Abstract
Proteins L7 and L12 from 50S ribosomal subunits of Escherichia coli are required for peptidechain termination. This termination process is inhibited by thiostrepton. Since both thiostrepton-treated ribosomes and those depleted of L7 and L12 have a markedly reduced ability to form release factor·UA[3H]A·ribosome complexes, the binding of release factors to the ribosome appears to be the primary site of inhibition.
Keywords: E. coli, thiostrepton, ribosomal protein
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballesta J. P.G., Vazquez D. Reconstitution of the 50S ribosome subunit. Role of proteins L 7 and L 12 in the GTPase activities. Site of action of thiostrepton. FEBS Lett. 1972 Dec 15;28(3):337–342. doi: 10.1016/0014-5793(72)80745-2. [DOI] [PubMed] [Google Scholar]
- Beaudet A. L., Caskey C. T. Mammalian peptide chain termination. II. Codon specificity and GTPase activity of release factor. Proc Natl Acad Sci U S A. 1971 Mar;68(3):619–624. doi: 10.1073/pnas.68.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodley J. W., Lin L., Highland J. H. Studies on translocation. VI. Thiostrepton prevents the formation of a ribosome-G factor-guanine nucleotide complex. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1406–1411. doi: 10.1016/0006-291x(70)90543-7. [DOI] [PubMed] [Google Scholar]
- Brot N., Yamasaki E., Redfield B., Weissbach H. The binding of aminoacyl-tRNA and poly U to a soluble factor (S) extracted from ribosomes. Biochem Biophys Res Commun. 1970 Aug 11;40(3):698–707. doi: 10.1016/0006-291x(70)90960-5. [DOI] [PubMed] [Google Scholar]
- Brot N., Yamasaki E., Redfield B., Weissbach H. The properties of an E. coli ribosomal protein required for the function of factor G. Arch Biochem Biophys. 1972 Jan;148(1):148–155. doi: 10.1016/0003-9861(72)90125-7. [DOI] [PubMed] [Google Scholar]
- Cabrer B., Vázquez D., Modolell J. Inhibition by elongation factor EF G of aminoacyl-tRNA binding to ribosomes. Proc Natl Acad Sci U S A. 1972 Mar;69(3):733–736. doi: 10.1073/pnas.69.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey C. T., Tompkins R., Scolnick E., Caryk T., Nirenberg M. Sequential translation of trinucleotide codons for the initiation and termination of protein synthesis. Science. 1968 Oct 4;162(3849):135–138. doi: 10.1126/science.162.3849.135. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Caskey C. T. Peptide chain termination: effect of protein S on ribosomal binding of release factors. Proc Natl Acad Sci U S A. 1970 Oct;67(2):537–543. doi: 10.1073/pnas.67.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg-Manago M., Dondon J., Graffe M. Inhibition by thiostrepton of the IF-2-dependent ribosomal GTPase. FEBS Lett. 1972 May 1;22(2):217–221. doi: 10.1016/0014-5793(72)80049-8. [DOI] [PubMed] [Google Scholar]
- Hamel E., Koka M., Nakamoto T. Requirement of an Escherichia coli 50 S ribosomal protein component for effective interaction of the ribosome with T and G factors and with guanosine triphosphate. J Biol Chem. 1972 Feb 10;247(3):805–814. [PubMed] [Google Scholar]
- Hamel E., Nakamoto T. Studies on the role of an Escherichia coli 50 S ribosomal component in polypeptide chain elongation. J Biol Chem. 1972 Nov 10;247(21):6810–6817. [PubMed] [Google Scholar]
- Highland J. H., Bodley J. W., Gordon J., Hasenbank R., Stöffler G. Identity of the ribosomal proteins involved in the interaction with elongation factor G. Proc Natl Acad Sci U S A. 1973 Jan;70(1):147–150. doi: 10.1073/pnas.70.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A., Sander G., Grunberg-Manago M. Effect of ribosomal protein L12 upon initiation factor IF-2 activities. Biochem Biophys Res Commun. 1973 Apr 16;51(4):979–986. doi: 10.1016/0006-291x(73)90023-5. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Liou Y., Tanaka N. Inhibition by thiopeptin of ribosomal functions associated with T and G factors. Biochem Biophys Res Commun. 1971 Aug 20;44(4):859–863. doi: 10.1016/0006-291x(71)90790-x. [DOI] [PubMed] [Google Scholar]
- Kischa K., Möller W., Stöffler G. Reconstitution of a GTPase activity by a 50S ribosomal protein and E. coli. Nat New Biol. 1971 Sep 8;233(36):62–63. doi: 10.1038/newbio233062a0. [DOI] [PubMed] [Google Scholar]
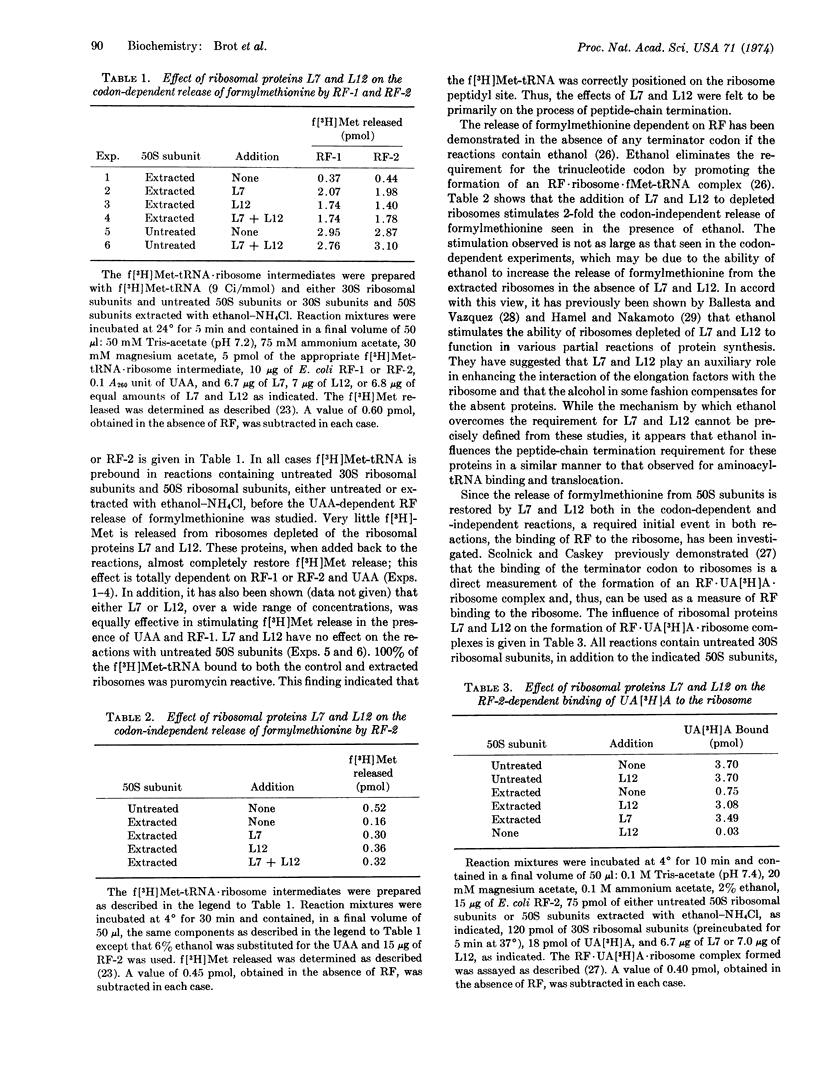
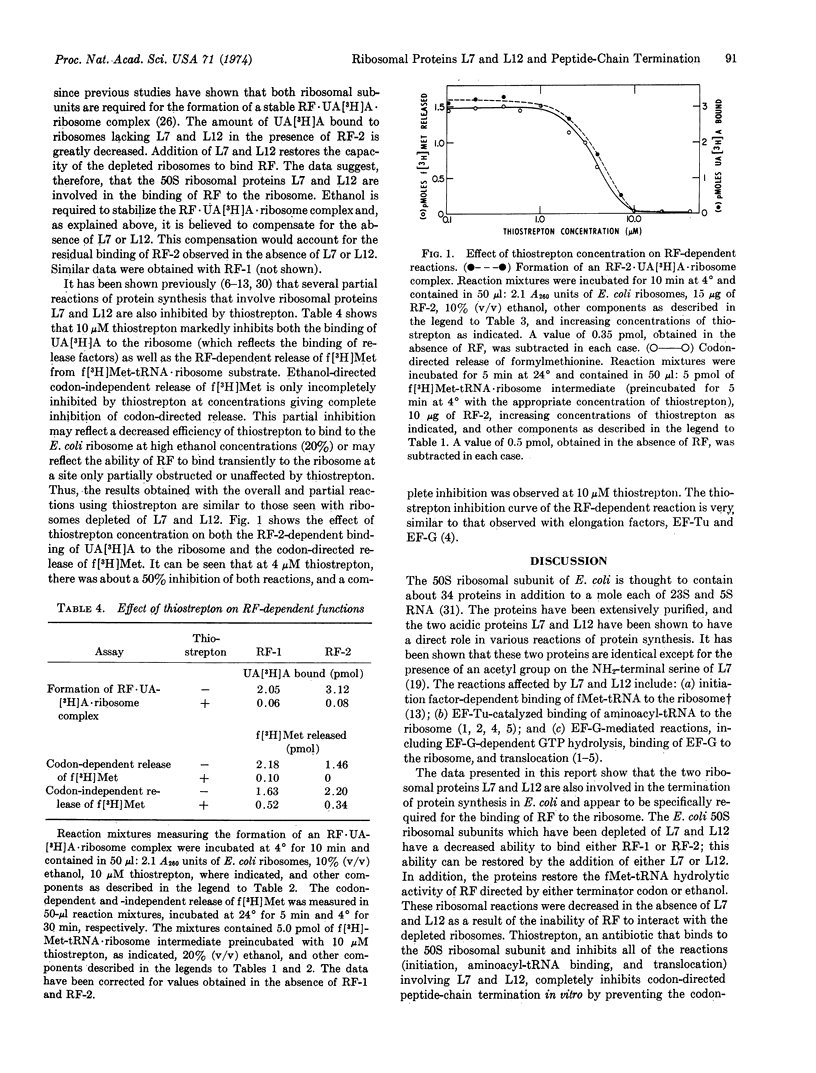
- Kurland C. G. Structure and function of the bacterial ribosome. Annu Rev Biochem. 1972;41(10):377–408. doi: 10.1146/annurev.bi.41.070172.002113. [DOI] [PubMed] [Google Scholar]
- Leder P., Bursztyn H. Initiation of protein synthesis II. A convenient assay for the ribosome-dependent synthesis of N-formyl-C14-methionylpuromycin. Biochem Biophys Res Commun. 1966 Oct 20;25(2):233–238. doi: 10.1016/0006-291x(66)90586-9. [DOI] [PubMed] [Google Scholar]
- Miller D. L. Elongation factors EF Tu and EF G interact at related sites on ribosomes. Proc Natl Acad Sci U S A. 1972 Mar;69(3):752–755. doi: 10.1073/pnas.69.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman G., Goldstein J., Scolnick E., Caskey T. Peptide chain termination. 3. Stimulation of in vitro termination. Proc Natl Acad Sci U S A. 1969 May;63(1):183–190. doi: 10.1073/pnas.63.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Cabrer B., Parmeggiani A., Vazquez D. Inhibition by siomycin and thiostrepton of both aminoacyl-tRNA and factor G binding to ribosomes. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1796–1800. doi: 10.1073/pnas.68.8.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell J., Vazquez D. Inhibition by aminoacyl transfer ribonucleic acid of elongation factor G-dependent binding of guanosine nucleotide to ribosomes. J Biol Chem. 1973 Jan 25;248(2):488–493. [PubMed] [Google Scholar]
- Möller W., Groene A., Terhorst C., Amons R. 50-S ribosomal proteins. Purification and partial characterization of two acidic proteins, A 1 and A 2, isolated from 50-S ribosomes of Escherichia coli. Eur J Biochem. 1972 Jan 31;25(1):5–12. doi: 10.1111/j.1432-1033.1972.tb01660.x. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Pestka S., Brot N. Studies on the formation of transfer ribonucleic acid-ribosome complexes. IV. Effect of antibiotics on steps of bacterial protein synthesis: some new ribosomal inhibitors of translocation. J Biol Chem. 1971 Dec 25;246(24):7715–7722. [PubMed] [Google Scholar]
- Pestka S., Nirenberg M. Regulatory mechanisms and protein synthesis. X. Codon recognition on 30 S ribosomes. J Mol Biol. 1966 Oct 28;21(1):145–171. doi: 10.1016/0022-2836(66)90085-4. [DOI] [PubMed] [Google Scholar]
- Pestka S. Thiostrepton: a ribosomal inhibitor of translocation. Biochem Biophys Res Commun. 1970 Aug 11;40(3):667–674. doi: 10.1016/0006-291x(70)90956-3. [DOI] [PubMed] [Google Scholar]
- Richman N., Bodley J. W. Ribosomes cannot interact simultaneously with elongation factors EF Tu and EF G. Proc Natl Acad Sci U S A. 1972 Mar;69(3):686–689. doi: 10.1073/pnas.69.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D. Inability of E. coli ribosomes to interact simultaneously with the bacterial elongation factors EF Tu and EF G. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1850–1856. doi: 10.1016/0006-291x(72)90061-7. [DOI] [PubMed] [Google Scholar]
- Sander G., Marsh R. C., Parmeggiani A. Isolation and characterization of two acidic proteins from the 50S subunit required for GTPase activities of both EF G and EF T. Biochem Biophys Res Commun. 1972 May 26;47(4):866–873. doi: 10.1016/0006-291x(72)90573-6. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Caskey C. T. Peptide chain termination. V. The role of release factors in mRNA terminator codon recognition. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1235–1241. doi: 10.1073/pnas.64.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Watanabe S., Teraoka H., Tamaki M. Effect of siomycin on protein synthesizing activity of Escherichia coli ribosomes. Biochem Biophys Res Commun. 1970;39(6):1189–1193. doi: 10.1016/0006-291x(70)90686-8. [DOI] [PubMed] [Google Scholar]
- Tate W. P., Beaudet A. L., Caskey C. T. Influence of guanine nucleotides and elongation factors on interaction of release factors with the ribosome. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2350–2355. doi: 10.1073/pnas.70.8.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins R. K., Scolnick E. M., Caskey C. T. Peptide chain termination. VII. The ribosomal and release factor requirements for peptide release. Proc Natl Acad Sci U S A. 1970 Mar;65(3):702–708. doi: 10.1073/pnas.65.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Demohn V. Inhibition by thiostrepton of the formation of a ribosome-bound guanine nucleotide complex. FEBS Lett. 1970 Dec;11(3):149–152. doi: 10.1016/0014-5793(70)80515-4. [DOI] [PubMed] [Google Scholar]
- Weissbach H., Redfield B., Yamasaki E., Davis R. C., Jr, Pestka S., Brot N. Studies on the ribosomal sites involved in factors Tu and G-dependent reactions. Arch Biochem Biophys. 1972 Mar;149(1):110–117. doi: 10.1016/0003-9861(72)90304-9. [DOI] [PubMed] [Google Scholar]



