuclear volume of cells per unit area”/Testis weight expressed as percentage was the “Relative percentage of nuclear volume of cells” of each testis. In order to calculate the total number of Leydig cells per testis, the mean nuclear diameter of 100 randomly selected circular profiles of Leydig cells was determined for each testis of the mice used for stereometry (n = 7 per group). The mean diameter in pixels was converted to µm and the weight ( = volume) of each testis was expressed in µm3. Then the Floderus equation NV = NA/(T+D−2 h) was used to transform the “Nuclear volume of cells per unit area” (NA). T is the section thickness, D is the mean Leydig cell diameter and h is the height of the smallest nuclear profile found in the section. The latter was arbitrarily set at the 0.1× mean nuclear diameter [58]. The total number of Leydig cells per unit area (NV) of each testis was multiplied by the testis volume (assuming that each testis volume equals to its weight) to determine the total number of Leydig cells per testis.
Statistical analyses
The Mann-Whitney U test was used for body and testes weights and histomorphometry. A p-value <0.05 was statistically significant.
Supporting Information
L. reuteri increases testicular weight/body weight ratio. Dietary supplementation with L. reuteri, increased testicular weight-body weight ratio when compared to age- and diet-matched controls. A similar effect was observed with the neutralization of IL17. Numbers on the y-axis of bar graphs correspond to the mean±SEM of the testicular weight/body weight ratio. *p<0.05, **p<0.001, ***p<0.0001.
(TIF)
Effects of L. reuteri on Leydig cells. a. L. reuteri-fed mouse testes and mice undergoing depletion of IL-17 have a significantly increased Leydig cell nuclear volume per size unit area and b. increased relative percentage of Leydig cell nuclear volume compared to control mice. Numbers on the y axis of bar graphs correspond to the mean±SEM of “relative percentage of Leydig cell nuclear volume”;. c. In a similar manner, the same mice show an increased Leydig cell nuclear diameter compare to their age-, diet- and treatment-matched controls. Numbers on the y axis of bar graphs correspond to the mean±SEM of Leydig cell nuclear diameter. *p<0.05, **p<0.001, ***p<0.0001
(TIF)
Dietary L. reuteri increases spermatogenesis in mice. L. reuteri has a beneficial effect in sperm concentration and sperm activity of 12-month-old outbred Swiss mice. *p<0.05, **p<0.001.
(TIF)
Acknowledgments
We thank James Versalovic for the gift of ATCC 6475 Lactobacillus reuteri, and special thanks to James G. Fox for encouragement and support.
Funding Statement
This work was supported by National Institutes of Health grants P30-ES002109 (pilot project award to SEE and EJA), U01 CA164337 (to SEE and EJA), and RO1CA108854 (to SEE). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Haider SG (2004) Cell biology of Leydig cells in the testis. Int Rev Cytol 233: 181–241. [DOI] [PubMed] [Google Scholar]
- 2. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86: 724–731. [DOI] [PubMed] [Google Scholar]
- 3. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, et al. (2002) Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87: 589–598. [DOI] [PubMed] [Google Scholar]
- 4.Payne AH, Hardy MP (2007) The Leydig cell in health and disease. Totowa, NJ: Humana Press.
- 5. Midzak AS, Chen H, Papadopoulos V, Zirkin BR (2009) Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol 299: 23–31. [DOI] [PubMed] [Google Scholar]
- 6. Chen H, Ge RS, Zirkin BR (2009) Leydig cells: From stem cells to aging. Mol Cell Endocrinol 306: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takano H, Abe K (1987) Age-related histologic changes in the adult mouse testis. Arch Histol Jpn 50: 533–544. [DOI] [PubMed] [Google Scholar]
- 8. Matsumoto AM (2002) Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci 57: M76–99. [DOI] [PubMed] [Google Scholar]
- 9. Stanworth RD, Jones TH (2008) Testosterone for the aging male; current evidence and recommended practice. Clin Interv Aging 3: 25–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bassil N, Morley JE (2010) Late-life onset hypogonadism: a review. Clin Geriatr Med 26: 197–222. [DOI] [PubMed] [Google Scholar]
- 11. Baer JT (2012) Testosterone replacement therapy to improve health in older males. Nurse Pract 37: 39–44. [DOI] [PubMed] [Google Scholar]
- 12. Pines A (2011) Male menopause: is it a real clinical syndrome? Climacteric 14: 15–17. [DOI] [PubMed] [Google Scholar]
- 13. Hijazi RA, Cunningham GR (2005) Andropause: is androgen replacement therapy indicated for the aging male? Annu Rev Med 56: 117–137. [DOI] [PubMed] [Google Scholar]
- 14. Mah PM, Wittert GA (2010) Obesity and testicular function. Mol Cell Endocrinol 316: 180–186. [DOI] [PubMed] [Google Scholar]
- 15. Haffner SM, Valdez RA, Stern MP, Katz MS (1993) Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disord 17: 643–649. [PubMed] [Google Scholar]
- 16. Kelly DM, Jones TH (2013) Testosterone: a metabolic hormone in health and disease. J Endocrinol 217: R25–45. [DOI] [PubMed] [Google Scholar]
- 17. Ghanayem BI, Bai R, Kissling GE, Travlos G, Hoffler U (2010) Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod 82: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levkovich T, Poutahidis T, Smillie C, Varian BJ, Ibrahim YM, et al. (2013) Probiotic bacteria induce a ‘glow of health’. PLoS One 8: e53867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, et al. (2007) Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis 28: 2614–2623. [DOI] [PubMed] [Google Scholar]
- 20. Bassil N, Alkaade S, Morley JE (2009) The benefits and risks of testosterone replacement therapy: a review. Ther Clin Risk Manag 5: 427–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, et al. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238. [DOI] [PubMed] [Google Scholar]
- 22. Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, et al. (2006) IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med 203: 2485–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park EJ, Lee JH, Yu GY, He G, Ali SR, et al. (2010) Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140(2): 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, et al. (2003) CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol 162: 691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, et al. (2005) CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res 65: 3998–4004. [DOI] [PubMed] [Google Scholar]
- 26. Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, et al. (2009) T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res 69: 5490–5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salama P, Phillips M, Grieu F, Morris M, Zeps N, et al. (2009) Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 27: 186–192. [DOI] [PubMed] [Google Scholar]
- 28. Rook GA (2009) Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 126: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang SH, Chung Y, Dong C (2010) Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem 285(50): 38751–38755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12: 5–9. [DOI] [PubMed] [Google Scholar]
- 31. Litonjua AA, Weiss ST (2007) Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol 120: 1031–1035. [DOI] [PubMed] [Google Scholar]
- 32. Harris DM, Go VL (2004) Vitamin D and colon carcinogenesis. J Nutr 134: 3463S–3471S. [DOI] [PubMed] [Google Scholar]
- 33. Liva SM, Voskuhl RR (2001) Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol 167: 2060–2067. [DOI] [PubMed] [Google Scholar]
- 34. Powrie F, Maloy KJ (2003) Immunology. Regulating the regulators. Science 299: 1030–1031. [DOI] [PubMed] [Google Scholar]
- 35. Lee YK, Mazmanian SK (2010) Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 330: 1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ibrahim HA, Zhu Y, Wu C, Lu C, Ezekwe MO, et al. (2012) Selenium-enriched probiotics improves murine male fertility compromised by high fat diet. Biol Trace Elem Res 147: 251–260. [DOI] [PubMed] [Google Scholar]
- 37. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB (2011) Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 364: 2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim SW, Park KY, Kim B, Kim E, Hyun CK (2013) Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem Biophys Res Commun 431: 258–263. [DOI] [PubMed] [Google Scholar]
- 39.Oksaharju A, Kooistra T, Kleemann R, van Duyvenvoorde W, Miettinen M, et al.. (2012) Effects of probiotic Lactobacillus rhamnosus GG and Propionibacterium freudenreichii ssp. shermanii JS supplementation on intestinal and systemic markers of inflammation in ApoE*3Leiden mice consuming a high-fat diet. Br J Nutr: 1–9. [DOI] [PubMed]
- 40. Kang JH, Yun SI, Park HO (2010) Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol 48: 712–714. [DOI] [PubMed] [Google Scholar]
- 41. Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, et al. (2011) Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol 110: 650–657. [DOI] [PubMed] [Google Scholar]
- 42. Axling U, Olsson C, Xu J, Fernandez C, Larsson S, et al. (2012) Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 9: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fak F, Backhed F (2012) Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− Mice. PLoS One 7: e46837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walter J, Britton RA, Roos S (2011) Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci U S A 108 Suppl 14645–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kerr JB, Sharpe RM (1985) Stimularoty effect of follicle-stimulating hormone on rat Leydig cells. A morphometric and ultrastructural study. Cell Tissue Res 239: 405–145. [DOI] [PubMed] [Google Scholar]
- 46. Christensen AK, Peacock KC (1980) Increase in Leydig cell number in testes of adult rats treated chronically with an excess of human chorionic gonadotropin. Biol Reprod 22: 383–391. [DOI] [PubMed] [Google Scholar]
- 47. Dombrowicz D, Sente B, Reiter E, Closset J, Hennen G (1996) Pituitary control of proliferation and differentiation of Leydig cells and their putative precursors in immature hypophysectomized rat testis. J Androl 17: 639–650. [PubMed] [Google Scholar]
- 48. Duckett RJ, Hedger MP, McLachlan RI, Wreford NG (1997) The effects of gonadotropin-releasing hormone immunization and recombinant follicle-stimulating hormone on the Leydig cell and macrophage populations of the adult rat testis. J Androl 18: 417–423. [PubMed] [Google Scholar]
- 49. Baker PJ, O'Shaughnessy PJ (2001) Role of gonadotrophins in regulating number of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction 122: 227–234. [DOI] [PubMed] [Google Scholar]
- 50. Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, et al. (2013) Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLoS One 8: e78898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108: 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, et al. (2010) Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 95(4): 1810–18. [DOI] [PubMed] [Google Scholar]
- 53. Saulnier DM, Santos F, Roos S, Mistretta TA, Spinler JK, et al. (2011) Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS One 6: e18783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thornton CE, Brown SD, Glenister PH (1999) Large numbers of mice established by in vitro fertilization with cryopreserved spermatozoa: implications and applications for genetic resource banks, mutagenesis screens, and mouse backcrosses. Mamm Genome 10: 987–992. [DOI] [PubMed] [Google Scholar]
- 55. Nakagata N (1994) [Cryopreservation of embryos and gametes in mice]. Jikken Dobutsu 43: 11–18. [DOI] [PubMed] [Google Scholar]
- 56. Nakagata N, Takeshima T (1992) High Fertilizing Ability of Mouse Spermatozoa Diluted Slowly after Cryopreservation. Theriogenology 37: 1283–1291. [Google Scholar]
- 57. Atanassova N, McKinnell C, Walker M, Turner K, Fisher J, et al. (1999) Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number, and the efficiency of spermatogenesis in adulthood. Endocrinology 140: 5364–5373. [DOI] [PubMed] [Google Scholar]
- 58. Mori H, Christensen AK (1980) Morphometric analysis of Leydig cells in the normal rat testis. J Cell Biol 84: 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
L. reuteri increases testicular weight/body weight ratio. Dietary supplementation with L. reuteri, increased testicular weight-body weight ratio when compared to age- and diet-matched controls. A similar effect was observed with the neutralization of IL17. Numbers on the y-axis of bar graphs correspond to the mean±SEM of the testicular weight/body weight ratio. *p<0.05, **p<0.001, ***p<0.0001.
(TIF)
Effects of L. reuteri on Leydig cells. a. L. reuteri-fed mouse testes and mice undergoing depletion of IL-17 have a significantly increased Leydig cell nuclear volume per size unit area and b. increased relative percentage of Leydig cell nuclear volume compared to control mice. Numbers on the y axis of bar graphs correspond to the mean±SEM of “relative percentage of Leydig cell nuclear volume”;. c. In a similar manner, the same mice show an increased Leydig cell nuclear diameter compare to their age-, diet- and treatment-matched controls. Numbers on the y axis of bar graphs correspond to the mean±SEM of Leydig cell nuclear diameter. *p<0.05, **p<0.001, ***p<0.0001
(TIF)
Dietary L. reuteri increases spermatogenesis in mice. L. reuteri has a beneficial effect in sperm concentration and sperm activity of 12-month-old outbred Swiss mice. *p<0.05, **p<0.001.
(TIF)