Abstract
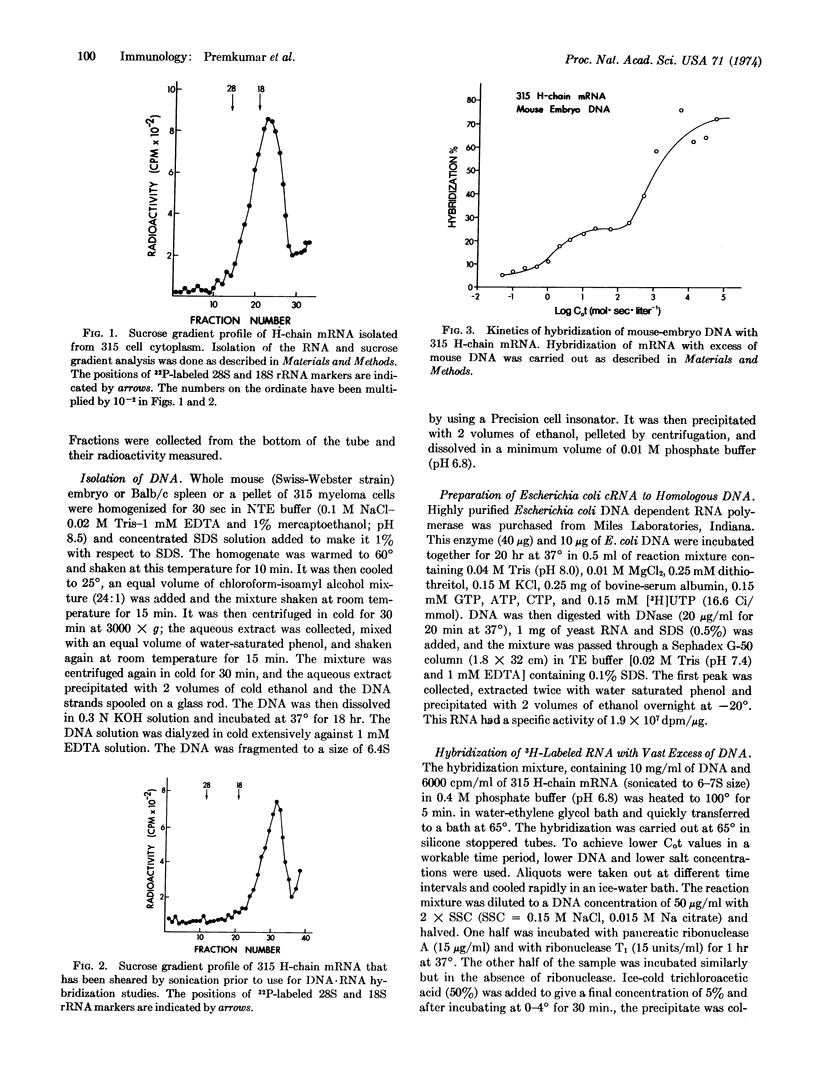
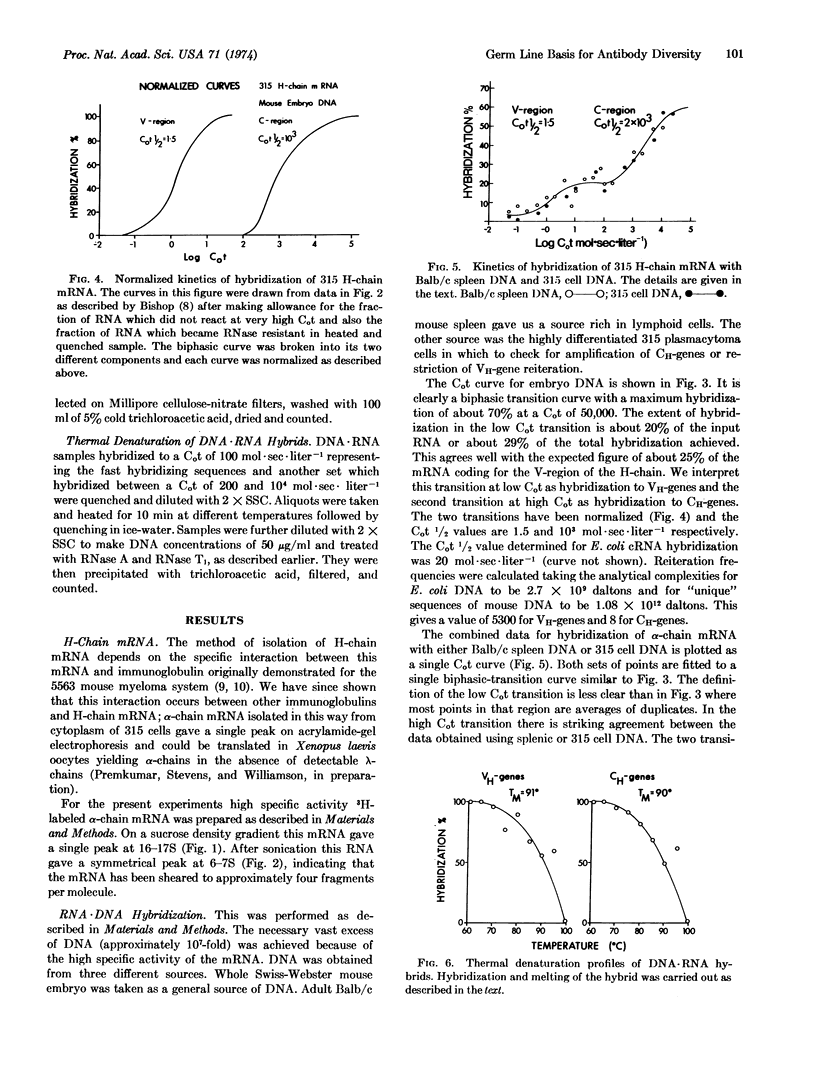
The reiteration frequency for mouse immunoglobulin VH-genes and CH-genes has been directly estimated by hybridization of purified MOPC 315 α-chain mRNA with a vast excess of mouse DNA. A biphasic Cot curve resulted. The low Cot transition (Cot1/2 about 1.5) was interpreted as hybridization to VH-genes and the high Cot transition (Cot1/2 about 103) as hybridization to CH-genes. These values correspond to about 5000 VH-genes and less than 8 CH-genes. This germ-line content of VH-genes is sufficient to account for antibody diversity given a comparable set of VL-genes. Minimal-gene models are invalidated and there is no need to invoke somatic generators of diversity.
Keywords: mouse myeloma, H-chain mRNA, variable region, constant region
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birshtein B. K., Cebra J. J. Primary structure of C-1-a 1 --a cyanogen bromide fragment of heavy chain from inbred guinea pig immunoglobulin G(2), which contains a markedly variable segment. Biochemistry. 1971 Dec 21;10(26):4930–4937. doi: 10.1021/bi00802a015. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Wasserman R. L., Kehoe J. M. Phylogenetically associated residues within the VH3 subgroup of several mammalian species. Evidence for a "pauci-gene" basis for antibody diversity. J Exp Med. 1973 Aug 1;138(2):410–427. doi: 10.1084/jem.138.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delovitch T. L., Baglioni C. Estimation of light-chain gene reiteration of mouse immunoglobulin by DNA-RNA hybridization. Proc Natl Acad Sci U S A. 1973 Jan;70(1):173–178. doi: 10.1073/pnas.70.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer W. J., Bennett J. C. The molecular basis of antibody formation: a paradox. Proc Natl Acad Sci U S A. 1965 Sep;54(3):864–869. doi: 10.1073/pnas.54.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Idiotypic identity of antibodies to streptococcal carbohydrate in inbred mice. Eur J Immunol. 1972 Aug;2(4):301–307. doi: 10.1002/eji.1830020402. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Kindt T. J. The inheritance of individual antigenic specificities of rabbit antibodies to streptococcal carbohydrates. J Exp Med. 1971 Aug 1;134(2):532–552. doi: 10.1084/jem.134.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally J. A., Edelman G. M. Somatic translocation of antibody genes. Nature. 1970 Jul 25;227(5256):341–348. doi: 10.1038/227341a0. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Kinetics of labeling and isolation of a labeled peptide. Biochemistry. 1970 Mar 3;9(5):1267–1278. doi: 10.1021/bi00807a031. [DOI] [PubMed] [Google Scholar]
- Hood L., Talmage D. W. Mechanism of antibody diversity: germ line basis for variability. Science. 1970 Apr 17;168(3929):325–334. doi: 10.1126/science.168.3929.325. [DOI] [PubMed] [Google Scholar]
- Pawlak L. L., Nisonoff A. Distribution of a cross-reactive idiotypic specificity in inbred strains of mice. J Exp Med. 1973 Apr 1;137(4):855–869. doi: 10.1084/jem.137.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz G., Beato M., Feigelson P. Isolation of eukaryotic messenger RNA on cellulose and its translation in vitro. Biochem Biophys Res Commun. 1972 Nov 1;49(3):680–689. doi: 10.1016/0006-291x(72)90465-2. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Isolation of messenger RNA coding for mouse heavy-chain immunoglobulin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1127–1131. doi: 10.1073/pnas.70.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Specific IgG mRNA molecules from myeloma cells in heterogeneous nuclear and cytoplasmic RNA containing poly-A. Nature. 1972 Sep 15;239(5368):143–146. doi: 10.1038/239143a0. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Translational control of immunoglobulin synthesis. II. Cell-free interaction of myeloma immunoglobulin with mRNA. J Mol Biol. 1973 Aug 15;78(3):517–525. doi: 10.1016/0022-2836(73)90472-5. [DOI] [PubMed] [Google Scholar]
- Williamson A. R. Extent and control of antibody diversity. Biochem J. 1972 Nov;130(2):325–333. doi: 10.1042/bj1300325. [DOI] [PMC free article] [PubMed] [Google Scholar]



