Abstract
Melioidosis, infection caused by the Gram-negative bacterium Burkholderia pseudomallei, is a common cause of sepsis in northeast Thailand. In white North Americans, common functional genetic variation in TLR1 is associated with organ failure and death from sepsis. We hypothesized that TLR1 variants would be associated with outcomes in Thais with melioidosis. We collated the global frequencies of three TLR1 variants that are common in white North American populations: rs5743551 (-7202A/G), rs4833095 (742A/G), and rs5743618 (1804G/T). We noted a reversal of the minor allele from white North American subjects to Asian populations that was particularly pronounced for rs5743618. In the Utah residents of European ancestry, the frequency of the rs5743618 T allele was 17% whereas in Vietnamese subjects the frequency was >99%. We conducted a genetic association study in 427 patients with melioidosis to determine the association of TLR1 variation with organ failure or death. We genotyped rs5743551 and rs4833095. The variants were in high linkage disequilibrium but neither variant was associated with organ failure or in-hospital death. In 300 healthy Thai individuals we further tested the association of TLR1 variation with ex vivo blood responses to Pam3CSK4, a TLR1 agonist. Neither variant was robustly associated with blood cytokine responses induced by Pam3CSK4. We identified additional common variation in TLR1 by searching public databases and the published literature and screened three additional TLR1 variants for associations with Pam3CSK4-induced responses but found none. We conclude that the genetic architecture of TLR1 variation differs substantially in southeast Asians compared to other populations and common variation in TLR1 in Thais is not associated with outcome from melioidosis or with altered blood responses to Pam3CSK4. Our findings highlight the need for additional studies of TLR1 and other innate immune genetic modulators of the inflammatory host response and determinants of sepsis in southeast Asian populations.
Introduction
The global burden of sepsis is estimated at up to 19 million cases per year [1]. Much of this burden occurs in low resource settings where limited data suggest that outcomes are particularly poor [2]. In northeast Thailand, melioidosis - infection with the Gram-negative bacterium Burkholderia pseudomallei - is the second most common cause of bacteremia and a frequent cause of sepsis [3], [4]. In this setting and despite appropriate antimicrobial therapy, melioidosis mortality is 43% [5]. Melioidosis is endemic in southeast Asia and northern Australia but increasingly found elsewhere in the tropics [3]. As a systemic infection characterized by an inflammatory host response and poor outcomes, melioidosis serves as an informative example of severe Gram-negative sepsis [6]–[10].
Genetic variation accounts for susceptibility to and outcome from infectious disease, and provides a window into mechanisms that underlie the complex pathophysiology of sepsis [11], [12]. Innate immune signaling pathways that titrate the host inflammatory response are of particular interest. Toll-like receptors (TLRs) comprise a subset of innate immune sensors within the IL-1 receptor family [13]. TLR4 is the best-known TLR, initiating an inflammatory cascade in response to ligation of endotoxin (lipopolysaccharide) expressed by Gram-negative bacteria. We have previously examined innate immune host genetic variants that are associated with susceptibility to melioidosis, and found that TLR4 variants are associated with infection [14]. TLR5 is a bacterial flagellin sensor; we have also shown that a common genetic variant in TLR5 is highly associated with survival in individuals with melioidosis [15].
TLR1 is another sensor in the TLR family that forms a heterodimer with TLR2 and facilitates innate immune activation upon ligation of bacterial cell wall components such as lipopeptides, peptidoglycan, and lipotechoic acid [13]. Three TLR1 variants have been described as associated with sepsis, although the relationship is complex. rs5743551 (-7202A/G) is upstream of TLR1. In white North American subjects, this polymorphism tags two non-synonymous polymorphisms: rs5743618 and rs4833095 [16]. rs5743618 (1804G/T or 1805G/T), located in the trans-membrane domain of TLR1, changes a serine to an isoleucine at amino acid 602. The T allele has been demonstrated by multiple authors to be relatively hypermorphic [16]–[19]. rs4833095 (742A/G or 743A/G) is situated in the extracellular domain and replaces asparagine with serine at amino acid 248. Two studies have shown an association of rs5743551 and rs5743618 with mortality from sepsis in white medical/surgical and trauma patients in Washington, USA or British Columbia, Canada [16], [20]. Similar associations have been demonstrated for organ failure in sepsis patients in Spain and in white medical/surgical patients in the US [16], [21]. Homozygous carriers of the rs5743551 G allele have a higher mortality in sepsis, and, interestingly, the mortality association is stronger for the tag SNP rs5743551 G allele than for the coding rs5743618 T allele [16], [20]. Moreover, the rs4833095 G allele, but not the rs5743618 T allele, is associated with mortality from Gram-positive trauma-related sepsis [20], and the rs4833095 G allele has been associated with infections such as leprosy and malaria in populations where the rs5743618 T allele is infrequent [22], [23], suggesting an independent effect of this variant. The allele frequency of these TLR1 variants is markedly different around the world [24], potentially leading to differential associations with outcomes from sepsis.
B. pseudomallei is recognized by TLR2/1, inducing rapid upregulation of the innate immune response [25]. It is conceivable that functional variation in TLR1 may modulate host defense in melioidosis. In light of the data showing an important role for TLR1 in human sepsis and as a trigger for B. pseudomallei-induced immune system activation, we undertook an analysis of the association of common hypermorphic TLR1 genetic variants with outcome in a cohort of Thai subjects with culture-proven melioidosis. We also analyzed the association of TLR1 variants with blood cytokine responses to a TLR1 agonist in healthy Thai subjects. We hypothesized that hypermorphic TLR1 variation would be associated with altered outcome, including death and organ failure. Notably, we found that the genetic architecture of functional TLR1 variation is substantially different in southeast Asian populations, that common TLR1 variation in Thais is not hypermorphic, and that common variation in TLR1 in Thais is not associated with outcome in melioidosis. Our data suggest that immunogenetic determinants of outcome from Gram-negative infection in Thais differ from previously described determinants in white North American subjects with sepsis.
Materials and Methods
Human studies
Melioidosis cohort
Subjects with melioidosis were identified among inpatients at Sappasithiprasong Hospital, Ubon Ratchathani, northeast Thailand from 1999 to 2005. A study team screening patients cultured blood, urine, and other relevant samples (e.g. abscess aspirates) for B. pseudomallei [26]. Melioidosis status was defined by a positive culture for B. pseudomallei from a sample collected by the study team or independently by hospital clinicians. Demographic and clinical data from enrolment until discharge from hospital was recorded by the study team. All patients included in this analysis were Thai. Outcomes for this study were organ failure or death. Organ failure was defined as respiratory failure or shock. The definition for respiratory failure was hypoxia (PaO2 <60 mmHg) or hypercapnia (PaCO2 >50 mmHg) in conjunction with acidaemia (blood pH <7.30) or requirement for mechanical ventilation. The definition of shock was hypotension (systolic arterial blood pressure <90 mmHg, or 40 mmHg lower than patient's normal blood pressure) despite adequate fluid resuscitation or need for vasopressors to maintain systolic blood pressure > = 90 mmHg or mean arterial pressure > = 70 mmHg. Death was defined as in-hospital death or discharge in a moribund condition for palliative care at home. Written informed consent for enrollment into clinical studies of melioidosis was obtained from subjects or their representatives at the time of recruitment.
Immuno-assay studies
Three-hundred healthy subjects donating blood at the blood donation center at Sappasithiprasong Hospital in 2010 were recruited for participation as previously described [15] [Chantratita et al, in press]. Subjects were included if they indicated they were Thai and between the ages of 18 and 60 and did not report any history of immunodeficiency or inflammatory conditions, chronic diseases, pregnancy in the past six months, anti-inflammatory medication use in the past week, antibiotic use in the past month, vaccination in the past six months, heavy exercise or alcohol consumption in the past 24 h, or smoking in the past month. All subjects were born in the northeast region of Thailand. Enrolled subjects gave written informed consent to participate and provided a post-donation blood sample.
The Ethical Review Committee for Research in Human Subjects, Ministry of Public Health, Thailand, and the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, and the University of Washington Human Subjects Division Institutional Review Board approved the consent procedure and protocol of these studies.
Immuno-assays
Three hundred and eighty microliters of fresh whole blood in citrate mixed 1∶1 with RPMI media was added to pre-prepared plates containing 20 µL of Pam3CSK4 (Invivogen, San Diego, CA) for a final concentration of 100 ng/mL. Pam3CSK4 is a specific agonist for TLR2/1. Plates were incubated at 37°C on a shaking incubator under 5% CO2 for 6 h before being spun down and plasma removed and frozen at −80°C. IL-6, IL-8, TNF-α, IL-10, MCP-1, IL-1ra, G-CSF, and IL-1β were later assayed in duplicate on a multiplex bead system (Luminex, Austin, TX) using reagents from R&D Systems (Minneapolis, MN). A complete blood count with differential was performed in the hospital clinical laboratory for each subject at the time of phlebotomy.
Genotyping
DNA was extracted from whole blood of patients with melioidosis using Nucleon BACC3 kits (GE Healthcare, Buckinghamshire, UK) or from whole blood of healthy donors using the QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany). We selected TLR1 polymorphisms rs5743551 (-7202A/G) and rs4833095 (742A/G) to genotype in the melioidosis cohort using an allele-specific primer extension method (Sequenom, Inc.) with reads by a MALDI-TOF mass spectrometer [14], [15] and in the healthy blood donors using Fluidigm SNPtype assays on a Biomark microfluidics real time PCR system. Additional TLR1 variants were identified as follows: SNP selection of non-synonymous TLR1 coding variants was performed by searching the HapMap project database (http://hapmap.ncbi.nlm.nih.gov) and based on functional prediction using a FastSNP analysis (http://fastsnp.ibms.sinica.edu.tw) [27]. Tag TLR1 SNPs were selected from the Han Chinese in Beijing and Japanese in Tokyo populations in the HapMap database for variants with a minor allele frequency (MAF) at least 2% using the HapMap tag-SNP picker option. Genotyping was performed using Fluidigm SNPtype assays on the Biomark real time PCR system.
Statistical analysis
Deviation from Hardy-Weinberg equilibrium was calculated for each variant using the exact test. In the melioidosis cohort, the power to detect an association between genotype and death was estimated to be 99% for a risk allele frequency of 0.45-0.55, assuming a recessive model and genotype relative risk of 2, 40% mortality in the population, 100 nonsurvivors, 300 survivors, and alpha = 0.05 [28]. The crude association between genotype or allele count and outcome was performed using the Chi square test. The adjusted analysis was performed using logistic regression, assuming a recessive genetic model, adjusting for age, gender, pre-existing condition, and clinical presentation. The inclusion in the models of TLR5 variant rs5744168 (1174C/T) that we have previously shown to be associated with outcome from melioidosis [15] did not appreciably alter the TLR1 genotype associations. Survival analyses were performed with the log rank test. Healthy subject plasma cytokine levels were normalized to monocyte count and log10 transformed before analysis by linear regression, adjusting for age, gender, and batch. Analyses were performed with Stata version 11.2 (College Station, Texas). P values ≤0.05 were considered significant.
Results
Global variation in common TLR1 polymorphisms
We first examined data on the global frequency of three well described TLR1 variants (rs5743551, rs4833095, and rs5743618) in the published literature or publically accessible databases (Table S1) [14], [16], [17], [20], [21], [24], [29]–[32]. Each variant has been associated with susceptibility to infection or outcome from sepsis. We found marked differences in allele frequencies across populations. For each variant there was a general trend of reversal of the minor allele from North American subjects (who were white or of European ancestry) to Asian populations, which was most pronounced for rs5743618. In the CEU population (Utah residents of European ancestry), the frequency of the rs5743618 T allele was 17%. In contrast, in Vietnamese subjects, the frequency of this allele was >99%. The frequency of the rs5743551 G allele was 18% in CEU subjects but 52% in subjects from northeast Thailand. Similarly, the frequency of the rs4833095 G allele was 18% in CEU subjects but 53% in subjects from northeast Thailand.
rs5743551 or rs4833095 are not associated with outcome in melioidosis
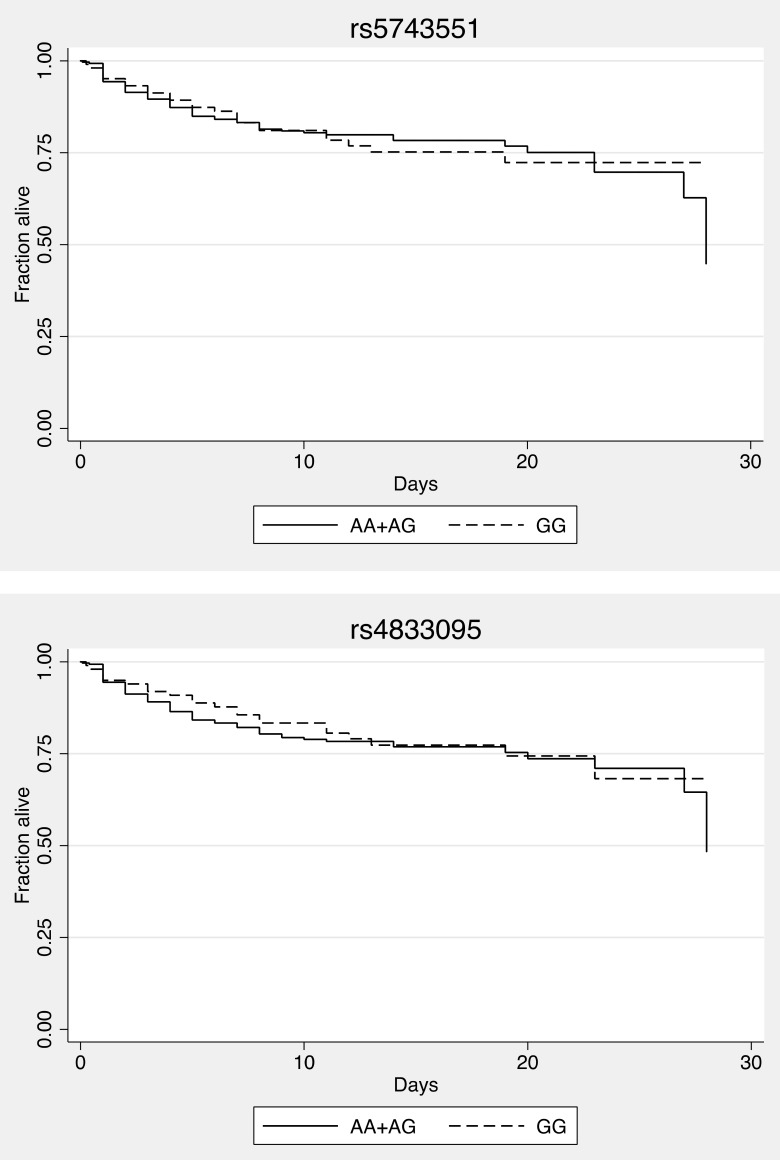
Given the different distribution of TLR1 alleles in Asia, it is not clear what the associations of these variants may be with outcomes from sepsis in a southeast Asian population. Consequently, we tested whether there was an association between TLR1 polymorphisms and death or organ failure in 427 Thai subjects with culture proven melioidosis admitted to Sappasithiprasong Hospital, Ubon Ratchathani, Thailand. We have previously reported on TLR variants in these and other subjects at this site [14], [15]. All individuals had culture-proven melioidosis and half were bacteremic. The median age was 49 (IQR 39–60) and 48% were female. The patients presented with bacteremia (50%), pneumonia (40%) and urinary tract infection (12%). Pre-existing conditions were diabetes (55%), chronic liver disease (1%), and kidney disease (6%). Thirty percent developed organ failure, defined as respiratory failure (17%) or shock (24%), and 23% died. We genotyped rs5743551 and rs4833095 but did not genotype rs5743618 as the minor allele (G) frequency was ∼1% in east Asian populations, suggesting that our power to detect an effect would be low. For both variants, we determined that there was no deviation from Hardy-Weinberg equilibrium in the survivors. The variants were in high linkage disequilibrium (R2 = 0.79). Following the genetic models described by Wurfel and Thompson [16], [20], we tested the association of homozygous carriage of the rs5743551 G allele with in-hospital death from melioidosis (Table 1) but found no significant effect (adjusted OR 0.99, 95% CI: 0.58–1.71, p = 0.98). Similarly, we found no association with death of homozygous carriage of the rs4833095 G allele (adjusted OR 0.94, 95% CI 0.54–1.63, p = 0.82). Survival curves showed no difference based on genotype (Figure 1). There was no association of either variant with organ failure (Table 1).
Table 1. TLR1 variant genotype and allele frequencies and associations with death in melioidosis.
| Marker | Outcomea | HWEb | Crudec | Adjustedd | |||
| P | P | OR | 95% CI | P | |||
| Death | |||||||
| rs5743551 | No | Yes | |||||
| AA | 86 (26.2) | 21 (21.9) | |||||
| AG | 151 (46.0) | 48 (50.0) | 0.67 | 0.99 | 0.58–1.71 | 0.98 | |
| GG | 91 (27.7) | 27 (28.1) | |||||
| 0.15 | |||||||
| A | 323 (49.2) | 90 (46.9) | 0.57 | ||||
| G | 333 (50.8) | 102 (53.1) | |||||
| rs4833095 | |||||||
| GG | 86 (26.2) | 25 (25.5) | |||||
| AG | 167 (50.9) | 51 (52.0) | 0.98 | 0.94 | 0.54–1.63 | 0.82 | |
| AA | 75 (22.9) | 22 (22.5) | |||||
| 0.82 | |||||||
| G | 339 (51.7) | 101 (51.5) | 0.97 | ||||
| A | 317 (48.3) | 95 (48.5) | |||||
| Organ failure | |||||||
| rs5743551 | No | Yes | |||||
| AA | 75 (26.0) | 27 (22.7) | |||||
| AG | 129 (44.8) | 63 (52.9) | 0.32 | 0.77 | 0.45–1.29 | 0.32 | |
| GG | 84 (29.2) | 29 (24.4) | |||||
| 0.08 | |||||||
| A | 279 (48.4) | 117 (49.2) | 0.85 | ||||
| G | 297 (51.6) | 121 (50.8) | |||||
| rs4833095 | |||||||
| GG | 80 (27.7) | 27 (22.5) | |||||
| AG | 142 (49.1) | 69 (57.5) | 0.23 | 0.73 | 0.43–1.25 | 0.26 | |
| AA | 67 (23.2) | 24 (20.0) | |||||
| 0.81 | |||||||
| G | 302 (52.3) | 123 (51.3) | 0.80 | ||||
| A | 276 (47.8) | 117 (48.8) | |||||
For each outcome, genotype or allele counts are given with percentages in parentheses.
Hardy-Weinberg equilibrium determined by exact test in survivors or in individuals without organ failure.
Crude genotypic or allelic association with outcome determined by Chi square test.
Adjusted association of genotype with outcome determined by logistic regression assuming a recessive model (G allele for each variant), adjusted for age, gender, pre-existing medical condition, and clinical presentation.
Figure 1. rs5743551 and rs4833095 are not associated with altered survival in melioidosis.
For each variant, Kaplan-Meier in-hospital survival curves are plotted for melioidosis patients, grouped by genotype. Curves are not significantly different by the logrank test (P>0.05).
rs5743551 and rs4833095 are not associated with Thai whole blood cytokine responses to a TLR1 agonist
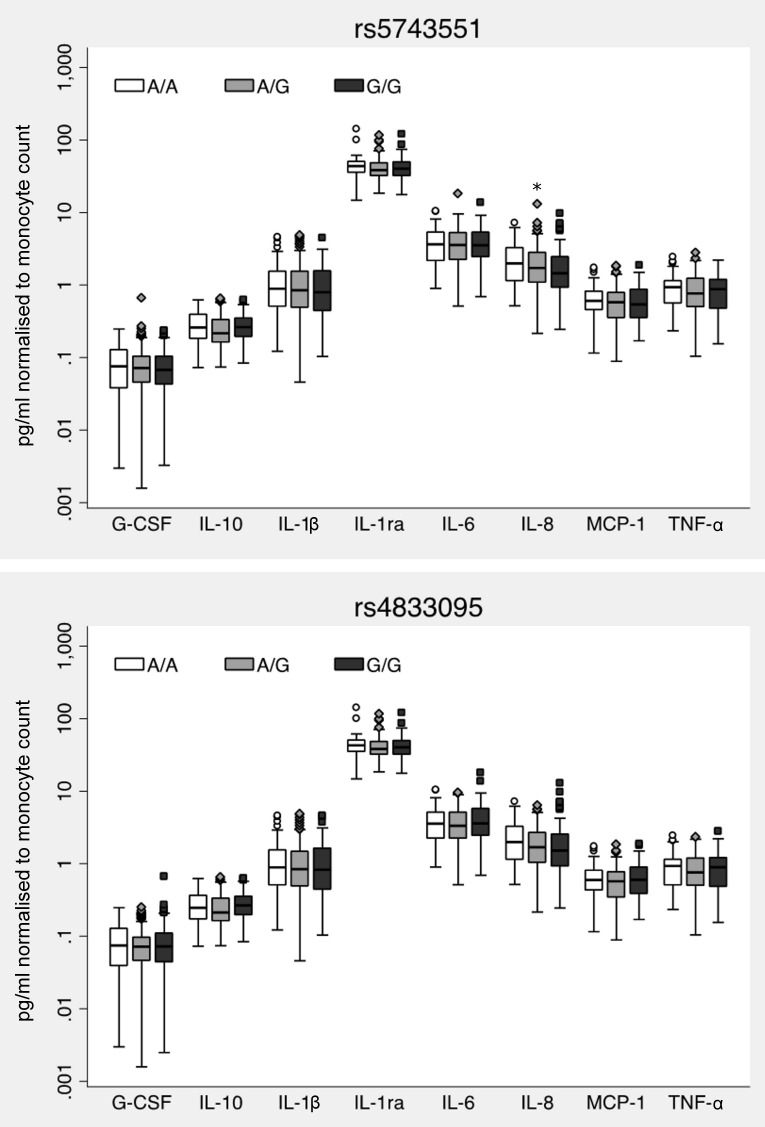
As rs5743551 is associated with altered immune responses by whole blood in white American and African American healthy subjects [16], we tested whether a similar phenotype was evident in Thais. We stimulated whole blood from 300 healthy Thai subjects with Pam3CSK4, a specific TLR1 agonist. We observed a range of up to one log in cytokine production suggesting substantial inter-individual variation in the innate immune response to a TLR1 agonist. We genotyped rs5743551 and rs4833095 in these individuals and found that LD was again high (R2 = 0.91). Genotype and allele frequencies for these variants are given in Table 2. No variant deviated from Hardy-Weinberg equilibrium. In contrast to previous associations of rs5743551 genotype with altered Pam3CSK4-induced G-CSF, IL-1ra, IL-1β, IL-6, IL-8, IL-10, and TNF-α responses in white American subjects [16], we found only a modest reduction in IL-8 level (P = 0.03) but no significant alteration in any other cytokine for rs5743551 (Figure 2). We observed no rs4833095-dependent difference in inflammatory mediator production induced by Pam3CSK4 (Figure 2).
Table 2. TLR1 variant genotype and allele frequencies in healthy Thai subjects.
| Marker | Counts (%) | HWEb |
| P | ||
| rs5743551 | ||
| AA | 57 (19.0) | |
| AG | 147 (49.0) | |
| GG | 96 (32.0) | |
| 1.0 | ||
| A | 261 (43.5) | |
| G | 339 (56.5) | |
| rs4833095 | ||
| GG | 103 (34.5) | |
| AG | 139 (46.5) | |
| AA | 57 (19.1) | |
| 0.41 | ||
| G | 345 (57.7) | |
| A | 253 (42.3) | |
| rs5743562 | ||
| AA | 163 (54.3) | |
| AG | 115 (38.3) | |
| GG | 22 (7.3) | |
| 0.77 | ||
| A | 441 (73.5) | |
| G | 159 (26.5) | |
| rs5743563 | ||
| TT | 163 (54.3) | |
| TC | 115 (38.3) | |
| CC | 22 (7.3) | |
| 0.77 | ||
| T | 441 (73.5) | |
| C | 159 (26.5) | |
| rs5743592 | ||
| TT | 159 (53.7) | |
| TC | 115 (38.9) | |
| CC | 22 (7.4) | |
| 0.88 | ||
| T | 433 (0.73) | |
| C | 159 (0.27) | |
| rs5743593 | ||
| TT | 164 (54.7) | |
| TC | 114 (38.0) | |
| CC | 22 (7.3) | |
| 0.77 | ||
| T | 442 (73.7) | |
| C | 158 (26.3) | |
| rs5743595 | ||
| TT | 163 (54.3) | |
| TC | 115 (38.3) | |
| CC | 22 (7.3) | |
| 0.77 | ||
| T | 441 (73.5) | |
| C | 159 (26.5) | |
| rs5743596 | ||
| CC | 252 (84.0) | |
| CT | 46 (15.3) | |
| TT | 2 (0.7) | |
| 1.0 | ||
| C | 550 (0.92) | |
| T | 50 (0.08) | |
| rs5743604 | ||
| TT | 81 (27.1) | |
| TC | 145 (48.5) | |
| CC | 73 (24.4) | |
| 0.64 | ||
| T | 307 (51.3) | |
| C | 291 (48.7) |
Hardy-Weinberg equilibrium determined by exact test in all subjects.
Figure 2. rs5743551 and rs4833095 are not associated with Thai whole blood cytokine responses to a TLR1 agonist.
Whole blood was stimulated with Pam3CSK4 100/mL for six hours. Cytokine and chemokines were measured in plasma by multiplex bead assay, normalized to monocyte count, and log10 transformed for statistical analysis by linear regression, adjusting for age, gender, and batch. Boxes show the median and interquartile range; whiskers show upper and lower adjacent values. For rs5743551, N = 57 (AA), 147 (A/G), 96 (GG). For rs4833095, N = 57 (AA), 139 (A/G), 103 (GG). Cytokine responses were not significantly different by either genotype (P>0.05 for all comparisons).
Other common TLR1 variants are not associated with Thai whole blood cytokine responses to a TLR1 agonist
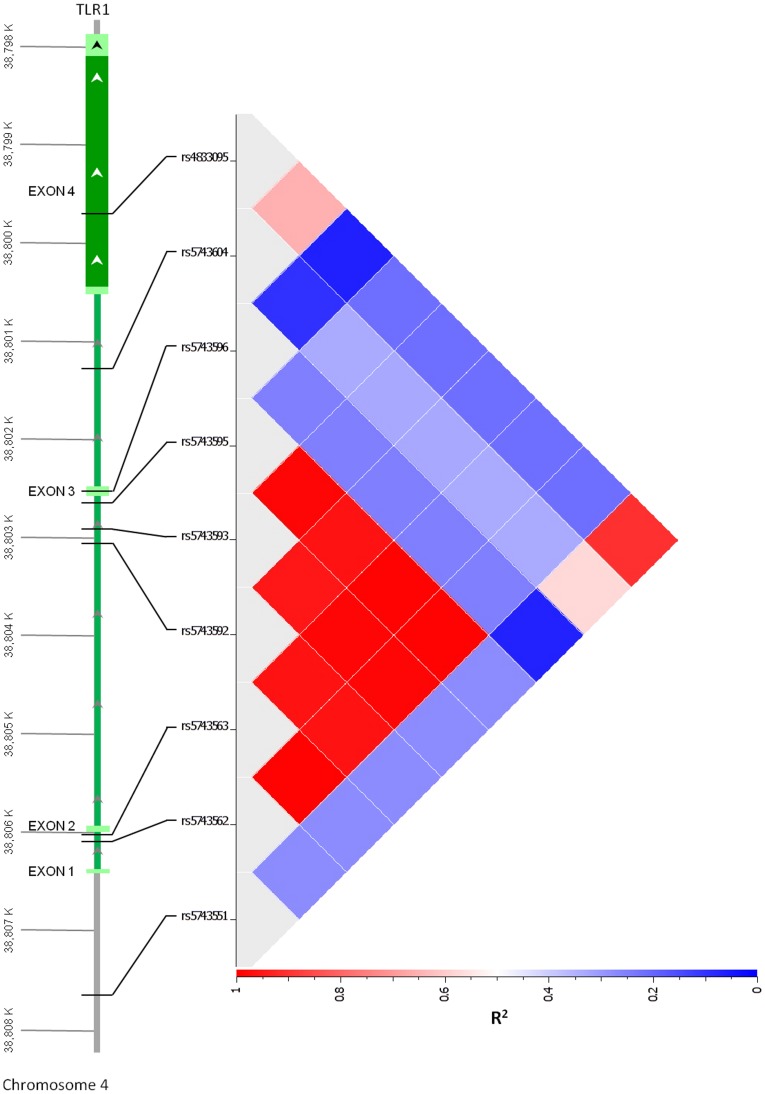
As these common variants did not have an apparent functional association in our cohort, we then searched for other TLR1 variants in Asian subjects that might underlie the variable innate immune response of Thai subjects to Pam3CSK4. We identified TLR1 region variants as described in the methods and genotyped seven additional polymorphisms (rs5743562, rs5743563, rs5743592, rs5743593, rs5743595, rs5743596, and rs5743604). Genotype and allele frequencies are given in Table 2. Five of these variants (rs5743593, rs5743595, rs5743563, rs5743562, and rs5743592) were in very high LD (R2≥0.96) (Figure 3) so we selected one tag SNP from this bin (rs5743595) and the two other variants (rs5743604 and rs5743596) for analysis. However, we found no consistent association of any of the three polymorphisms with cytokine level induced by Pam3CSK4 (Table S2).
Figure 3. Linkage disequilibrium plot of common TLR1 variants in Thais.
Linkage disequilibrium (R2) for nine common TLR1 variants in 300 Thai blood donors. Plot created using Golden Helix software.
Discussion
The results of this investigation show that common variation in TLR1 in Thais is not associated with altered inflammatory responses to Pam3CSK4 in blood or with outcome from melioidosis, a common cause of sepsis in northeast Thailand. Our findings also confirm that the genetic architecture of functional TLR1 variation is substantially different in southeast Asians compared to other global populations.
Sepsis remains a vexing syndrome to identify and treat [33], [34]. Targeting the innate immune response driven by activation of pathogen recognition sensors such as TLRs has been seen as a fruitful approach [35]. Indeed, numerous studies support the role of TLR pathway molecules in the pathogenesis of sepsis [36]. Only relatively recently, however, has TLR1 been carefully studied as an important regulator of the host response in sepsis, particularly in human disease. Our study of genetic variation in Thais with melioidosis offers an opportunity to examine the association of common TLR1 variants with clinical outcome from one of the major Gram-negative causes of sepsis in northeast Thailand. Immunoassays in subjects recruited from the same population can provide corroborating functional evidence of inflammatory effects attributable to genotype. However, our data do not implicate known common genetic variants in TLR1 in modulating the host response clinically or under experimental conditions.
In white Americans, rs5743551 is in moderate to high LD with the non-synonymous coding variants rs5743618 and rs4833095. All three variants (G, T, and G alleles, respectively) have been associated with greater inflammation or death from sepsis [16], [20], [21]. The rs5743618 T allele is also associated with susceptibility to leprosy [19], [24] but protection against tuberculosis and candidemia [37]–[39]. Yet, our findings suggest that in Thais, rs5743551 tags only rs4833095, the minor alleles of both polymorphisms are reversed compared to white Americans, and neither variant is associated with outcome from melioidosis. Although the G allele of rs5743618, encoding serine, has impaired cell surface trafficking and signaling [16], [17], [40], in Chinese and Vietnamese subjects this allele is very rare and, unlike in white American populations, is not tagged by rs5743551. We cannot exclude effects of rs5743618 in Thais, but the very low minor allele frequency in other southeast Asians argues against a significant explanatory effect of this genotype on clinical outcome. Furthermore, these data underscore the variability in TLR1 genetic architecture across populations.
Despite the documented functional effects of rs5743618, there is a stronger association of the tag SNP rs5743551 G allele with death from sepsis than for the rs5743618 T allele in white North Americans [16], [20]. In white Americans with Gram-positive trauma-related sepsis, the rs5743551 G allele, but not the rs5743618 T allele, is associated with death [20]. These data argue in favor of additional functional TLR1 variation tagged by rs5743551. rs4833095 is a possible candidate that is in fact associated with mortality in this Gram-positive trauma-related sepsis population [20]. The variant is also associated with susceptibility to infection. In a Brazilian cohort, the rs4833095 G allele (but not rs5743618) is associated with leprosy [31]. In addition, this allele is also associated with leprosy in Bangladeshi populations where the rs5743618 T allele occurs with a frequency of about 5% and the rs4833095 A allele is associated with placental malaria in Ghanaians where the rs5743618 G allele frequency is 2% [22], [23]. We have previously reported associations of rs5743351 and rs4833095 with susceptibility to melioidosis [14]. Yet, the mechanisms that modulate susceptibility to infection may well differ from those that regulate outcome once infection is established. For example, we have found that a common nonsense TLR5 polymorphism, rs5744168, is strongly protective against death in this cohort, but is not associated with susceptibility to melioidosis [14], [15].
In addition to the absence of an association of rs4833095 with death from melioidosis in Thais, our present functional studies do not support an effect of rs4833095 on the host inflammatory response. Notably, the complete lack of effect on Pam3CSK4-induced cytokine release by rs4833095 in Thai subjects stands in contrast to recent work by Mikacenic et al [41]. Using a similar immuno-assay design, these investigators found an extremely strong effect of rs4833095 on IL-6 and TNF-α release induced by stimulation of whole blood from white subjects with Pam3CSK4. Yet, assays of HEK293 cells transfected with this variant (independent of rs5743618) have not shown altered NF-κB activation in response to Pam3CSK4 [16], [17]. Combined, the data suggest that in white North Americans, rs4833095 may not itself be functional but rather may tag a causative variant. In contrast, in Thais, there is no clear evidence of functionality, intrinsic or otherwise, attributable to rs4833095.
Our screening analysis of additional common TLR1 variation did not reveal any other variants associated with altered inflammatory response to Pam3CSK4. Our approach was restricted to previously described variation, however, and it is clear that there is marked variation in the genetic architecture of TLR1 across populations, possibly driven by infectious disease [24], [40], that may account for our negative results. We speculate that uncharacterized functional TLR1 genetic variation in Asians remains to be discovered, and that this may regulate outcomes in sepsis. Although current genome wide association studies chips are characterized by a relative paucity of Asian variants, ongoing comprehensive sequencing efforts across a variety of populations are facilitating identification of potentially clinically relevant variants in relatively understudied populations [32].
While melioidosis is representative of Gram-negative sepsis, a prior study of TLR1 polymorphisms in sepsis noted a relationship (in several cohorts) between frequency of Gram-positive infection and rs5743551 genotype [16]. Although TLR1 augments TLR2-mediated NF-κB activation by B. pseudomallei in vitro [25], suggesting a role for TLR1 signaling in melioidosis, it is conceivable that clinical associations of rs5743551 or rs4833095 might be limited to Gram-positive sepsis in Thais. Ongoing studies by our group are addressing this question.
Potential limitations to all genetic association studies include population stratification due to ethnic admixture and other unmeasured confounding. Previously, however, we have not observed substantial population stratification in our Sappasithiprasong cohort [14]. In addition, we cannot exclude the possibility that some severely ill patients died before enrolment into our study. Our melioidosis cohort is notable for being one of the few large populations of patients infected with a single Gram-negative etiology of sepsis and is further strengthened by the large scale immuno-assay study of healthy subjects from the same northeastern Thai population.
Conclusion
In conclusion, in our cohort of Thai subjects with melioidosis we do not confirm the previously described associations in Spanish and white North American populations of TLR1 genetic variation with outcomes from sepsis. We are also unable to identify functional effects of selected common TLR1 variants in Thais. We confirm substantial differences in the genetic architecture of TLR1 variation of Thais compared to other populations. These findings underscore the need for additional studies of TLR1 and other innate immune genetic modulators of the inflammatory host response and determinants of sepsis in southeast Asian populations.
Supporting Information
TLR1 variant genotype frequencies in selected global populations.
(DOCX)
Minor allele frequencies and association of TLR1 variants with cytokine induced by stimulation of whole blood with Pam3CSK4 in healthy Thai subjects.
(DOCX)
Acknowledgments
The authors acknowledge the support of the staff and patients at Sappasithiprasong Hospital and Mahidol-Oxford Tropical Medicine Research Unit; DNA extraction by Premjit Amornchai, Aunchalee Thanwisai, and Malinee Oyuchua; graphics by Johanna Robertson; and assistance generating immuno-assays and advice from Mark Wurfel.
Funding Statement
This study was funded by a Wellcome Trust Career Development award in Public Health and Tropical Medicine, UK (grant 087769/Z/08/Z to NC), by the US National Institutes of Health award K08HL094759 (TEW), and by the Parker B. Francis Foundation (TEW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD (2010) Critical care and the global burden of critical illness in adults. Lancet 376: 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng AC, West TE, Limmathurotsakul D, Peacock SJ (2008) Strategies to reduce mortality from bacterial sepsis in adults in developing countries. PLoS Med 5: e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiersinga WJ, Currie BJ, Peacock SJ (2012) Melioidosis. N Engl J Med 367: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 4. Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D (2013) Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PloS One 8: e54714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, et al. (2010) Increasing incidence of human melioidosis in Northeast Thailand. Am J Trop Med Hyg 82: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suputtamongkol Y, Kwiatkowski D, Dance DA, Chaowagul W, White NJ (1992) Tumor necrosis factor in septicemic melioidosis. J Infect Dis 165: 561–564. [DOI] [PubMed] [Google Scholar]
- 7. Friedland JS, Suputtamongkol Y, Remick DG, Chaowagul W, Strieter RM, et al. (1992) Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect Immun 60: 2402–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simpson AJ, Smith MD, Weverling GJ, Suputtamongkol Y, Angus BJ, et al. (2000) Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J Infect Dis 181: 621–625. [DOI] [PubMed] [Google Scholar]
- 9. Lauw FN, Simpson AJ, Prins JM, Smith MD, Kurimoto M, et al. (1999) Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J Infect Dis 180: 1878–1885. [DOI] [PubMed] [Google Scholar]
- 10. Chaowagul W, White NJ, Dance DA, Wattanagoon Y, Naigowit P, et al. (1989) Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis 159: 890–899. [DOI] [PubMed] [Google Scholar]
- 11. Chapman SJ, Hill AV (2012) Human genetic susceptibility to infectious disease. Nat Rev Genet 13: 175–188. [DOI] [PubMed] [Google Scholar]
- 12. Wurfel MM (2008) Genetic insights into sepsis: what have we learned and how will it help? Curr Pharm Des 14: 1900–1911. [DOI] [PubMed] [Google Scholar]
- 13. Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 14. West TE, Chierakul W, Chantratita N, Limmathurotsakul D, Wuthiekanun V, et al. (2012) Toll-like receptor 4 region genetic variants are associated with susceptibility to melioidosis. Genes Immun 13: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. West TE, Chantratita N, Chierakul W, Limmathurotsakul D, Wuthiekanun V, et al. (2013) Impaired TLR5 functionality is associated with survival in melioidosis. J Immunol 190: 3373–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wurfel MM, Gordon AC, Holden TD, Radella F, Strout J, et al. (2008) Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med 178: 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hawn TR, Misch EA, Dunstan SJ, Thwaites GE, Lan NT, et al. (2007) A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur J Immunol 37: 2280–2289. [DOI] [PubMed] [Google Scholar]
- 18. Barreiro LB, Neyrolles O, Babb CL, Tailleux L, Quach H, et al. (2006) Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med 3: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson CM, Lyle EA, Omueti KO, Stepensky VA, Yegin O, et al. (2007) Cutting edge: A common polymorphism impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J Immunol 178: 7520–7524. [DOI] [PubMed] [Google Scholar]
- 20.Thompson CM, Holden TD, Rona G, Laxmanan B, Black RA, et al.. (2013) Toll-Like Receptor 1 Polymorphisms and Associated Outcomes in Sepsis After Traumatic Injury: A Candidate Gene Association Study. Ann Surg. [DOI] [PMC free article] [PubMed]
- 21. Pino-Yanes M, Corrales A, Casula M, Blanco J, Muriel A, et al. (2010) Common variants of TLR1 associate with organ dysfunction and sustained pro-inflammatory responses during sepsis. PloS One 5: e13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schuring RP, Hamann L, Faber WR, Pahan D, Richardus JH, et al. (2009) Polymorphism N248S in the human Toll-like receptor 1 gene is related to leprosy and leprosy reactions. J Infect Dis 199: 1816–1819. [DOI] [PubMed] [Google Scholar]
- 23. Hamann L, Bedu-Addo G, Eggelte TA, Schumann RR, Mockenhaupt FP (2010) The toll-like receptor 1 variant S248N influences placental malaria. Infect Genet Evol 10: 785–789. [DOI] [PubMed] [Google Scholar]
- 24. Wong SH, Gochhait S, Malhotra D, Pettersson FH, Teo YY, et al. (2010) Leprosy and the adaptation of human toll-like receptor 1. PLoS Pathog 6: e1000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. West TE, Ernst RK, Jansson-Hutson MJ, Skerrett SJ (2008) Activation of Toll-like receptors by Burkholderia pseudomallei. BMC Immunol 9: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng AC, Peacock SJ, Limmathurotsakul D, Wongsuvan G, Chierakul W, et al. (2006) Prospective evaluation of a rapid immunochromogenic cassette test for the diagnosis of melioidosis in northeast Thailand. Trans R Soc Trop Med Hyg 100: 64–67. [DOI] [PubMed] [Google Scholar]
- 27. Yuan HY, Chiou JJ, Tseng WH, Liu CH, Liu CK, et al. (2006) FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res 34: W635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Purcell S, Cherny SS, Sham PC (2003) Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19: 149–150. [DOI] [PubMed] [Google Scholar]
- 29. Misch EA, Hawn TR (2008) Toll-like receptor polymorphisms and susceptibility to human disease. Clin Sci (Lond) 114: 347–360. [DOI] [PubMed] [Google Scholar]
- 30. Randhawa AK, Shey MS, Keyser A, Peixoto B, Wells RD, et al. (2011) Association of human TLR1 and TLR6 deficiency with altered immune responses to BCG vaccination in South African infants. PLoS Pathog 7: e1002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Sales Marques C, Brito-de-Souza VN, Guerreiro LT, Martins JH, Amaral EP, et al. (2013) Toll-like Receptor 1 N248S Single-Nucleotide Polymorphism Is Associated With Leprosy Risk and Regulates Immune Activation During Mycobacterial Infection. J Infect Dis 208: 120–129. [DOI] [PubMed] [Google Scholar]
- 32. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, et al. (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 491: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hotchkiss RS, Monneret G, Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13: 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vincent JL, Opal SM, Marshall JC, Tracey KJ (2013) Sepsis definitions: time for change. Lancet 381: 774–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cinel I, Opal SM (2009) Molecular biology of inflammation and sepsis: a primer. Crit Care Med 37: 291–304. [DOI] [PubMed] [Google Scholar]
- 36. Weighardt H, Holzmann B (2007) Role of Toll-like receptor responses for sepsis pathogenesis. Immunobiology 212: 715–722. [DOI] [PubMed] [Google Scholar]
- 37. Uciechowski P, Imhoff H, Lange C, Meyer CG, Browne EN, et al. (2011) Susceptibility to tuberculosis is associated with TLR1 polymorphisms resulting in a lack of TLR1 cell surface expression. J Leukoc Biol 90: 377–388. [DOI] [PubMed] [Google Scholar]
- 38. Ocejo-Vinyals JG, Puente de Mateo E, Ausin F, Aguero R, Arroyo JL, et al. (2013) Human toll-like receptor 1 T1805G polymorphism and susceptibility to pulmonary tuberculosis in northern Spain. Int J Tuberc Lung Dis 17: 652–654. [DOI] [PubMed] [Google Scholar]
- 39. Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, et al. (2012) Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis 205: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barreiro LB, Ben-Ali M, Quach H, Laval G, Patin E, et al. (2009) Evolutionary dynamics of human Toll-like receptors and their different contributions to host defense. PLoS Genet 5: e1000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mikacenic C, Reiner AP, Holden TD, Nickerson DA, Wurfel MM (2013) Variation in the TLR10/TLR1/TLR6 locus is the major genetic determinant of interindividual difference in TLR1/2-mediated responses. Genes Immun 14: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TLR1 variant genotype frequencies in selected global populations.
(DOCX)
Minor allele frequencies and association of TLR1 variants with cytokine induced by stimulation of whole blood with Pam3CSK4 in healthy Thai subjects.
(DOCX)