Abstract
The so-called “burst abdomen” has been described for many years and is a well-known clinical condition, whereas the concept of the “open abdomen” is relatively new. In clinical practice, both nosological entities are characterized by a complex spectrum of symptoms apparently disconnected, which in many cases poses a great challenge for surgical repair. In order to assess the management of these disorders in a more comprehensive and integral fashion, the concept of “acute postoperative open abdominal wall” (acute POAW) is presented, which in turn can be divided into “intentional” or planned acute POAW and “unintentional” or unplanned POAW. The understanding of the acute POAW as a single clinical process not only allows a better optimization of the therapeutic approach in the surgical repair of abdominal wall-related disorders, but also the stratification and collection of data in different patient subsets, favoring a better knowledge of the wide spectrum of conditions involved in the surgical reconstruction of the abdominal wall.
Keywords: Burst abdomen, Open abdomen, Evisceration, Abdominal wall, Mesh, Negative pressure wound therapy, Incisional hernia, Enteroatmospheric fistula
Core tip: Burst abdomen and open abdomen are clinical conditions apparently disconnected. In order to assess the management of these disorders in a more comprehensive and integral fashion, the concept of “acute postoperative open abdominal wall” (acute POAW) is presented. The understanding of the acute POAW as a single clinical process allows stratification and collection of data in different patient subsets, favoring a better knowledge of conditions involved in the surgical reconstruction of the abdominal wall.
INTRODUCTION
Excluding the defects of the abdominal wall secondary to trauma, tumors or necrotizing infections, the ‘‘acute postoperative open abdominal wall’’ (acute POAW) embracing evisceration and the open abdomen, appears to include a number of heterogeneous and unrelated processes[1]. Different descriptors found in the PubMed database[2] may be applicable to the concept of acute POAW, such as ‘‘burst abdomen’’, “postoperative burst abdomen”, “abdominal evisceration”, “bowel evisceration”, “abdominal wall dehiscence”, “abdominal fascial dehiscence”, “acute abdominal wound failure”, “open abdomen”, “abdominal wound dehiscence”, “abdominal wall rupture” and “disruption of abdominal wall wounds”. In this previous context, definition of what constitutes an acute POAW becomes a maze.
We here propose that acute POAW is a single nosological entity formed by patients with different interrelated categories of treatment approaches. Therefore, the purpose of this article is to present the conceptual frame for an analysis of the acute POAW and their subgroup categories of treatment. For clarity purposes, the information is divided into definition of acute POAW, description and treatment of intentional (planned) and unintentional (unplanned) acute POAW, followed by some concluding remarks.
DEFINITION OF ACUTE POAW
Acute POAW consists of the separation of the cutaneous, muscular and aponeurotic layers of the abdominal wall that occurs immediately or within the first hours or days after laparotomy. It may be considered a unique nosological clinical entity resulting from intentional or unintentional surgical-related actions and composed by different interrelated clinico-therapeutical scenarios.
INTENTIONAL (PLANNED) ACUTE POAW
Intentional acute POAW is the result of a deliberate therapeutic procedure, the so-called “open abdomen”[3,4]. This entity was described for the first time in the context of patients with intra-abdominal infection due to pancreatitis or peritonitis[5,6], but the indications for the use of the open abdomen technique have expanded to patients without intra-abdominal infection[7]. Nowadays the main indications are (1) damage control for life-threatening intra-abdominal bleeding; (2) management of severe intra-abdominal sepsis; and (3) prevention or treatment of intra-abdominal hypertension.
Once the therapeutic objective has been achieved, closure of the musculofascial layers should be performed[3,4,8-10]. However, closure of the open abdomen depends on the method used for temporary abdominal wall closure[3,8,9], the capacity of tissues for healing without tension, and whether or not enteroatmospheric fistulas are present.
The ideal temporary abdominal wall closure should protect the abdominal contents, prevent evisceration, allow removal of infected or toxic fluid from the peritoneal cavity, avoid damage to the musculofascial tissue, preserve the abdominal wall domain, facilitate reoperation for definitive closure and, very importantly, prevent the formation of enterocutaneous fistulas[11]. Different methods for temporary abdominal closure have been used, including among others: skin approximation with towel clips or running suture, application of a plastic silo (the Bogota bag), absorbable synthetic meshes [Safil®Mesh (BBraun, Rubí, Barcelona, Spain); BIO-A Tissue Reinforcement® mesh (Gore and Associates, Flagstaff, AZ)], non-absorbable synthetic meshes (polypropylene, e-PTFE), dynamic methods [ABRA® (Canica Design Inc, Almonte, Ontario, Canada); Wittmann Patch® (Starsurgical, Burlington, WI)], biological implants or negative pressure dressing systems [RENASYS AB® Abdominal Kit (Smith and Nephew, Hull, United Kingdom); ABThera® (KCI International, San Antonio, TX)][12]. The capacity of tissues for healing without tension depends on wound-related factors and the patient’s general condition[11]. Independently of the technique used for temporary abdominal wall closure, there is a limited window of 2-3 wk to assess early vs delayed closure[8-11,13,14]. Early definitive closure (final closure of the abdominal defect within the window of 2-3 wk) is based on the resolution of interstitial edema and the evidence of non-adherence between the bowel loops and the abdominal wall. In contrast, when the abdominal content adheres to the undersurface of the anterior abdominal wall (“frozen abdomen” generally beyond 2-3 wk), delayed closure (“planned” incisional hernia repair) is the only realistic alternative in the operative management of the open abdomen. There are mixed situations between non-adherent loops and abdominal wall and the “frozen abdomen”.
The development of enteroatmospheric fistulas is the most serious and challenging local complication[15], with an overall incidence still reported between 5% and 75%. Mortality of patients with fistula can be still high, up to 42% according to a review of different studies[15].
Treatment options
According to the aforementioned features, we have four different subgroup categories of treatment options: (1) Patients within the 2-3 wk time window with non-adherent bowel loops/abdominal wall and without intestinal fistula are candidates for definitive fascia-to-fascia closure using a continuous slowly absorbable monofilament suture and following the 4:1 suture length (SL): wound length (WL) ratio[16,17]. Also, autologous tissue reconstruction procedures (component separation technique, anterior rectus sheath flaps, oblique muscles) to improve closure or to further reduce tension have been reported[13,18-20]. There are no data in the literature on the usefulness of synthetic (absorbable or non-absorbable) meshes or biological implants to reinforce the repair, which mostly relies on the surgeon’s experience and decision and the risk factors present in each individual patient; (2) Patients within the 2-3 wk time window with partially non-adherent bowel loops/abdominal wall and without enteroatmospheric fistula are candidates for a definitive early progressive abdominal wall closure, which will depend on the progressive improvement of the patient’s general condition and the interstitial edema. In these cases, combinations of non-absorbable synthetic meshes and negative pressure wound therapy (NPWT) are generally indicated. NPWT and non-absorbable synthetic mesh traction (pleating or serial excision of the mesh as the fascial edges are re-approximated) have been reported to be a practical wound closure system for the treatment of the open abdomen[21-25]. In addition, several types of extracellular matrix-derived biological implants have been used[26,27], although they are not recommended to bridge a fascial defect, and the long-term durability and functional outcome of biological implants is still unknown[28]. Other techniques for progressive closure of the abdominal wall, in combination or not with NPWT, include dynamic wound closure systems based on continuous dynamic tension to achieve re-approximation of the fascial edges of the abdominal wall[29,30] or the use of patches of synthetic material as a temporary, gradual means for abdominal closure[31]; (3) Patients beyond the 2-3 wk window without progress towards closure or improvement of general condition and interstitial edema (“frozen abdomen”) and without bowel fistulization. In these cases, the treatment options include skin cover over the defect or allow wound granulation (absorbable synthetic mesh implant, NPWT) and thereafter cover with skin grafts and subsequent definitive delayed closure (after 6-12 mo) in the context of a “planned” incisional hernia repair[32-37]; and (4) Patients with enteroatmospheric fistula. In these cases, the constant leak of enteric contents on the open abdomen aggravates the inflammation and encourages the formation of new fistulas. The control is extremely difficult[3]. Management includes systemic treatment (nutritional support) and temporary local control to prevent spillage of the enteric contents and excoriation of the surrounding skin while planning for definitive closure of the fistula. Due to the large variability of enterocutaneous fistulas, treatment should be individualized[15,38-40].
UNINTENTIONAL (UNPLANNED) ACUTE POAW
Unintentional acute POAW or acute wound failure (also known as burst abdomen, evisceration, wound dehiscence, wound disruption and fascial dehiscence) is a postoperative complication after primary closure of an abdominal laparotomy incision[41]. The incidence of fascial dehiscence ranges between 0.5% and 3% of all laparotomies[42,43]. The morbidity is high, with prolonged hospital stay and an increase in direct costs[44-48]. The dehiscence-associated mortality rate (range 34%-44%) does not appear to be declining[49,50]. Moreover, unintentional acute POAW is associated with a high incidence of subsequent incisional hernia (40%-60%)[49,50]. Wound breakdown may be complete, affecting all layers of the abdominal wall including the skin[51,43] or incomplete when the skin remains intact. Drainage of serosanguinous fluid from the incision precedes dehiscence in up to 84% of cases[41].
Predisposing factors to the development of wound disruption include the technique of wound closure, type of incision, indication for operation (emergency operations, malignant tumors, infectious diseases), raised intra-abdominal pressure (coughing, vomiting, abdominal distension from ileus or vigorous postoperative ventilation), age > 65 years, chronic obstructive pulmonary disease (COPD), hemodynamic instability, malnutrition, diabetes, obesity, ascites, jaundice and steroid use[43]. However, wound infection due to intra-abdominal infection (20%-40% of cases)[52,53] or wound contamination (up to 52% of cases)[52] is the single most important risk factor for abdominal wound disruption[43].
Unintentional acute POAW may occur during the first 24 h after surgery[43], although it may range from 1 to more than 23 d[41,47], with an average of 7 d postoperatively[41]. The preferred treatment of unplanned acute POAW regarding definitive early or delayed closure[43-45,47,48,51-53] should be established according to the possibility of early reclosure without tension during the window period of 2-3 wk (as in planned acute POAW), the identification and proper treatment of intra-abdominal infection including intra-abdominal abscesses (appropriate antibiotic treatment and drainage preferably by the percutaneous route) and the presence or absence of enterocutaneous fistulas.
Treatment options
According to the aforementioned features, we have different subgroup categories of treatment which are also closely interrelated with the subgroup categories of intentional acute POAW. (1) Patients with unintentional acute POAW with complete wound dehiscence shared the same characteristics and should be managed as patients with intentional acute POAW; (2) Patients with incomplete unintentional acute POAW with non-adherent bowel loops/abdominal wall and without fistula are candidates for fascia-to-fascia closure using a continuous slowly absorbable monofilament suture and following the 4:1 SL:WL ratio[16,17,41-48,51-53]. Placement of retention sutures is controversial and negative side-effects of the retention closure technique have been reported[41,54-58]. Development of recurrence of unintentional acute POAW has been described with a 5% incidence and development in long term follow-up of incisional hernia in 40%-60% of the cases[49,50]. For this reason, reinforcement with a synthetic mesh may be useful, especially in the absence of intra-abdominal infection, although mesh closure has also been recommended in clean-contaminated/contaminated wounds[59-63]. Use of absorbable mesh is discouraged by the high incidence of incisional hernias in the long-term[64]. In contaminated/dirty fields, other methods such as NPWT or dynamic wound closure systems are more appropriate[65]. The usefulness and long-term results of biological implants is uncertain and are not recommended in cases of large bacterial inocula[28]; (3) Patients within the 2-3 wk time window with incomplete unintentional acute POAW and partially non-adherent bowel loops/abdominal wall and without enteroatmospheric fistula are candidates for a definitive early progressive abdominal wall closure in the same way as planned acute POAW; (4) In patients with incomplete wound dehiscence and bowel loops adherent to the abdominal wall beyond 2-3 wk (frozen abdomen), abdominal girdles may be used before planning a delayed closure method (after 6-12 mo) in the context of an incisional hernia repair[49,50]; (5) Patients with incomplete wound failure and enterocutaneous fistula should be managed individually and the technique of closure of the wound depends on the surgeon’s discretion (as in planned acute POAW); and (6) In highly selected patients at high risk for surgery, the use of some type of compression garment (such as a girdle) is recommended and attempts of closure of the musculofascial layers are contraindicated.
Treatment strategies and relationships of acute POAW (intentional and unintentional) for the different clinical/therapeutic scenarios are summarized in Figure 1 and Tables 1 and 2. However, the description of different options do not lead to the definitive concept of “how I do it” in each scenario because of a lack of a systematic approach (low level of evidence) in the management of this serious and heterogeneous surgical problem. In addition, the use of different techniques is still dependent on the individual surgeon’s decision and experience.
Figure 1.
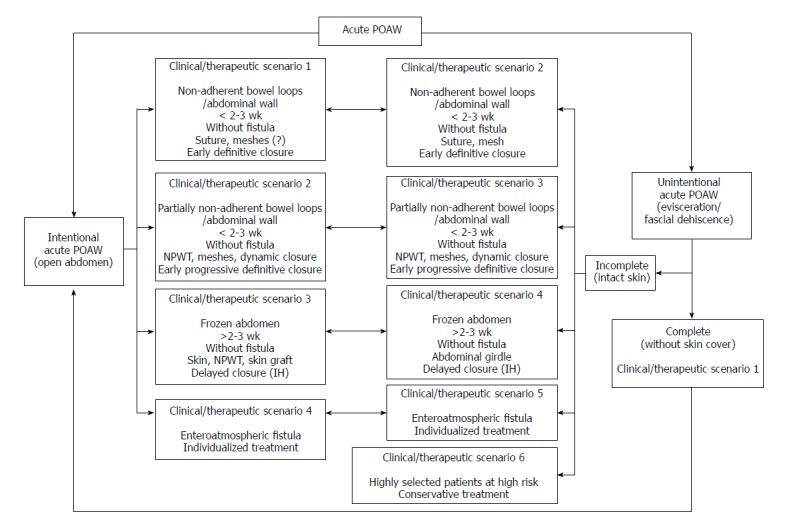
Treatment strategies of acute postoperative open abdominal wall (intentional and unintentional) for the different clinical/therapeutic scenarios. POAW: Postoperative open abdominal wall; NPWT: Negative pressure wound therapy; IH: Incisional hernia.
Table 1.
Groups and therapeutic options in complete intentional and unintentional acute postoperative open abdominal wall
| Clinical/therapeutic scenario | Intestinal fistula | Non-adherent bowel loops | Free inner abdominal wall | Window ≤ 2-3 wk | Window > 2-3 wk | Therapeutic option |
| 1 | No | Yes | Yes | Yes Early definitive closure | - | Fascia to fascia closure. Continuous slowly absorbable monofilament suture, 4:1 rule |
| 2 | No | Partially | Partially | Yes Definitive early progressive closure | - | Vacuum-assisted wound closure and mesh traction or dynamic wound closure systems |
| 3 | No | No “Frozen abdomen” | No “Frozen abdomen” | - | Yes Delayed closure | Skin cover or after granulation skin graft “Planned” incisional hernia repair |
| 4 | Yes | - | - | - | - | Individualized |
Table 2.
Groups and therapeutic options in incomplete (intact skin) unintentional acute postoperative open abdominal wall
| Clinical/therapeutic scenario | Intestinal fistula | Non-adherent bowel loops | Free inner abdominal wall | Window ≤ 2-3 wk | Window > 2-3 wk | Therapeutic option |
| 2 | No | Yes | Yes | Yes Early definitive closure | - | Fascia to fascia closure, 4:1 No retention sutures Mesh depending contamination Biologics doubtful |
| 3 | No | Partially | Partially | Yes definitive early progressive closure | - | Vacuum-assisted wound closure and mesh traction Or dynamic wound closure systems |
| 4 | No | No “Frozen abdomen” | No “Frozen abdomen” | Yes Delayed closure | Abdominal girdles Planned incisional hernia repair | |
| 5 | Yes | - | - | - | - | Individualized |
| 6 High surgical risk | - | - | - | - | - | Abdominal girdle |
CONCLUSION
We believe that in daily surgical practice, burst abdomen/evisceration/fascial dehiscence and the open abdomen are viewed as different and unrelated processes, possibly because the first is considered a complication of surgery[41,43] and the second as a procedure of surgery[1,3]. On the other hand, the abdominal wall is a complex and unique biological “organ”/mechanism contributing to the correct maintenance of the organism homeostatic balance through contention of the abdominal viscera in the right position, dynamics of respiratory activity[66], movement of the trunk[67], statics of the spine[68,69] and generation of intra-abdominal pressure for physiological functions such as cough, micturition and defecation. In this context, acute postoperative open abdominal wall as a result of unintended complications of surgery (i.e., burst abdomen/evisceration/fascial dehiscence) or intended surgical options (i.e., the open abdomen) originates from different and interrelated groups of patients with a common characteristic: impaired abdominal wall, which in turn may be grouped together under the term of acute POAW. Conceptual understanding of acute POAW as a nosological entity would allow stratification and collection of data in different patient subsets, favoring a better knowledge and optimization of the therapeutic approach of patients with this kind of abdominal wall-related disorders. In addition, it allows considering the abdominal wall system as an independent “organ” involved in other pathological and/or therapeutic conditions with a final common challenge: closure of the abdominal wall.
ACKNOWLEDGMENTS
The authors thank Marta Pulido, MD, for editing the manuscript and editorial assistance.
Footnotes
P- Reviewers: Hotta T, Nespoli A S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Wang CH
References
- 1.Leppäniemi AK. Laparostomy: why and when. Crit Care. 2010;14:216. doi: 10.1186/cc8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Available from: http: //www.ncbi.nlm.nih.gov/pubmed.
- 3.Demetriades D. Total management of the open abdomen. Int Wound J. 2012;9 Suppl 1:17–24. doi: 10.1111/j.1742-481X.2012.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friese RS. The open abdomen: definitions, management principles, and nutrition support considerations. Nutr Clin Pract. 2012;27:492–498. doi: 10.1177/0884533612446197. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg D. On leaving the peritoneal cavity open in acute generalized suppurative peritonitis. Am J Surg. 1979;137:216–220. doi: 10.1016/0002-9610(79)90148-x. [DOI] [PubMed] [Google Scholar]
- 6.Schein M, Saadia R, Decker GG. The open management of the septic abdomen. Surg Gynecol Obstet. 1986;163:587–592. [PubMed] [Google Scholar]
- 7.Rotondo MF, Schwab CW, McGonigal MD, Phillips GR, Fruchterman TM, Kauder DR, Latenser BA, Angood PA. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375–382; discussion 382-383. [PubMed] [Google Scholar]
- 8.Dubose JJ, Scalea TM, Holcomb JB, Shrestha B, Okoye O, Inaba K, Bee TK, Fabian TC, Whelan J, Ivatury RR. Open abdominal management after damage-control laparotomy for trauma: a prospective observational American Association for the Surgery of Trauma multicenter study. J Trauma Acute Care Surg. 2013;74:113–120; discussion 1120-1122. doi: 10.1097/TA.0b013e31827891ce. [DOI] [PubMed] [Google Scholar]
- 9.MacLean AA, O’Keeffe T, Augenstein J. Management strategies for the open abdomen: survey of the American Association for the Surgery of Trauma membership. Acta Chir Belg. 2008;108:212–218. doi: 10.1080/00015458.2008.11680206. [DOI] [PubMed] [Google Scholar]
- 10.Goussous N, Kim BD, Jenkins DH, Zielinski MD. Factors affecting primary fascial closure of the open abdomen in the nontrauma patient. Surgery. 2012;152:777–783; discussion 783-784. doi: 10.1016/j.surg.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Campbell A, Chang M, Fabian T, Franz M, Kaplan M, Moore F, Reed RL, Scott B, Silverman R. Management of the open abdomen: from initial operation to definitive closure. Am Surg. 2009;75:S1–22. [PubMed] [Google Scholar]
- 12.Quyn AJ, Johnston C, Hall D, Chambers A, Arapova N, Ogston S, Amin AI. The open abdomen and temporary abdominal closure systems--historical evolution and systematic review. Colorectal Dis. 2012;14:e429–e438. doi: 10.1111/j.1463-1318.2012.03045.x. [DOI] [PubMed] [Google Scholar]
- 13.Kushimoto S, Yamamoto Y, Aiboshi J, Ogawa F, Koido Y, Yoshida R, Kawai M. Usefulness of the bilateral anterior rectus abdominis sheath turnover flap method for early fascial closure in patients requiring open abdominal management. World J Surg. 2007;31:2–8; discussion 9-10. doi: 10.1007/s00268-006-0282-3. [DOI] [PubMed] [Google Scholar]
- 14.Kreis BE, de Mol van Otterloo AJ, Kreis RW. Open abdomen management: a review of its history and a proposed management algorithm. Med Sci Monit. 2013;19:524–533. doi: 10.12659/MSM.883966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker HP, Willms A, Schwab R. Small bowel fistulas and the open abdomen. Scand J Surg. 2007;96:263–271. doi: 10.1177/145749690709600402. [DOI] [PubMed] [Google Scholar]
- 16.Diener MK, Voss S, Jensen K, Büchler MW, Seiler CM. Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg. 2010;251:843–856. doi: 10.1097/SLA.0b013e3181d973e4. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins TP. Closure of the abdominal wound. J R Soc Med. 1979;72:472–473. doi: 10.1177/014107687907200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez OM, Ruas E, Dellon AL. “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg. 1990;86:519–526. doi: 10.1097/00006534-199009000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Poulakidas S, Kowal-Vern A. Component separation technique for abdominal wall reconstruction in burn patients with decompressive laparotomies. J Trauma. 2009;67:1435–1438. doi: 10.1097/TA.0b013e3181b5f346. [DOI] [PubMed] [Google Scholar]
- 20.Gutarra F, Asensio JR, Kohan G, Quarin C, Petrelli L, Quesada BM. Closure of a contained open abdomen using a bipedicled myofascial oblique rectus abdominis flap technique. J Plast Reconstr Aesthet Surg. 2009;62:1490–1496. doi: 10.1016/j.bjps.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 21.Dietz UA, Wichelmann C, Wunder C, Kauczok J, Spor L, Strauß A, Wildenauer R, Jurowich C, Germer CT. Early repair of open abdomen with a tailored two-component mesh and conditioning vacuum packing: a safe alternative to the planned giant ventral hernia. Hernia. 2012;16:451–460. doi: 10.1007/s10029-012-0919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Björck M. Management of the tense abdomen or difficult abdominal closure after operation for ruptured abdominal aortic aneurysms. Semin Vasc Surg. 2012;25:35–38. doi: 10.1053/j.semvascsurg.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Seternes A, Myhre HO, Dahl T. Early results after treatment of open abdomen after aortic surgery with mesh traction and vacuum-assisted wound closure. Eur J Vasc Endovasc Surg. 2010;40:60–64. doi: 10.1016/j.ejvs.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Petersson U, Acosta S, Björck M. Vacuum-assisted wound closure and mesh-mediated fascial traction--a novel technique for late closure of the open abdomen. World J Surg. 2007;31:2133–2137. doi: 10.1007/s00268-007-9222-0. [DOI] [PubMed] [Google Scholar]
- 25.Kleif J, Fabricius R, Bertelsen CA, Bruun J, Gögenur I. Promising results after vacuum-assisted wound closure and mesh-mediated fascial traction. Dan Med J. 2012;59:A4495. [PubMed] [Google Scholar]
- 26.de Moya MA, Dunham M, Inaba K, Bahouth H, Alam HB, Sultan B, Namias N. Long-term outcome of acellular dermal matrix when used for large traumatic open abdomen. J Trauma. 2008;65:349–353. doi: 10.1097/TA.0b013e31817fb782. [DOI] [PubMed] [Google Scholar]
- 27.Antoniou GA, Antoniou SA, Dodd DP. Use of porcine dermal collagen implant for definite early closure of the open abdomen in aortic surgery. Int Angiol. 2012;31:303–304. [PubMed] [Google Scholar]
- 28.López Cano M, Armengol Carrasco M, Quiles Pérez MT, Arbós Vía MA. Biological implants in abdominal wall hernia surgery. Cir Esp. 2013;91:217–223. doi: 10.1016/j.ciresp.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Salman AE, Yetişir F, Aksoy M, Tokaç M, Yildirim MB, Kiliç M. Use of dynamic wound closure system in conjunction with vacuum-assisted closure therapy in delayed closure of open abdomen. Hernia. 2012:Epub ahead of print. doi: 10.1007/s10029-012-1008-0. [DOI] [PubMed] [Google Scholar]
- 30.Verdam FJ, Dolmans DE, Loos MJ, Raber MH, de Wit RJ, Charbon JA, Vroemen JP. Delayed primary closure of the septic open abdomen with a dynamic closure system. World J Surg. 2011;35:2348–2355. doi: 10.1007/s00268-011-1210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keramati M, Srivastava A, Sakabu S, Rumbolo P, Smock M, Pollack J, Troop B. The Wittmann Patch s a temporary abdominal closure device after decompressive celiotomy for abdominal compartment syndrome following burn. Burns. 2008;34:493–497. doi: 10.1016/j.burns.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Mischinger HJ, Kornprat P, Werkgartner G, El Shabrawi A, Spendel S. Abdominal wall closure by incisional hernia and herniation after laparostoma. Chirurg. 2010;81:201–210. doi: 10.1007/s00104-009-1818-5. [DOI] [PubMed] [Google Scholar]
- 33.Burlew CC. The open abdomen: practical implications for the practicing surgeon. Am J Surg. 2012;204:826–835. doi: 10.1016/j.amjsurg.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Drumond DA. Skin-adipose tissue detachment for laparotomy closure: a simple and effective technique for a complex problem. Rev Col Bras Cir. 2010;37:175–183. doi: 10.1590/s0100-69912010000300004. [DOI] [PubMed] [Google Scholar]
- 35.Jernigan TW, Fabian TC, Croce MA, Moore N, Pritchard FE, Minard G, Bee TK. Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg. 2003;238:349–355; discussion 355-357. doi: 10.1097/01.sla.0000086544.42647.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekeh AP, McCarthy MC, Woods RJ, Walusimbi M, Saxe JM, Patterson LA. Delayed closure of ventral abdominal hernias after severe trauma. Am J Surg. 2006;191:391–395. doi: 10.1016/j.amjsurg.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 37.Schachtrupp A, Fackeldey V, Klinge U, Hoer J, Tittel A, Toens C, Schumpelick V. Temporary closure of the abdominal wall (laparostomy) Hernia. 2002;6:155–162. doi: 10.1007/s10029-002-0085-x. [DOI] [PubMed] [Google Scholar]
- 38.Jamshidi R, Schecter WP. Biological dressings for the management of enteric fistulas in the open abdomen: a preliminary report. Arch Surg. 2007;142:793–796. doi: 10.1001/archsurg.142.8.793. [DOI] [PubMed] [Google Scholar]
- 39.Evenson AR, Fischer JE. Current management of enterocutaneous fistula. J Gastrointest Surg. 2006;10:455–464. doi: 10.1016/j.gassur.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Draus JM, Huss SA, Harty NJ, Cheadle WG, Larson GM. Enterocutaneous fistula: are treatments improving. Surgery. 2006;140:570–576; discussion 576-578. doi: 10.1016/j.surg.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Carlson MA. Acute wound failure. Surg Clin North Am. 1997;77:607–636. doi: 10.1016/s0039-6109(05)70571-5. [DOI] [PubMed] [Google Scholar]
- 42.Webster C, Neumayer L, Smout R, Horn S, Daley J, Henderson W, Khuri S. Prognostic models of abdominal wound dehiscence after laparotomy. J Surg Res. 2003;109:130–137. doi: 10.1016/s0022-4804(02)00097-5. [DOI] [PubMed] [Google Scholar]
- 43.Eke N, Jebbin NJ. Abdominal wound dehiscence: A review. Int Surg. 2006;91:276–287. [PubMed] [Google Scholar]
- 44.Wahl W, Menke H, Schnütgen M, Junginger T. Fascia dehiscence--cause and prognosis. Chirurg. 1992;63:666–671. [PubMed] [Google Scholar]
- 45.Pavlidis TE, Galatianos IN, Papaziogas BT, Lazaridis CN, Atmatzidis KS, Makris JG, Papaziogas TB. Complete dehiscence of the abdominal wound and incriminating factors. Eur J Surg. 2001;167:351–354; discussion 355. doi: 10.1080/110241501750215221. [DOI] [PubMed] [Google Scholar]
- 46.Tera H, Aberg C. Relaparotomy. A ten-year series. Acta Chir Scand. 1975;141:637–644. [PubMed] [Google Scholar]
- 47.Graham DJ, Stevenson JT, McHenry CR. The association of intra-abdominal infection and abdominal wound dehiscence. Am Surg. 1998;64:660–665. [PubMed] [Google Scholar]
- 48.Keill RH, Keitzer WF, Nichols WK, Henzel J, DeWeese MS. Abdominal wound dehiscence. Arch Surg. 1973;106:573–577. doi: 10.1001/archsurg.1973.01350160185032. [DOI] [PubMed] [Google Scholar]
- 49.Grace RH, Cox S. Incidence of incisional hernai after dehiscence of the abdominal wound. Am J Surg. 1976;131:210–212. doi: 10.1016/0002-9610(76)90099-4. [DOI] [PubMed] [Google Scholar]
- 50.van’t RM, De Vos Van Steenwijk PJ, Bonjer HJ, Steyerberg EW, Jeekel J. Incisional hernia after repair of wound dehiscence: incidence and risk factors. Am Surg. 2004;70:281–286. [PubMed] [Google Scholar]
- 51.Schessel ES, Ger R, Ambrose G, Kim R. The management of the postoperative disrupted abdominal wall. Am J Surg. 2002;184:263–268. doi: 10.1016/s0002-9610(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 52.van Ramshorst GH, Nieuwenhuizen J, Hop WC, Arends P, Boom J, Jeekel J, Lange JF. Abdominal wound dehiscence in adults: development and validation of a risk model. World J Surg. 2010;34:20–27. doi: 10.1007/s00268-009-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papaziogas B, Koutelidakis I, Tsaousis P, Atmatzidis S, Ananiadis A, Papadakis G, Christopoulos P, Atmatzidis K, Makris I. Use of mesh for management of post-operative evisceration. Surg Chron. 2012;17:103–107. [Google Scholar]
- 54.van Geldere D. Abdominal Wound Dehiscence. In: Bendavid R, Abrahamson J, Arregui ME, Flament JB, Phillips EH, et al., editors. Abdominal Wall Hernias. Principles and Management. New York: Springer; 2001. pp. 569–576. [Google Scholar]
- 55.van Ramshorst GH, Eker HH, Harlaar JJ, Nijens KJ, Jeekel J, Lange JF. Therapeutic alternatives for burst abdomen. Surg Technol Int. 2010;19:111–119. [PubMed] [Google Scholar]
- 56.Rink AD, Goldschmidt D, Dietrich J, Nagelschmidt M, Vestweber KH. Negative side-effects of retention sutures for abdominal wound closure. A prospective randomised study. Eur J Surg. 2000;166:932–937. doi: 10.1080/110241500447083. [DOI] [PubMed] [Google Scholar]
- 57.Gislason H, Grønbech JE, Søreide O. Burst abdomen and incisional hernia after major gastrointestinal operations--comparison of three closure techniques. Eur J Surg. 1995;161:349–354. [PubMed] [Google Scholar]
- 58.Hubbard TB, Rever WB. Retention sutures in the closure of abdominal incisions. Am J Surg. 1972;124:378–380. doi: 10.1016/0002-9610(72)90045-1. [DOI] [PubMed] [Google Scholar]
- 59.Scholtes M, Kurmann A, Seiler CA, Candinas D, Beldi G. Intraperitoneal mesh implantation for fascial dehiscence and open abdomen. World J Surg. 2012;36:1557–1561. doi: 10.1007/s00268-012-1534-z. [DOI] [PubMed] [Google Scholar]
- 60.Machairas A, Liakakos T, Patapis P, Petropoulos C, Tsapralis D, Misiakos EP. Prosthetic repair of incisional hernia combined with elective bowel operation. Surgeon. 2008;6:274–277. doi: 10.1016/s1479-666x(08)80050-9. [DOI] [PubMed] [Google Scholar]
- 61.Antonopoulos IM, Nahas WC, Mazzucchi E, Piovesan AC, Birolini C, Lucon AM. Is polypropylene mesh safe and effective for repairing infected incisional hernia in renal transplant recipients. Urology. 2005;66:874–877. doi: 10.1016/j.urology.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 62.Kelly ME, Behrman SW. The safety and efficacy of prosthetic hernia repair in clean-contaminated and contaminated wounds. Am Surg. 2002;68:524–528; discussion 528-529. [PubMed] [Google Scholar]
- 63.Birolini C, Utiyama EM, Rodrigues AJ, Birolini D. Elective colonic operation and prosthetic repair of incisional hernia: does contamination contraindicate abdominal wall prosthesis use. J Am Coll Surg. 2000;191:366–372. doi: 10.1016/s1072-7515(00)00703-1. [DOI] [PubMed] [Google Scholar]
- 64.Abbott DE, Dumanian GA, Halverson AL. Management of laparotomy wound dehiscence. Am Surg. 2007;73:1224–1227. [PubMed] [Google Scholar]
- 65.van’t Riet M, de Vos van Steenwijk PJ, Bonjer HJ, Steyerberg EW, Jeekel J. Mesh repair for postoperative wound dehiscence in the presence of infection: is absorbable mesh safer than non-absorbable mesh. Hernia. 2007;11:409–413. doi: 10.1007/s10029-007-0240-5. [DOI] [PubMed] [Google Scholar]
- 66.Puckree T, Cerny F, Bishop B. Abdominal motor unit activity during respiratory and nonrespiratory tasks. J Appl Physiol (1985) 1998;84:1707–1715. doi: 10.1152/jappl.1998.84.5.1707. [DOI] [PubMed] [Google Scholar]
- 67.Myriknas SE, Beith ID, Harrison PJ. Stretch reflexes in the rectus abdominis muscle in man. Exp Physiol. 2000;85:445–450. [PubMed] [Google Scholar]
- 68.Gracovetsky S, Farfan H, Helleur C. The abdominal mechanism. Spine (Phila Pa 1976) 1985;10:317–324. doi: 10.1097/00007632-198505000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Lam KS, Mehdian H. The importance of an intact abdominal musculature mechanism in maintaining spinal sagittal balance. Case illustration in prune-belly syndrome. Spine (Phila Pa 1976) 1999;24:719–722. doi: 10.1097/00007632-199904010-00022. [DOI] [PubMed] [Google Scholar]


