Abstract
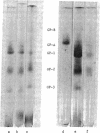
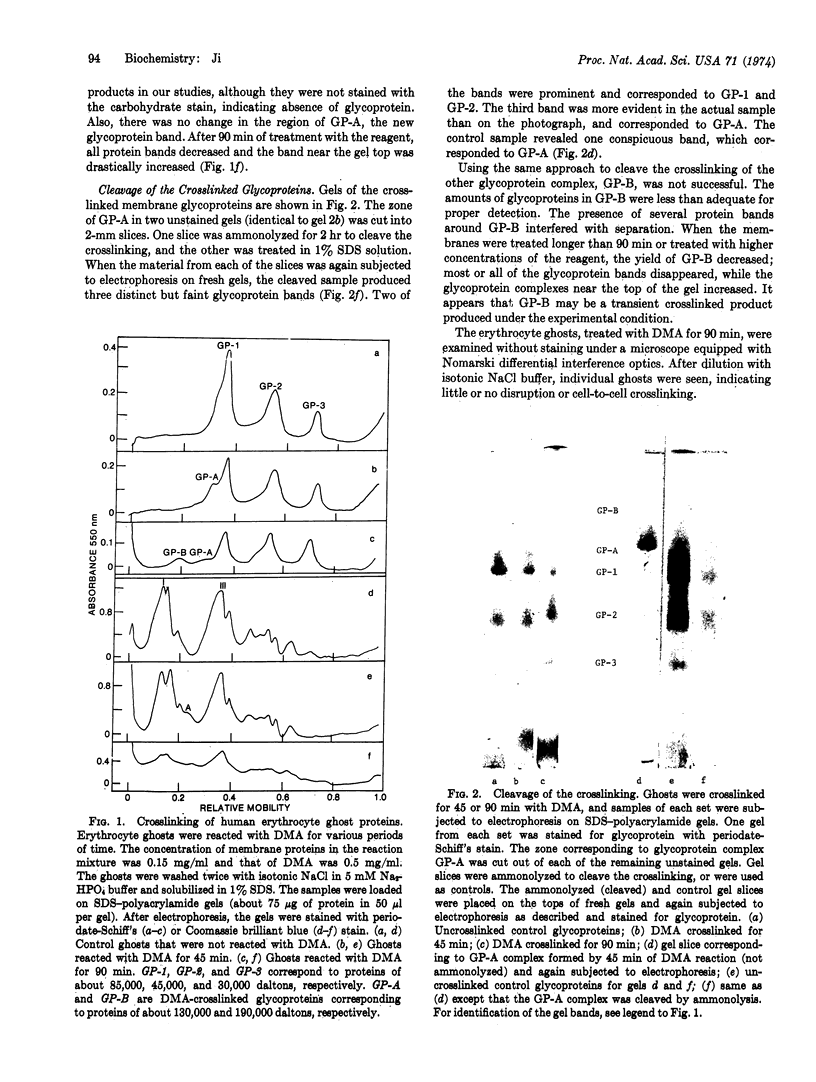
The glycoproteins of human erythrocyte membranes were crosslinked with dimethyl adipimidate dihydrochloride. On sodium dodecyl sulfate-polyacrylamide gel electrophoregrams of the crosslinked solubilized membranes, at least three new glycoprotein complexes appeared in addition to the normal glycoprotein species. One of the new glycoprotein complexes was shown to contain two of the three species of membrane glycoproteins.
Keywords: dimethyl adipimidate dihydrochloride, membrane structure
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher M. S. Major human erythrocyte glycoprotein spans the cell membrane. Nat New Biol. 1971 Jun 23;231(25):229–232. doi: 10.1038/newbio231229a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A. A cross-linking study of the beef erythrocyte membrane: extensive interaction of all the proteins of the membrane except for the glycoproteins. Biochem Biophys Res Commun. 1973 Feb 5;50(3):656–661. doi: 10.1016/0006-291x(73)91294-1. [DOI] [PubMed] [Google Scholar]
- Carpenter F. H., Harrington K. T. Intermolecular cross-linking of monomeric proteins and cross-linking of oligomeric proteins as a probe of quaternary structure. Application to leucine aminopeptidase (bovine lens). J Biol Chem. 1972 Sep 10;247(17):5580–5586. [PubMed] [Google Scholar]
- Clarke M. Isolation and characterization of a water-soluble protein from bovine erythrocyte membranes. Biochem Biophys Res Commun. 1971 Nov;45(4):1063–1070. doi: 10.1016/0006-291x(71)90445-1. [DOI] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Guidotti G. Membrane proteins. Annu Rev Biochem. 1972;41:731–752. doi: 10.1146/annurev.bi.41.070172.003503. [DOI] [PubMed] [Google Scholar]
- Hulla F. W., Gratzer W. B. Association of high-molecular weight proteins in the red cell membrane. FEBS Lett. 1972 Sep 15;25(2):275–278. doi: 10.1016/0014-5793(72)80502-7. [DOI] [PubMed] [Google Scholar]
- Inbar M., Ben-Bassat H., Sachs L. Membrane changes associated with malignancy. Nat New Biol. 1972 Mar 1;236(61):3–passim. doi: 10.1038/newbio236003a0. [DOI] [PubMed] [Google Scholar]
- Ji T. H. Cross-linking sialoglycoproteins of human erythrocyte membranes. Biochem Biophys Res Commun. 1973 Jul 17;53(2):508–514. doi: 10.1016/0006-291x(73)90691-8. [DOI] [PubMed] [Google Scholar]
- Ji T. H. Interference by detergents, chelating agents, and buffers with the Lowry protein determination. Anal Biochem. 1973 Apr;52(2):517–521. doi: 10.1016/0003-2697(73)90056-0. [DOI] [PubMed] [Google Scholar]
- Marton L. S., Garvin L. E. Subunit structure of the major human erythrocytes glycoprotein: depolymerization by heating ghosts with sodium dodecyl sulfate. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1457–1462. doi: 10.1016/0006-291x(73)90664-5. [DOI] [PubMed] [Google Scholar]
- McQuiddy P., Lilien J. Sialic acid and cell aggregation. J Cell Sci. 1971 Nov;9(3):823–833. doi: 10.1242/jcs.9.3.823. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Anionic sites of human erythrocyte membranes. I. Effects of trypsin, phospholipase C, and pH on the topography of bound positively charged colloidal particles. J Cell Biol. 1973 May;57(2):373–387. doi: 10.1083/jcb.57.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Topography of membrane concanavalin A sites modified by proteolysis. Nat New Biol. 1972 Oct 18;239(94):193–197. doi: 10.1038/newbio239193a0. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S. B. Utilization of L-glutamine in intercellular adhesion: ascites tumor and embryonic cells. Exp Cell Res. 1973 Mar 15;77(1):175–182. doi: 10.1016/0014-4827(73)90566-1. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P. Translational mobility of the membrane intercalated particles of human erythrocyte ghosts. pH-dependent, reversible aggregation. J Cell Biol. 1972 Jun;53(3):777–787. doi: 10.1083/jcb.53.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., White D. Intercellular contact and cell-surface galactosyl transferase activity (cell culture-mouse-radioautography-contact inhibition-cis-and trans-galactosylation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):485–489. doi: 10.1073/pnas.69.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Nagai Y., Tegtmeyer H. Isolation and properties of human blood-group NN and meconium-Vg antigens. Biochemistry. 1966 Oct;5(10):3254–3272. doi: 10.1021/bi00874a028. [DOI] [PubMed] [Google Scholar]
- Steck T. L. Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol. 1972 May 14;66(2):295–305. doi: 10.1016/0022-2836(72)90481-0. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H. Separation and some properties of the major proteins of the human erythrocyte membrane. Biochem J. 1972 Sep;129(2):333–347. doi: 10.1042/bj1290333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]