Abstract
A relatively small number of signaling pathways govern the early patterning processes of metazoan development. The architectural changes over time to these signaling pathways offer unique insights into their evolution. In the case of Hedgehog (Hh) signaling, two very divergent mechanisms of pathway transduction have evolved. In vertebrates, signaling relies on trafficking of Hh pathway components to nonmotile specialized primary cilia. In contrast, protostomes do not use cilia of any kind for Hh signal transduction. How these divergent lineages adapted such dramatically different ways of activating the signaling pathway is an unanswered question. Here, we present evidence that in the sea urchin, a basal deuterostome, motile cilia are required for embryonic Hh signal transduction, and the Hh receptor Smoothened (Smo) localizes to cilia during active Hh signaling. This is the first evidence that Hh signaling requires motile cilia and the first case of an organism requiring cilia outside of the vertebrate lineage.
Keywords: cell biology, evolution and development, cell signaling
The field of Hh signaling was revolutionized in 2003 with the discovery that effective signal transduction relies on the presence of primary cilia in vertebrates (Huangfu et al. 2003). This differs sharply from the mode of transduction in protostomes such as fruit flies and worms in which cilia are dispensable. In mice (Corbit et al. 2005; May et al. 2005; Rohatgi et al. 2007) and zebrafish (Aanstad et al. 2009; Huang and Schier 2009), Hh pathway members, including Smo and Patched (Ptc), are trafficked into and out of sensory cilia during the transduction process. Intraflagellar transport (IFT) proteins and motor proteins such as Kif3a, a vertebrate homolog of Kinesin-2, are required for this movement (Liu 2005; Rohatgi et al. 2007). In contrast, this trafficking is not necessary in protostomes (Avidor-Reiss et al. 2004; Rink et al. 2009; Glazer et al. 2010). How two divergent mechanisms of transduction arose is unclear. Examining the sea urchin, a more basal deutersotome, provides a crucial missing link to the evolution of this pathway. In the sea urchin genome, single Hh, Ptc, Smo, and Gli homologs exist (Walton et al. 2006). Our lab reported the role of Hh signaling in sea urchins where it acts to pattern embryonic musculature (Walton et al. 2009). Although most of the ciliary trafficking genes have been identified in the sea urchin genome (Morris et al. 2006), it has remained unclear whether Hh signaling depended on cilia for signal transduction.
Results and Discussion
To begin investigating the role of cilia in Hh signaling in urchin, we examined the Hh-receiving cells for the presence of cilia. In the sea urchin, the Hh ligand is expressed in and secreted from endoderm cells in the primitive gut of gastrula stage embryos and diffuses to a subset of adjacent mesodermal cells that express Ptc and Smo (fig. 1A–D). These latter cells will form specialized structures termed the coelomic pouches and contribute to the musculature of the embryo (Burke and Alvarez 1988). To assess the presence of cilia, we labeled the presumptive muscle cells with a myosin heavy chain (αMHC) antibody and co-stained with an acetylated tubulin (αAcTub) antibody that labels the cilia axonemes. We observed that the presumptive muscle cells, which are known to receive Hh signal, have short monocilia on their apical surface (fig. 1E and F). This finding demonstrated that the Hh-receiving cells of the sea urchin embryo are indeed ciliated.
Fig. 1.
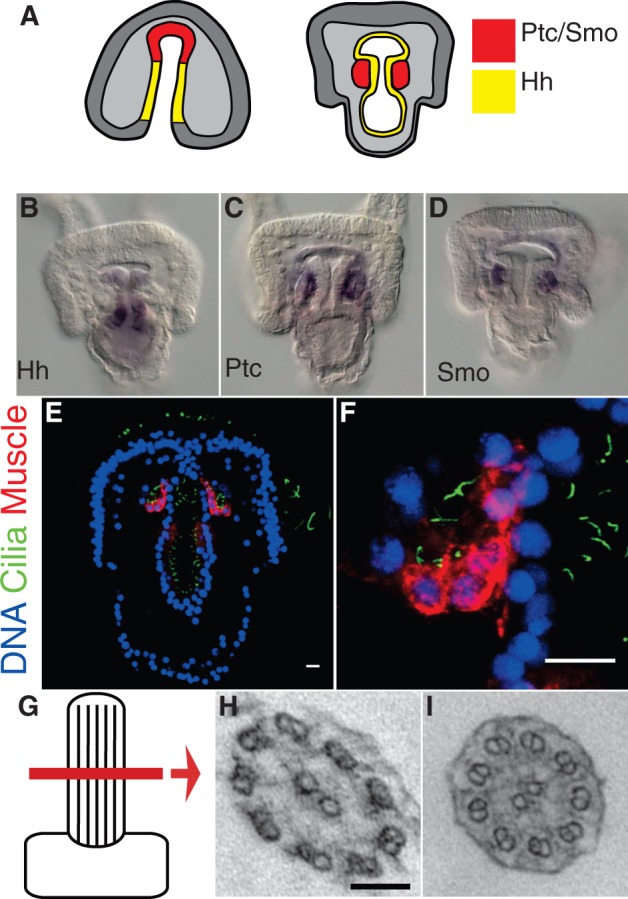
Hh-receiving cells exhibit motile cilia. (A) Model of Hh signaling in the sea urchin. Hh is secreted from the gut endoderm (yellow) and diffuses to the adjacent mesoderm where Ptc and Smo are expressed (red). (B–D) In situ mRNA hybridization of Hh (B), Ptc (C), and Smo (D). (E and F) Presumptive muscle cells display monocilia. (E) Hh-receiving cells are labeled by the muscle-specific MHC antibody (red), and cilia are labeled with an acetylated tubulin antibody (green). Scale bars = 10 μm. (F) Enlargement. (G–I) Transmission electron micrographs of cilia ultrastructure. (G) Cartoon representing the lateral region of the cilium scanned. (H) Cross-section of cilium from ectoderm showing 9 + 2 microtubule arrangement. Scale bar = 100 nm. (I) Cross-section of cilium on Hh-receiving cells of coelomic pouch also with a 9 + 2 arrangement.
We were next interested in determining whether or not the cilia present on Hh-receiving cells are more like primary cilia or motile cilia, because in vertebrates, Hh signal transduction occurs via specialized nonmotile primary cilia (Davenport and Yoder 2005). In contrast, motile or beating cilia have yet to be linked to the reception and transduction of any developmental signaling pathway. To determine the type of cilia present on sea urchin Hh-receiving cells, we examined their ciliary microtubule arrangement by transmission electron microscopy. The axoneme of a primary cilium consists of nine doublets of microtubules (9 + 0), whereas motile cilia typically include a central pair of microtubules in addition to the nine outer doublets (9 + 2) (Satir and Christensen 2007). To our surprise, we found that the cilia present on Hh-receiving cells exhibited a 9 + 2 microtubule arrangement identical to the microtubule arrangement of ectodermal motile cilia used for locomotion (fig. 1G–I). To assess the motility of these cilia, we performed timelapse microscopy and observed that they were indeed motile (supplementary movie S1, Supplementary Material online). Thus, the cilia present on Hh-receiving cells in the sea urchin are not immotile primary cilia like those associated with Hh signaling in vertebrates but are motile cilia.
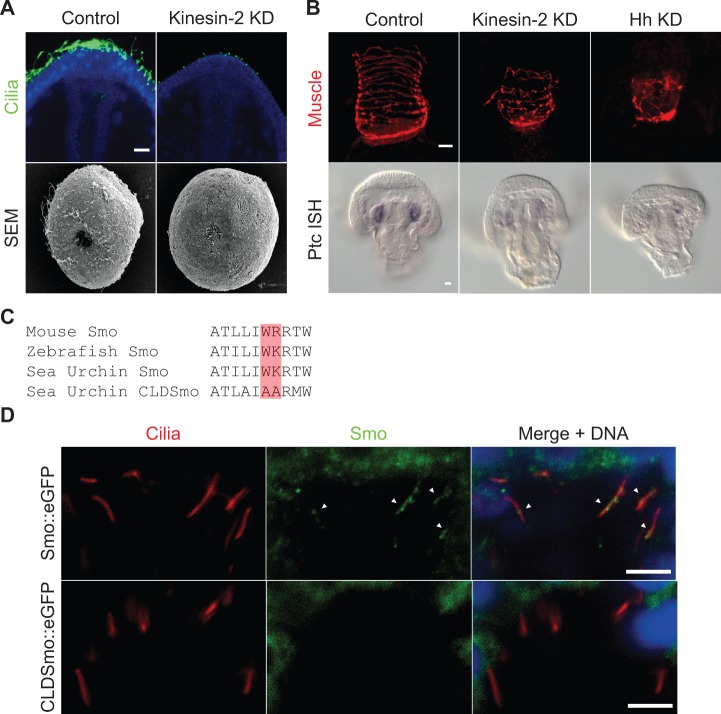
After establishing that Hh-receiving cells in the sea urchin embryo are ciliated, we next tested the functionality of these cilia in the context of Hh signaling. To do this, we first perturbed Kinesin-2, a conserved motor protein that is necessary for cilia assembly and trafficking (Cole et al. 1998). In the sea urchin, the function of Kinesin-2 protein can be inhibited by injecting a monoclonal antibody against Kinesin-2 into the fertilized egg (Morris and Scholey 1997). Sea urchin embryos injected with this Kinesin-2 antibody fail to assemble full-length cilia as demonstrated with the αAcTub antibody and by scanning electron microscopy (fig. 2A), consistent with Kinesin-2 inhibition in mice and Chlamydomonas (Cole et al. 1998; Marszalek et al. 1999). In contrast, inhibition of Hh has no effect on cilia length (supplementary fig. S1, Supplementary Material online). To assess Hh function in Kinesin-2-deficient embryos, we compared the muscle patterns and the expression of the Hh receptor and target, Ptc, in Kinesin-2 knockdown, Hh knockdown, and control embryos (fig. 2B). We previously reported that Hh signaling is necessary for muscle patterning in the sea urchin embryo; inhibition of Hh signaling results in smaller, mispatterned foregut muscles (Walton et al. 2009). To assess the musculature of Kinesin-2 knockdown embryos, we labeled the muscle cells using the αMHC antibody. In control embryos, the musculature is organized in distinct circumesophageal rings (fig. 2B). When we blocked cilia assembly by injecting a Kinesin-2 antibody, the embryos showed mispatterned musculature identical to the phenotype seen in Hh knockdown embryos (fig. 2B). Then, we examined the expression of Ptc in Kinesin-2 knockdown and Hh knockdown embryos. Ptc is the receptor of Hh signaling, but it is also a highly conserved transcriptional target of active signaling (Hidalgo and Ingham 1990; Concordet et al. 1996; Marigo and Tabin 1996; Marigo et al. 1996; Rink et al. 2009). When the Hh ligand was knocked down with a morpholino, the expression of Ptc was greatly reduced compared with controls when assessed by in situ mRNA hybridization (fig. 2B). Likewise, when we prevented cilia assembly by knocking down Kinesin-2, Ptc expression was reduced, consistent with a loss of Hh signaling. Thus, from the knockdown phenotypes and Ptc expression, we conclude that Hh signal transduction depends on the presence of cilia.
Fig. 2.
Hh signal transduction requires the presence of cilia, and Smo localizes to cilia. (A) Cilia assembly is prevented by Kinesin-2 knockdown (Kinesin-2 KD) seen by immunofluorescence of the cilia-specific acetylated tubulin antibody (green) in control and Kinesin-2 KD embryos. Scale bar = 10 μm. Scanning electron micrographs of control, and Kinesin-2 KD embryos at the gastrula stage also show the absence of cilia. Scale bar = 10 μm. (B) Immunofluorescence of the muscle-specific MHC antibody (red) in Kinesin-2 KD and Hh KD embryos shows mispatterning of muscle compared with controls consistent with Hh signaling inhibition. Scale bar = 10 μm. In situ mRNA hybridization of Hh target Ptc (Ptc ISH) in Kinesin-2 KD, and Hh knockdown (Hh KD) embryos shows reduced expression compared with controls. Scale bar = 10 μm. (C) Smo across taxa contains a conserved ciliary localization motif of a hydrophobic and basic residue (red bar). These residues were mutated to alanines to create a ciliary localization defective Smo (CLDSmo::eGFP). (D) Localization of the Hh receptor Smo to cilia using a GFP antibody (green) on embryos expressing a Smo::eGFP or CLDSmo::eGFP fusion construct. Cilia are visualized with an acetylated tubulin antibody (red) on Hh-receiving cells corresponding to the area highlighted in red in figure 1A. Smo::eGFP can be seen localizing to the cilia (arrowheads) but CLDSmo::eGFP does not. Scale bar = 5 μm.
In vertebrates, for Hh to signal via cilia, Smo must be actively trafficked into and out of the cilium by motor and IFT proteins (Corbit et al. 2005). In the absence of the Hh ligand, Smo is excluded from the cilium until Hh binds the receptor Ptc (Rohatgi et al. 2007). Upon binding, Smo is transported into the cilium. In order to test whether Smo can be trafficked into cilia in sea urchin embryos, we expressed a Smo C-terminal eGFP fusion construct (Smo::eGFP) in sea urchin embryos. We then double labeled these embryos with αAcTub to visualize cilia and an αGFP antibody to detect the Smo::eGFP protein. Close examination of the mesodermal cells showed Smo::eGFP being localized to the cilia (fig. 2D). Both vertebrate Smo and sea urchin Smo protein sequences contain a conserved ciliary localization motif consisting of a hydrophobic and basic amino acid (fig. 2C). These residues can be mutated to create a ciliary localization defective form of Smo (CLDSmo) (Corbit et al. 2005). When we expressed an eGFP tagged form of this mutant construct (CLDSmo::eGFP) in the embryo, we observed no localization of Smo to the cilia (fig. 2D). These results demonstrate that sea urchin Smo is actively trafficked into the motile cilia of Hh-receiving cells using the same ciliary localization motif found in vertebrates.
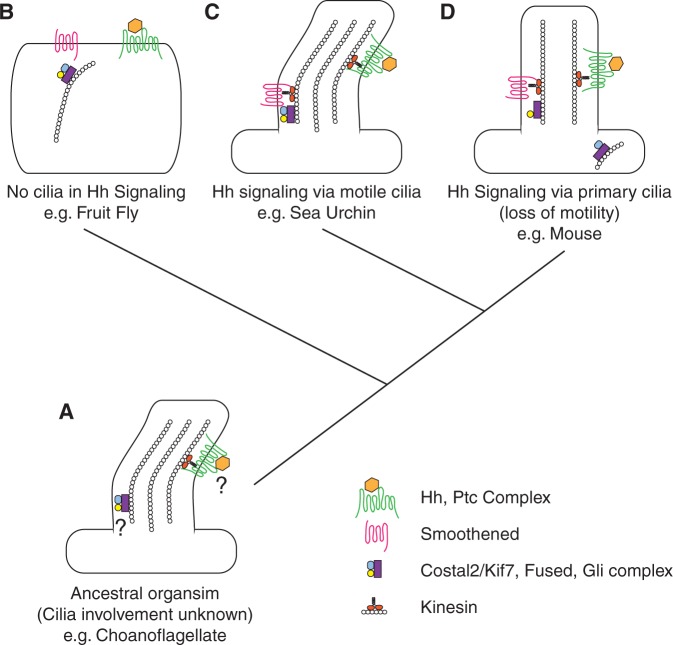
Together, these experiments demonstrate that in sea urchins, Hh signaling relies on the presence of motile cilia. Our findings challenge the assumption that the requirement of cilia for Hh signaling is a recent evolutionary acquisition unique to vertebrates. They also introduce the possibility that motile cilia have long been an important intracellular signaling organelle. Thus, we propose an evolutionary model in which a single-celled flagellated eukaryote, containing homologs of Hh pathway members, may have served as the common ancestor and utilized motor proteins associated with flagellar assembly to traffic the receptor Ptc (fig. 3A). The genome of the choanoflagellate Monosiga brevicollis, for example, contains homologs of Hh, Ptc, and Fused (Hausmann et al. 2009). As the pathway diverged, some organisms such as Drosophila dispensed with, or never required, the necessity of cilia for Hh transduction (fig. 3B). Among those that retained the requirement of cilia, additional divergence resulted in a lineage that continues to utilize motile cilia, as is the case with echinoderms (fig. 3C). Subsequent modifications affecting the dependence of Hh pathway function upon cilia assembly/maintenance proteins may have wholly or partially separated these functions. This could have lead to a vestigial involvement of cilia through immotile primary cilia (fig. 3D), resulting in the pathway seen in mice for example. Further studies of pathway members such as Fused, whose function appears to have concomitantly diverged with cilia-dependent Hh signaling, may yield important clues regarding the link between Hh signaling and cilia.
Fig. 3.
A model of Hh pathway evolution. (A) A single-celled flagellated eukaryote, containing homologs of Hh pathway members, may have served as the common ancestor. (B) As the pathway diverged, some organisms dispensed with the necessity of cilia for transduction. (C) Among those that retained the requirement of cilia, additional divergence resulted in a lineage that continues to utilize motile cilia and (D) another that has lost ciliary motility in Hh-receiving cells.
The kinase, Fused, is a positive regulator of the pathway in fruit flies and zebrafish. However, although it is required for Hh signaling in flies, loss or reduction of Fused in zebrafish recapitulates only some aspects of the Hh phenotype (Lum et al. 2003; Wolff et al. 2003; Wilson et al. 2009). Interestingly, Fused is required for cilia motility in both zebrafish and mice, and it appears to have lost its connection to Hh signaling or is compensated by another kinase in the latter (Wilson et al. 2009). The fact that Fused evolved dual functions in cilia motility and in Hh signaling supports the model in which Hh signaling and Fused evolved together in motile cilia. Future work in sea urchins and choanoflagellates should address the role of Fused in both Hh signaling and cilia motility and shed light on the ancestral state of the pathway.
The role of cilia in Hh pathway signal transduction represents a curious divergence in the deuterostome and protostome lineages and a unique opportunity to study the evolutionary connection between cilia and signal transduction, in general. Unlike protostomes, vertebrates and sea urchins require cilia for effective Hh signaling (Huangfu et al. 2003; Avidor-Reiss et al. 2004; Corbit et al. 2005; Liu 2005; Rohatgi et al. 2007; Aanstad et al. 2009; Huang and Schier 2009; Rink et al. 2009; Glazer et al. 2010). Primary cilia have been linked to intercellular transduction of several developmental pathways, but the origin of these roles has been poorly understood. Our finding that Hh signaling requires motile cilia in sea urchins, along with a recent finding that motile cilia are capable of responding to chemical signals in the human airway (Shah et al. 2009), challenge the conventional thinking that primary cilia are utilized for intercellular signaling and motile cilia are only used to generate mechanical forces. It may even be the case that motile cilia evolutionarily predate primary cilia in terms of developmental signal transduction. Additional studies in diverse taxa, particularly basal deuterostomes and single-celled eukaryotes with Hh pathway homologs, should provide opportunities to test this model and to better understand the origins of this critical signaling pathway.
Materials and Methods
See supplementary materials and methods summary, Supplementary Material online.
Supplementary Material
Supplementary text, figure S1, and movie S1 are available at Molecular Biology and Evolution online (http://www.mbe.oxfordjournals.org/).
Acknowledgments
This work was supported by the National Institutes of Health (grant numbers P01-HD-37105 and R01-HD-14483).
References
- Aanstad P, Santos N, Corbit KC, Scherz PJ, Trinh LA, Salvenmoser W, Huisken J, Reiter JF, Stainier DYR. The extracellular domain of smoothened regulates ciliary localization and is required for high-level Hh signaling. Curr Biol. 2009;19:1034–1039. doi: 10.1016/j.cub.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell. 2004;117:527–539. doi: 10.1016/s0092-8674(04)00412-x. [DOI] [PubMed] [Google Scholar]
- Burke RD, Alvarez CM. Development of the esophageal muscles in embryos of the sea urchin Strongylocentrotus purpuratus. Cell Tissue Res. 1988;252:411–417. doi: 10.1007/BF00214384. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concordet JP, Lewis KE, Moore JW, Goodrich LV, Johnson RL, Scott MP, Ingham PW. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122:2835–2846. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–F1169. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- Glazer AM, Wilkinson AW, Backer CB, Lapan SW, Gutzman JH, Cheeseman IM, Reddien PW. The Zn Finger protein Iguana impacts Hedgehog signaling by promoting ciliogenesis. Dev Biol. 2010;337:148–156. doi: 10.1016/j.ydbio.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann G, von Mering C, Basler K. The hedgehog signaling pathway: where did it come from? PLoS Biol. 2009;7:e1000146. doi: 10.1371/journal.pbio.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo A, Ingham P. Cell patterning in the Drosophila segment: spatial regulation of the segment polarity gene patched. Development. 1990;110:291–301. doi: 10.1242/dev.110.1.291. [DOI] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–3098. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- Liu A. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Lum L, Zhang C, Oh S, et al. (10 co-authors) Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–1274. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- Marigo V, Scott MP, Johnson RL, Goodrich LV, Tabin CJ. Conservation in hedgehog signaling: induction of a chicken patched homolog by Sonic hedgehog in the developing limb. Development. 1996;122:1225–1233. doi: 10.1242/dev.122.4.1225. [DOI] [PubMed] [Google Scholar]
- Marigo V, Tabin CJ. Regulation of patched by sonic hedgehog in the developing neural tube. Proc Natl Acad Sci U S A. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci U S A. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, Peterson AS. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol. 2005;287(2):378–389. doi: 10.1016/j.ydbio.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hoffman MP, Obar RA, et al. (13 co-authors) Analysis of cytoskeletal and motility proteins in the sea urchin genome assembly. Dev Biol. 2006;300:219–237. doi: 10.1016/j.ydbio.2006.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RL, Scholey JM. Heterotrimeric kinesin-II is required for the assembly of motile 9 + 2 ciliary axonemes on sea urchin embryos. J Cell Biol. 1997;138:1009–1022. doi: 10.1083/jcb.138.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink JC, Gurley KA, Elliott SA, Sanchez Alvarado A. Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science. 2009;326:1406–1410. doi: 10.1126/science.1178712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Croce JC, Glenn TD, Wu S-Y, McClay DR. Genomics and expression profiles of the Hedgehog and Notch signaling pathways in sea urchin development. Dev Biol. 2006;300:153–164. doi: 10.1016/j.ydbio.2006.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Warner J, Hertzler PH, McClay DR. Hedgehog signaling patterns mesoderm in the sea urchin. Dev Biol. 2009;331:26–37. doi: 10.1016/j.ydbio.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, Nguyen CT, Chen M-H, Yang J-H, Gacayan R, Huang J, Chen J-N, Chuang P-T. Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature. 2009;459:98–102. doi: 10.1038/nature07883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff C, Roy S, Ingham PW. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–1181. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.