Abstract
Characterizing working memory (WM) abnormalities represents a fundamental challenge in schizophrenia research given the impact of cognitive deficits on life outcome in patients. In prior work we demonstrated that dorsolateral prefrontal cortex (DLPFC) activation was related to successful distracter resistance during WM in healthy controls, but not in schizophrenia. Although understanding the impact of regional functional deficits is critical, functional connectivity abnormalities among nodes within WM networks may constitute a final common pathway for WM impairment. Therefore, this study tested the hypothesis that schizophrenia is associated with functional connectivity abnormalities within DLPFC networks during distraction conditions in WM. 28 patients and 24 controls completed a delayed non-verbal WM task that included transient visual distraction during the WM maintenance phase. We computed DLPFC whole-brain task-based functional connectivity (tb-fcMRI) specifically during the maintenance phase in the presence or absence of distraction. Results revealed that patients failed to modulate tb-fcMRI during distracter presentation in both cortical and sub-cortical regions. Specifically, controls demonstrated reductions in tb-fcMRI between DLPFC and the extended amygdala when distraction was present. Conversely, patients failed to demonstrate a change in coupling with the amygdala, but showed greater connectivity with medio-dorsal thalamus. While controls showed more positive coupling between DLPFC and other prefrontal cortical regions during distracter presentation, patients failed to exhibit such a modulation. Taken together, these findings support the notion that observed distracter resistance deficit involves a breakdown in coupling between DLPFC and distributed regions, encompassing both subcortical (thalamic/limbic) and control region connectivity.
Keywords: Schizophrenia, DLPFC, amygdala, thalamus, fMRI, Functional connectivity, Working memory, Distraction
1. Introduction
Cognitive impairments associated with schizophrenia compromise social and vocational function and are not effectively treated by available therapies (Cornblatt et al., 1999; Niendam et al., 2003; Green, 2006). Impairments in working memory (WM), the temporary storage and manipulation of information held ‘on-line’ in the service of some goal (Jonides et al., 2008), are prominent in schizophrenia (Elvevag and Goldberg, 2000). WM deficits are present prior to the onset of illness and in medication-free individuals in their first episode of illness (Delawalla et al., 2006).
Schizophrenia is associated with deficits in component processes of WM (Lee and Park, 2005), but how breakdowns in distinct aspects of WM function may contribute to the overall profile of impairment in this illness remains unclear. WM can be broken down into distinct temporal components: i) encoding of information in WM, ii) maintenance of information in WM including protection against decay and distraction; and iii) retrieval and manipulation of memoranda when needed (Baddeley and Hitch, 1974; Baddeley, 2000; Jonides et al., 2008). Studies of schizophrenia have focused particularly on WM encoding and maintenance deficits (Lee and Park, 2005; Johnson et al., 2006; Driesen et al., 2008; Schlösser et al., 2008). However, while there is a rich behavioral literature showing sensory gating problems in patients (Geyer et al., 2001; Turetsky et al., 2007), less work has been done to understand neural mechanisms underlying deficits in ‘protection’ of WM stores against disruption by distracters in schizophrenia.
In a recent investigation, we identified a dorsal-lateral prefrontal cortex (DLPFC) region centered on the medial frontal gyrus that healthy subjects engaged specifically when distracters appeared during delayed WM (Anticevic et al., 2011c). Furthermore, there was a significant relationship between the degree of DLPFC activation in response to distraction and successful WM performance in healthy individuals. In contrast, individuals with schizophrenia failed to: i) recruit the DLPFC region in response to distraction; ii) failed to show a relationship between DLPFC activation and WM performance — suggesting a possible breakdown in filtering operations during WM. Critically, a similar region has been previously shown to be involved in resistance of distraction in healthy adults in the context of WM (Postle, 2005).
While individual brain regions in DLPFC networks probably contribute uniquely to WM processes, there is growing interest in properties of WM dependent upon functional connectivity among these nodes (Glahn et al., 2005). One way to characterize this connectivity deficit is to examine task-based functional connectivity (tb-fcMRI) specifically during WM with and without distraction. In our prior work, tb-fcMRI revealed how regional coupling differs across WM phases and conditions in healthy subjects (Anticevic et al., 2010b) — this approach can readily be applied to comparisons with patient groups (Anticevic et al., 2011a). Here we extend this approach to schizophrenia to specifically test the hypothesis that group differences in DLPFC tb-fcMRI during WM are heightened in the presence of distracters. We hypothesized two distinct patterns of findings based on differential roles of subcortical regions (e.g. amygdala) in ‘bottom-up’ operations (Pessoa and Adolphs, 2010) and prefrontal cortical regions involvement in ‘top-down’ processes relevant to interference resolution (e.g. inferior frontal gyrus) (Thompson-Schill et al., 2002). Specifically, we predicted that patients may exhibit two types of anomalous connectivity patterns: i) ‘over-connectivity’ between DLPFC and regions involved ‘bottom up’ stimulus processing; and ii) ‘under-connectivity’ between DLPFC and cortical areas typically involved in cognitive control.
2. Methods
2.1. Subjects
Recruitment details are provided in our prior studies (Anticevic et al., 2011c). Briefly, we recruited 28 patients and 24 demographically matched healthy controls. All subjects underwent clinical interviewing and diagnostics by a Master’s level clinician using the Structured Clinical Interview for DSM-IV-TR (First et al., 2002), symptom ratings (Andreasen, 1983a, b) and IQ assessment (Wechsler, 1997). Exclusion criteria: i) lifetime history of Axis I psychiatric disorder or a first-degree relative with a psychotic disorder for controls; ii) All subjects were excluded for presence of DSM-IV substance abuse/dependence, anxiety or depression within the past 6 months or mental retardation; iii) serious medical conditions; iv) head injury (past or present) with neurological symptoms or disrupted consciousness or history of neurological disorders. Patients were receiving a stable level of medication for a period of at least 2 weeks; we converted all medication dosages to chloropromazine equivalents (Woods, 2003; Bazire, 2005) and verified that medication dosage did not alter reported effects (for additional covariate analyses see Supplement). At the time of assessment patients did not present with co-morbid axis I diagnoses. Groups were well-matched across demographic criteria (handedness, gender, age, parental education, and parental socioeconomic status) except on standard measures of verbal and non-verbal IQ (Wechsler, 1997) (Table 1) (although differences in IQ did not alter reported effects, see Supplement & Discussion for treatment of IQ differences).
Table 1.
Demographics. Positive symptoms were the sum of global scores for hallucinations and delusions; negative symptoms were the sum of global scores for alogia, anhedonia, avolition, affective flattening, and attentional impairment; and disorganization symptoms were the sum of global scores for bizarre behavior, positive thought disorder, and inappropriate affect.
| Characteristic | Controls
|
Patients
|
Significance
|
|||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | T value/chi-square | P value (two-tailed) | |
| Age (in years) | 37.18 | 7.59 | 36.39 | 9.54 | 0.31 | 0.759 |
| Gender (% male) | 74 | 78 | 0.34 | 0.737 | ||
| Paternal education (in years) | 12.70 | 1.46 | 13.26 | 2.61 | 0.90 | 0.370 |
| Maternal education | 12.48 | 1.53 | 13.50 | 3.07 | 1.42 | 0.162 |
| Paternal SES | 21.59 | 8.92 | 26.59 | 10.73 | 1.67 | 0.100 |
| Maternal SES | 17.27 | 8.55 | 25.24 | 11.88 | 2.51 | 0.015 |
| Participant’s education (in years) | 15.26 | 2.12 | 13.04 | 2.14 | 3.50 | 0.001 |
| Handedness (% right) | 100.00 | 86.96 | 1.45 | 0.152 | ||
| IQ Verbal | 110.23 | 10.85 | 95.23 | 14.18 | 3.88 | 0.000 |
| IQ Performance | 115.45 | 11.64 | 101.82 | 15.24 | 3.30 | 0.002 |
| Medication (CPZ equivalents) | – | – | 584.63 | 563.63 | – | – |
| Mean SAPS Global Item Score | – | – | 1.91 | 1.21 | – | – |
| Mean SANS Global Item Score | – | – | 2.50 | 0.78 | – | – |
| Disorganization | – | – | 5.48 | 2.71 | – | – |
| Poverty | – | – | 10.43 | 3.53 | – | – |
| Reality Disotortion | – | – | 4.26 | 3.53 | – | – |
SAPS, scale for assessment of positive symptoms; SANS, scale for the assessment of negative symptoms; CPZ, chlorpromazine; SES, socioeconomic status.
2.2. fMRI acquisition and stimuli
Images were acquired using a 3 T Tim-TRIO scanner at Washington University. Functional images were acquired using an asymmetric spin-echo, echo-planar sequence maximally sensitive to blood-oxygenation-level-dependent (BOLD) contrast (T2*) (repetition time [TR]=2200 ms, echo time [TE]=27 ms, field of view [FOV]=256 mm, flip=90°, voxel size=4×4×4mm). Structural images were acquired using a sagittal MP-RAGE 3D T1-weighted sequence (TR=2400 ms, TE=3.16 ms, flip=8°; voxel size=1 mm3). The task is described comprehensively elsewhere (Anticevic et al., 2010a, 2011c) (Fig. 1, see Supplement for more detail). Briefly, subjects completed 24 trials (three 5.09-min runs) to estimate distracter-free maintenance activity and 72 trials (six 7.44-min runs) with one of the following distracters presented during the delay period: a) negative images; b) neutral images; and c) task-related geometric shapes (these distracters resembled the memoranda). For the purpose of the current analyses, we collapsed across distracter types given prior results indicating that patients were more distracted relative to controls irrespective of distracter category (Anticevic et al., 2011c) (for details on behavioral results and task design choices see Supplement).
Fig. 1.
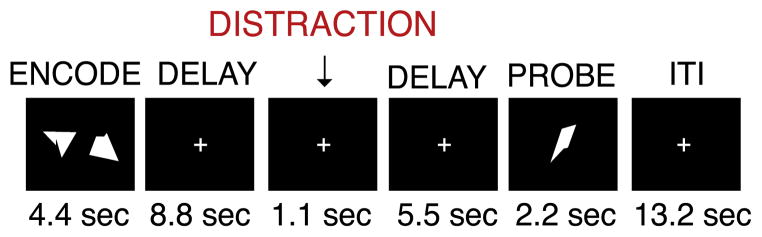
Task Design. Overall task design is shown. For the purposes of the present investigation we collapsed across different distracter conditions (see Method) since patients were more distracted than controls across all distracter types irrespective of distracter condition (Anticevic et al., 2011c). Complete details regarding the task were described previously (Anticevic et al., 2011b, 2011c). We also provide additional task details and considerations in the Supplement.
2.3. fMRI preprocessing
Preprocessing included: i) slice-time correction; ii) first 5 images removed to reach steady state tissue magnetization; iii) odd/even slice intensity differences removed due to interpolated acquisition; iv) rigid body motion correction and inspection (5 patients and 1 control were excluded given excessive motion) (Ojemann et al., 1997); v) intensity normalization to a whole brain mode value of 1,000 without bias or gain field correction; vi) registration of structural images to a template image in the Talairach coordinate system (12-parameter affine transform) (Talairach and Tournoux, 1988); vii) co-registration of BOLD images to the structural image with re-sampling to 3 mm3 (Ojemann et al., 1997; Buckner et al., 2004). There were no significant group differences in SNR across all BOLD runs [t(44)=0.08, p=0.93, NS].
2.4. Task-based functional connectivity (tb-fcMRI) analyses
To remove possible sources of spurious correlations (Fox et al., 2005; Anticevic Anticevic et al., 2010a, 2010b) additional preprocessing was conducted: i) spatial smoothing by 6-mm FWHM Gaussian filter; ii) high-pass filtering (>0.009 Hz) to remove low frequencies and scanner drift; iii) removal of motion correction parameters, ventricle, deep white matter, and global mean (GMS) signals and their first derivatives using a general linear model framework. All subsequent tb-fcMRI analyses were conducted on the residual signal. We acknowledge that GMS removal can possibly induce some negative relationships (Murphy et al., 2009). However, competing evidence illustrates that this pre-processing step is critical for optimizing fcMRI specificity (Fox et al., 2009) and is widely used (Biswal et al., 2010). Nevertheless, both groups underwent identical preprocessing. Thus, observed differences cannot be driven by GMS removal. However, we acknowledge that this can possibly complicate interpretation of obtained results.
Next, to examine tb-fcMRI, we followed an approach used in our previously published studies (Anticevic et al., 2010a, 2010b). Briefly, we computed the average BOLD signal value related to distracter onset (average of time points 8&9) at each trial for each voxel in the image. We averaged two time-points to reduce variability due to possible outlier frames. We did so for both distracter and distracter-free trials. Next, we concatenated values into two 4-D (brain volume×trial) time series that represented trial-to-trial variability in response to distracter vs. distracter-free trials (Fig. 2). Extracting only specific time-locked time series components, as argued previously (Anticevic et al., 2010b), ensured that the correlations are driven primarily by trial-to-trial variability and not overall task response. Furthermore, the issue of task response driving the variability is minimized given the slow event-related nature of the design. Also, this approach circumvents the need to assume and/or fit a hemodynamic response function (Rissman et al., 2004); however it is still limited by the number of time points available for analysis.
Fig. 2.
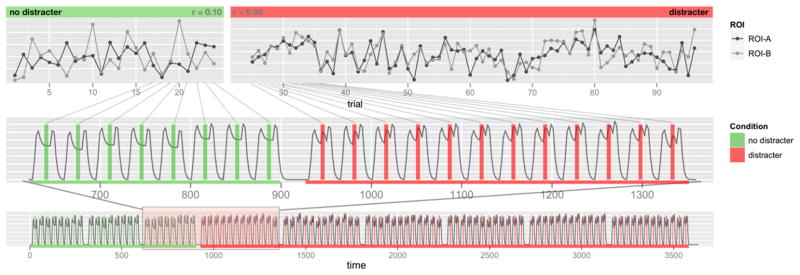
tb-fcMRI time-point selection approach. We illustrate the tb-fcMRI analysis strategy using the slow event-related design. This approach closely follows our previously published work (Anticevic et al., 2010b). The bottom panel shows the time series across the entire experiment. The initial time series marked in green indicates trials with no distraction, followed by trials with distraction marked in red. The middle panel focuses on a sub-set of the trials to more closely illustrate the time-point selection strategy. The vertical bars mark the corresponding ‘middle’ portion of each trial where activity is sampled by averaging across two frames following the onset of distraction. The top panel illustrates how these frames are concatenated into a time-series representing distracter-related signal across all trials. All tb-fcMRI analyses are performed on these extracted time courses, which reflect variation in peak response – as indicated by obtained correlation coefficients shown in corners of each top panel. This analytic strategy largely circumvents the concern that correlations are being driven by overall task response.
As noted, this investigation focused exclusively on the right DLPFC region that was functionally defined in our prior work (Anticevic et al., 2011c): the region was active in response to distracters for controls, but not patients and this activity predicted performance for controls but not patients. That is, given this region’s possible involvement in ‘filtering’ we examined its whole-brain connectivity. Given concerns about independence of region selection, we placed a sphere at the coordinates identified in our prior investigation (x=42;y=27; z=29) instead of using identical voxels. We obtained the DLPFC tb-fcMRI maps by extracting average values across all DLPFC voxels for each subject and computing their correlation with each voxel in the brain. We ascertained group-level statistical significance by converting individual correlation maps to Fisher-Z maps and computing voxel-wise 2nd-level statistics (analysis details are outlined in the results section). Given no a priori predictions with regard to connectivity differences as a function of performance or speed we combined correct and incorrect trials to maximize power. All reported foci met whole-brain type-I-error family-wise error correction as determined via AlphaSim [p<0.01 and >37 contiguously active voxels, estimated 6 mm smoothness and 5000 simulations within a whole-brain mask] (Cox, 1996).
3. Results
We hypothesized two major patterns of results: i) ‘over-connectivity’ between DLPFC and ‘bottom up’ regions; and ii) ‘under-connectivity’ between DLPFC and cortical areas typically involved in cognitive control. To test these hypothesized differences we computed a Diagnosis (patients vs. controls) ×Distraction (WM trials with distraction vs. no distraction) interaction using voxel-wise Fisher’s Z values as the dependent variable. We report regions showing a significant Diagnosis×Distraction interaction (i.e. differential connectivity patterns across groups as a function of task condition). All reported t-tests are two-tailed.
3.1. Group connectivity differences in subcortical regions
The ANOVA results revealed 3 subcortical regions exhibiting a significant interaction. One region was localized around the left paralimbic cortex proximal to the amygdaloid complex (Fig. 3a). The other two areas were localized around the bilateral medio-dorsal thalamus (Fig. 3b). For the extended amygdala region, controls showed more negative tb-fcMRI with DLPFC in response to distraction [t(23)=2.33, p<.03] but patients failed to show this connectivity modulation [t(23)=.57, p=57, NS]. For the thalamic region, the pattern was consistent across both hemispheres; therefore, we collapsed results bilaterally. The source of the interaction was driven by ‘over-connectivity’ between thalamic regions and DLPFC for patients, specifically in response to distraction [t(23)=2.23, p<.04]. Conversely, control subjects exhibited no modulation of DLPFC-thalamic connectivity as a function of distraction [t(23)=0.38, p=.7, NS]. Furthermore, patients showed significantly greater DLPFC-thalamus connectivity than controls in the distraction condition [t(52)=4.5, p<.001], but not in the no-distraction condition [t(52)=1.38, p=.17, NS]. These findings reveal that patients exhibit DLPFC-thalamus ‘over-connectivity, but fail to show a task-induced change in connectivity between DLPFC and the region proximal to the amygdala.
Fig. 3.
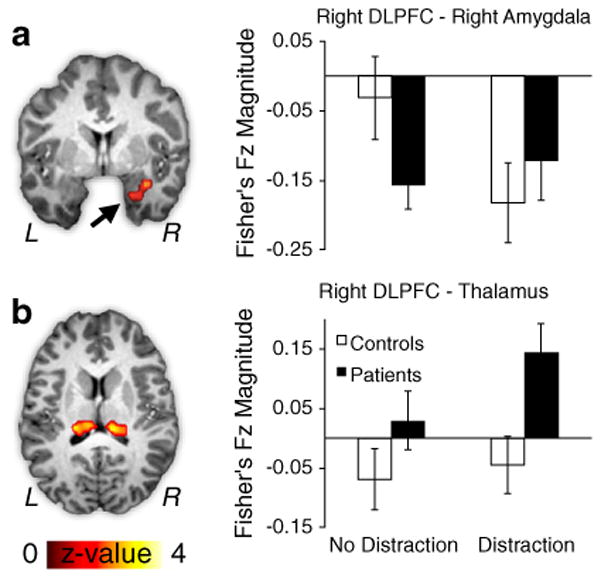
Subcortical regions showing significant tb-fcMRI group differences with right DLPFC following WM interference. All regions exhibited a significant Diagnosis (patients vs. controls) x Distraction (no distraction vs. distraction) interaction at the whole-brain level. (a) tb-fcMRI is shown between right DLPFC and right limbic cortex, proximal to the right amygdala (x=29, y=−3, z=−20). Controls (white bars) showed more negative coupling between right DLPFC and right amygdala, whereas patients (black bars) failed to exhibit such modulation. (b) tb-fcMRI is shown between right DLPFC and bilateral dorsal thalamic region (right: x=15, y=−26, z=15; left: x=12, y=−24, z=14). Patients (black bars) exhibited increases in right DLPFC-thalamic connectivity specifically following WM interference, whereas for controls this connectivity was attenuated (white bars). Error bars reflect +/− 1 standard error of the mean. For a vertical scatterplot showing the full distribution of all participants please see Supplement.
3.2. Group connectivity differences in cortical regions
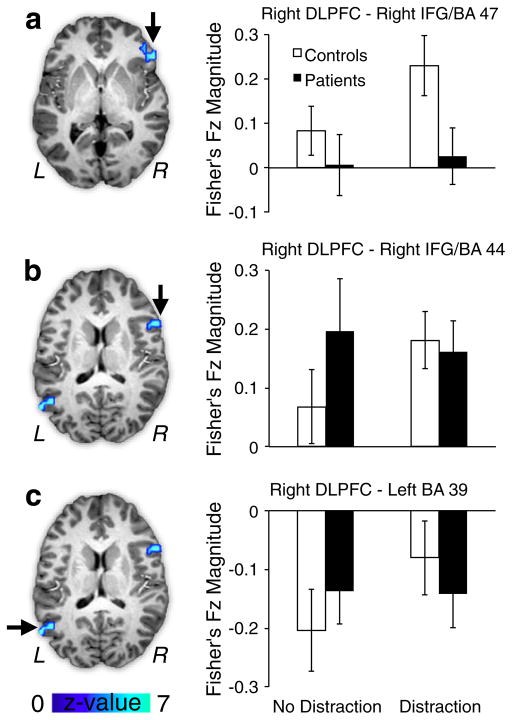
The ANOVA analysis identified three additional cortical regions exhibiting a significant Diagnosis x Distraction interaction (Fig. 4). Two of the foci were localized around right prefrontal cortex (inferior frontal gyrus/Brodmann’s Area 47 — Fig. 4a; Inferior-middle frontal gyrus/Brodmann’s Area 44 — Fig. 4b), whereas another region was centered on left parietal lobe (Brodmann’s Area 39 — Fig. 4c). The source of the interaction for the prefrontal regions was similar: for both foci control subjects exhibited a significant connectivity increase with DLPFC in response to distraction [IFG−t(23)=2.97, p<.007; MFG−t(23)=1.86, p<.08, trend], whereas patients failed to show such a modulation [IFG−t(23)=0.34, p=.73, NS; MFG−t(23)=.76, p=.46, NS]. The pattern of results for the parietal region was somewhat different: control subjects showed a significant reduction of a negative correlation with DLPFC in response to distraction [t(23)=2.18, p<.04], That is, in the absence of distraction, the correlation between DLPFC-parietal cortex activity was negative, but this relationship became less negative in response to distraction. In contrast, patients failed to show a modulation of this negative correlation [t(23)=0.18, p= 0.85, NS]. Taken together, present results indicate that patients do not modulate DLPFC cortical connectivity following WM interference, whereas control subjects show a clear task-dependent change between DLPFC and prefrontal/parietal cortical regions.
Fig. 4.
Cortical regions showing significant tb-fcMRI group differences with right DLPFC following WM interference. As in Fig. 1, all regions exhibited a significant Diagnosis (patients vs. controls)×Distraction (no distraction vs. distraction) interaction at the whole-brain level. (a) tb-fcMRI is shown between right DLPFC and right inferior frontal gyrus (IFG), corresponding to Brodmann’s area 47 (x=52, y=28, z=0). Patients (black bars) failed to show an increase in right DLPFC-IFG connectivity, whereas controls (white bars) showed a clear increase in coupling following WM interference. (b) tb-fcMRI is shown between right DLPFC and a more inferior portion of the right IFG, proximal to Brodmann’s area 44 (x=53, y=11, z=15). Again, controls showed an increase in positive coupling following interference, whereas patients failed to exhibit this modulation. (c) tb-fcMRI is shown between right DLPFC and left parietal cortex proximal to Brodmann’s area 39 (x=−53, y=−60, z=18). Controls showed a reduction of negative coupling in response to distraction, but patients failed to show a modulation of DLPFC-parietal coupling. Error bars reflect +/− 1 standard error of the mean. For a vertical scatterplot showing the full distribution of all participants please see Supplement.
4. Discussion
We directly examined deficits in functional connectivity of a key control region – DLPFC – previously associated with WM deficits in schizophrenia. We demonstrated that, when presented with distraction while maintaining information in WM, patients exhibited a failure to modulate DLPFC-amygdala connectivity and showed greater connectivity between the DLPFC and thalamus as compared to controls. These results are consistent with the hypothesis that in schizophrenia a distributed DLPFC network involved in both “bottom up” and “top down” processes may contribute to the increased interference susceptibility during WM.
4.1. Aberrant DLPFC connectivity with cortical vs. subcortical circuits
We observed a clear difference in the pattern of DLPFC connectivity impairments in schizophrenia best described as DLPFC ‘over-connectivity’ with subcortical regions, but ‘under-connectivity’ with prefrontal and parietal regions. This suggests that during WM interference, patients may exhibit ‘dysconnectivity’ between DLPFC and other control regions in pre-frontal cortex, but also aberrant communication with limbic circuits — both connectivity abnormalities demonstrated in other task contexts in schizophrenia (Fornito et al., 2011).
A particularly compelling finding was ‘over-connectivity’ in patients, specifically following distraction, between DLPFC and medio-dorsal thalamus. This finding is in accord with a body of preclinical and post-mortem evidence suggesting breakdowns in DLFPC-thalamic gating in psychosis (Cronenwett and Csernansky, 2010). Furthermore, this result is in line with the predictions of the thalamic filter model proposed by Carlsson and colleagues (Carlsson and Carlsson, 1990a, b; Carlsson et al., 2001). The model postulates that in schizophrenia there exists a breakdown in cortical glutamatergic control of the striato-thalamic filtering of sensory information. When functioning properly this mechanism is postulated to protect the cortex from excessive thalamic sensory drive, fostering a selection of purposeful behavioral programs (e.g. WM), a process compromised in schizophrenia (Carlsson and Carlsson, 1990b). Present findings are in support of excessive cortical-thalamic drive and highlight that such abnormalities in schizophrenia may be particularly manifest when interference protection is required.
One aspect of present results that complicates this interpretation is that controls did not exhibit a significant reduction of DLPFC-thalamic connectivity in response to distraction. It is possible that the actual lack of DLPC-thalamic connectivity modulation in controls is indicative of ‘successful’ gating. Another possibility is that the amount of distraction in the present study was not robust enough to modulate DLPFC-thalamic connectivity in healthy controls, but affected patients. If so, prospective studies should examine whether there is a parametric change of DLPFC-thalamus connectivity as a function of stronger WM interference.
We observed clear reductions in DLPFC-amygdala tb-fcMRI in controls during distraction; but patients failed to exhibit this downward modulation of DPFC-amygdala tb-fcMRI. This finding is consistent with the hypothesis suggesting disruptions in fronto-limbic circuits in psychosis (Williams et al., 2007; Hoptman et al., 2009; Dichter et al., 2010). Perhaps, in the face of interference of cognitive operations, healthy individuals down-regulate amygdala circuitry, which may minimize the degree to which salient information is able to interfere with the contents of WM (Pessoa, 2008). This possibility is consistent with disruptive effects of amygdala activation on PFC activity during WM (Anticevic et al., 2010a; Yun et al., 2010) and this lack of modulation may contribute to persisting WM deficits in schizophrenia. That is, there may be a breakdown in such prefrontally-mediated task-dependent modulation of extended amygdala signals in schizophrenia — a deficit that may contribute to aberrant attributions of salience (Kapur, 2003).
A less intuitive pattern of findings was observed for DLPFC-parietal connectivity, whereby patients exhibited more negative coupling between DLPFC and the parietal node irrespective of task condition, but controls showed less negative connectivity during distraction (see Fig. 4 and Supplement). One possibility is that this pattern could reflect a compensatory mechanism on part of the patients by suppressing signals in regions that control participants may not need to regulate during distraction. More work is needed to fully elucidate this pattern. For instance, to more fully characterize the functional significance of detected changes future work may want to examine intra-regional connectivity (e.g., regional homogeneity) in the nodes of interest as well as repeat the connectivity analysis by seeding identified regions.
4.2. Role of DLPFC in interference resolution deficits in schizophrenia
Deficits in DLPFC function in schizophrenia have typically been associated with abnormalities in information maintenance and/or manipulation during WM (Glahn et al., 2005). However, these findings suggest that DLPFC computations may be involved in protection of WM stores from external interference via modulation of distributed neural circuits. Indeed, findings from basic cognitive neuroscience (Sakai et al., 2002) and biophysically realistic computational models (Fredrik et al., 2009) raise the possibility that aspects of lateral PFC may operate in a broader way to protect WM from outside interference and that information maintenance may rely on regions other than DLPFC.
Our results focus exclusively on interference effects during WM maintenance. However, behavioral results from a recent study by Hahn and colleagues (Hahn et al., 2010) show that interference problems in schizophrenia – in the context of WM – may operate across stages. They showed that, in contrast to controls, patients were unable to override pre-potent bottom-up visual distraction during WM encoding and bias their attention away from such interference. In fact, individuals with schizophrenia more robustly encoded items that co-occurred with salient distracters, whereas controls successfully filtered such distraction (Hahn et al., 2010). It remains unclear whether DLPFC operates by protecting WM during encoding and whether such abnormalities would resemble present observations.
We examined functional connectivity differences in response to interference — that is, once distraction appeared. However, interference resolution during cognitive operations may depend on a combination of ‘preparatory’ and ‘reactive’ control signals. It remains unclear whether distinct abnormalities in preparatory and reactive control exist in schizophrenia – possibly reliant on unique neural circuits – that interactively compromise WM in this illness (Fletcher, 2011). Consistent with the role of prefrontal cortex in both processes, McNab & Klingberg have demonstrated the importance of prefrontal activation in ‘gating’ subcortical signals during WM prior to the onset of distraction in healthy adults (McNab and Klingberg, 2008). It will be important for future task-based and connectivity studies to ascertain whether lateral prefrontal cortex exhibits deficits across both preparatory and reactive control in schizophrenia.
4.3. Limitations
Patients in this sample were medicated. Thus, it cannot be ruled out that medication effects may be driving some observed effects, especially considering that D2 receptors in the striatum gate information flow through the thalamus (Carlsson et al., 2001). To examine this possibility we converted current medication levels to neuroleptic equivalents, which however did not explain observed effects. Nevertheless, due to long-term effects of various medications received over the course of the illness, it will be important to replicate these findings in un-medicated, at-risk or 1st degree relatives of patients with schizophrenia. Another limitation is that we did not find any relationships with individual differences in symptom severity (see Supplement). It may be possible that reported results constitute a trait or a marker for disease risk, but do not necessarily scale with reported symptoms. However, because our sample size was not powered for subtle individual difference tests, we cannot fully rule out statistical power issues. Additionally, history of substance abuse in the patient group may have impacted present findings (while likely limited by requiring sobriety for past 6 months). Thus, given the heterogeneity of the patient group future studies with 1st episode patients and more homogenous samples will be necessary to replicate the specificity of present findings to schizophrenia diagnosis. We took great care to match the groups on educational achievement. Nevertheless, cognitive deficits are prevalent in schizophrenia and often confounded with this diagnosis (Reichenberg and Harvey, 2007), therefore, it is also critical to verify present findings with samples that are more carefully matched on IQ profiles. Notably, in the present experimental task we ensured between-group performance matching during distracter-free trials (for reasons described previously (Anticevic et al., 2011c)). Therefore, despite differences in cognitive ability, present results cannot be attributed purely to performance confounds (see Supplement for detailed covariate analyses). Lastly, in our tb-fcMRI approach is reliant on the number of time points across which the correlation is estimated and therefore is a limitation that should be considered as it can impact the strength of the tb-fcMRI estimate.
4.4. Conclusion
Present findings demonstrate that schizophrenia is associated with DLPFC connectivity abnormalities during WM maintenance, specifically when faced with distraction. These differences were evident in cortical ‘control’ regions and subcortical ‘bottom-up’ regions. Taken together, present results offer evidence consistent with the hypothesis that a distributed network may be contributing to WM filtering deficits in schizophrenia, extending beyond lateral PFC.
Supplementary Material
Acknowledgments
Role of funding source
This research was supported by the McDonnell Center for Systems Neuroscience at Washington University in St. Louis. The funding source had no further role in the current study with regard to data collection, data analysis and interpretation of findings or in manuscript preparation and the submission decision.
We thank John Murray for useful discussions regarding present results. We thank the Washington University CONTE Center staff for assistance with recruitment and diagnostics. We also thank two anonymous Reviewers for their constructing and insightful feedback.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.schres.2012.07.007.
Footnotes
Contributors
AA & DMB conceptualized and designed the study. AA collected the data. AA and GR performed data analyses. AA examined and interpreted the results in consultation with DMB and JHK. AA wrote the first draft of the manuscript, which all co-authors commented on and edited.
Conflict of Interest
John H. Krystal, MD 2012 financial disclosure.
Note: the individual consultant agreements listed below are less than $10,000 per year.
Consultant
- Aisling Capital, LLC
- Astellas Pharma Global Development, Inc.
- AstraZeneca Pharmaceuticals
- Biocortech
- Brintnall & Nicolini, Inc.
- Easton Associates
- Gilead Sciences, Inc.
- GlaxoSmithKline
- Janssen Pharmaceuticals
- Lundbeck Research USA
- Medivation, Inc.
- Merz Pharmaceuticals
- MK Medical Communications
- F. Hoffmann-La Roche Ltd
- Sage Therapeutics, Inc.
- SK Holdings Co., Ltd
- Sunovion Pharmaceuticals, Inc.
- Takeda Industries
- Teva Pharmaceutical Industries, Ltd.
Scientific Advisory Board
- Abbott Laboratories
- Bristol-Myers Squibb
- CHDI Foundation, Inc.
- Eisai, Inc.
- Eli Lilly and Co.
- Forest Laboratories, Inc.
- Lohocla Research Corporation
- Mnemosyne Pharmaceuticals, Inc.
- Naurex, Inc.
- Pfizer Pharmaceuticals
- Shire Pharmaceuticals
- StratNeuro Research Program at Karolinska Institute (International Advisory Board)
Board of Directors
- Coalition for Translational Research in Alcohol and Substance Use Disorders
President
- American College of Neuropsychopharmacology
Income greater than $10,000
Editorial Board
- Editor - Biological Psychiatry
Employment
- Yale University School of Medicine
- VA CT Healthcare System
Patents and inventions
- Seibyl JP, Krystal JH, Charney DS. Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia. Patent #:5,447,948.September 5, 1995
- I am a co-inventor with Dr. Gerard Sanacora on a filed patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (PCTWO06108055A1).
- Intranasal administration of ketamine to treat depression (pending)
References
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) University of Iowa, Iowa City; 1983a. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) University of Iowa, Iowa City; 1983b. [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Resisting Emotional Interference: Brain Regions Facilitating Working Memory Performance During Negative Distraction. Cogn Affect Behav Neurosci. 2010a;10 (2):159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. NeuroImage. 2010b;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Corlett PR, Barch DM. Negative and Non-emotional Interference with Visual Working Memory in Schizophrenia. Biol Psychiatry. 2011a;70 (12):1159–1168. doi: 10.1016/j.biopsych.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Emotion Effects on Attention, Amygdala Activation, and Functional Connectivity in Schizophrenia. Schizophr Bull. 2011b doi: 10.1093/schbul/sbq168. http://dx.doi.org/10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed]
- Anticevic A, Repovs G, Barch DM. Working Memory Encoding and Maintenance Deficits in Schizophrenia: Neural Evidence for Activation and Deactivation Abnormalities. Schizophr Bull. 2011c doi: 10.1093/schbul/sbr107. http://dx.doi.org/10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed]
- Baddeley AD. The episodic buffer: A new component of working memory? Trends Cogn Sci. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ. Working memory. In: Bower G, editor. The psychology of Learning & Motivation. Academic Press; New York: 1974. pp. 47–89. [Google Scholar]
- Bazire S. Psychotropic Drug Directory. Fivepin Limited; Salisbury: 2005. [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107 (10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23 (2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia - implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990a;13 (7):272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull. 1990b;16:425–432. doi: 10.1093/schbul/16.3.425. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML. Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol. 2001;41:237–260. doi: 10.1146/annurev.pharmtox.41.1.237. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol. 1999;11:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Barch DM, Fisher-Eastep JL, Thomason ES, Hanewinkel MJ, Thompson PA, Csernansky JG. Factors mediating cognitive deficits and psychopathology among siblings of individuals with schizophrenia. Schizophr Bull. 2006;32:525–537. doi: 10.1093/schbul/sbj082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter G, Bellion C, Casp M, Belger A. Impaired Modulation of Attention and Emotion in Schizophrenia. Schizophr Bull. 2010;36 (3):595–606. doi: 10.1093/schbul/sbn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, Leung HC, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, Skudlarski P, Goldman-Rakic PS, Krystal JH. Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64 (12):1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) [Google Scholar]
- Fletcher PC. Hurry Up and Wait: Action, Distraction, and Inhibition in Schizophrenia. Biol Psychiatry. 2011;70 (12):1104–1106. doi: 10.1016/j.biopsych.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70 (1):64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102 (27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M, Zhang D, Snyder A, Raichle M. The Global Signal and Observed Anticorrelated Resting State Brain Networks. J Neurophysiol. 2009;101 (6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrik E, Klingberg T, Johansson P, McNab F, Tegnér J, Compte A. Mechanism for top-down control of working memory capacity. Proc Natl Acad Sci U S A. 2009;106 (16):6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156 (2–3):117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25 (1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67 (10):e12. [PubMed] [Google Scholar]
- Hahn B, Robinson BM, Kaiser ST, Harvey AN, Beck VM, Leonard CJ, Kappenman ES, Luck SJ, Gold JM. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiatry. 2010;68 (7):603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, D’Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AMC, Castellanos FX, Javitt DC, Milham MP. Amygdalofrontal Functional Disconnectivity and Aggression in Schizophrenia. Schizophr Bull. 2009;36 (5):1020–1028. doi: 10.1093/schbul/sbp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MR, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, Pearlson GD. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry. 2006;60 (1):11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, Moore KS. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160 (1):13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114 (4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11 (1):103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44 (3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Ojemann J, Akbudak E, Snyder A, McKinstry R, Raichle M, Conturo T. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. NeuroImage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9 (2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11 (11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR. Delay-period activity in the prefrontal cortex: one function is sensory gating. J Cogn Neurosci. 2005;17 (11):1679–1690. doi: 10.1162/089892905774589208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133 (5):833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage. 2004:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nat Neurosci. 2002;5:479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Schlösser RGM, Koch K, Wagner G, Nenadic I, Roebel M, Schachtzabel C, Axer M, Schultz C, Reichenbach JR, Sauer H. Inefficient executive cognitive control in schizophrenia is preceded by altered functional activation during information encoding: an fMRI study. Neuropsychologia. 2008;46 (1):336–347. doi: 10.1016/j.neuropsychologia.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Thompson-Schill SL, Jonides J, Marshuetz C, Smith EE, D’Esposito M, Kan IP, Knight RT, Swick D. Effects of frontal lobe damage on interference effects in working memory. Cogn Affect Behav Neurosci. 2002:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33 (1):69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. The Psychological Corporation; San Antonio: 1997. [Google Scholar]
- Williams LM, Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, Gordon E, Harris AW. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007;155 (1):29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64 (6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yun RJ, Krystal JH, Mathalon DH. Working Memory Overload: Fronto-Limbic Interactions and Effects on Subsequent Working Memory Function. Brain Imaging Behav. 2010;4 (1):96–108. doi: 10.1007/s11682-010-9089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.