Abstract
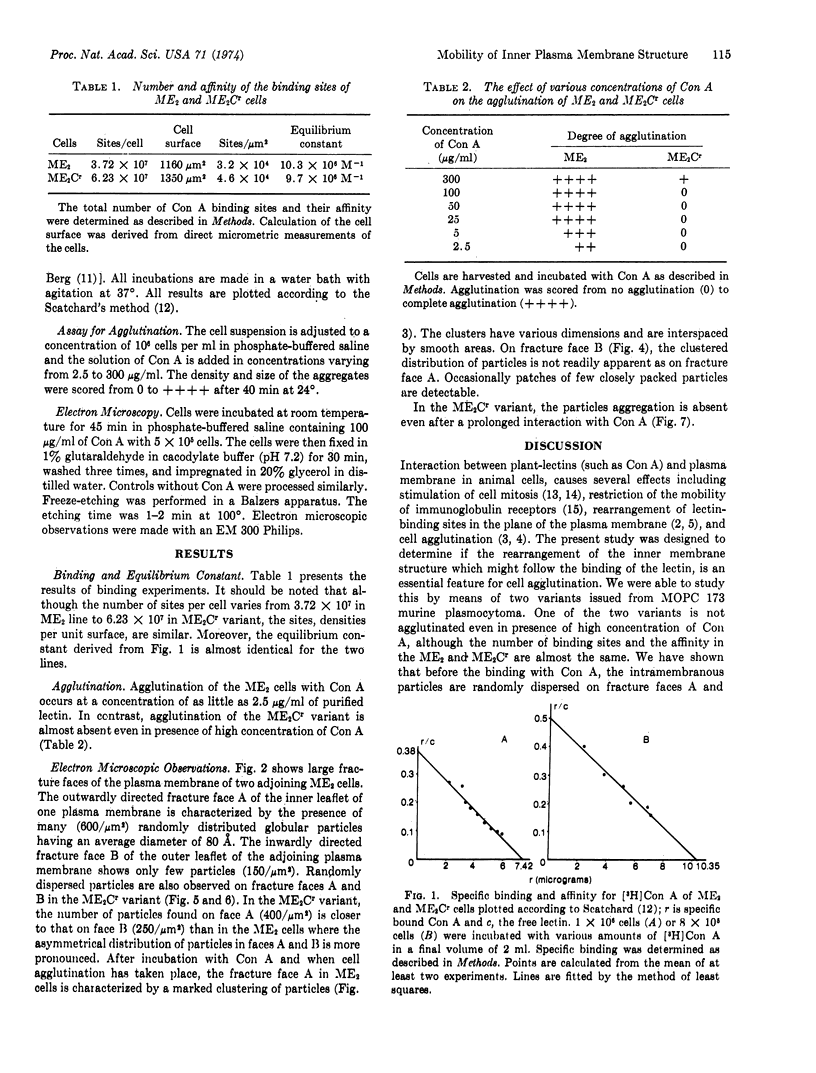
Both the distribution of the concanavalin A-binding sites and the rearrangement of the intramembranous particles revealed by the freeze-etching technique, have been studied by means of two variants of the same cell line issued from MOPC 173 murine plasmocytoma. One variant does not agglutinate even in presence of high lectin concentration. It has been shown that the number of binding sites and affinity are almost the same in the two variants. The clustered distribution of intramembranous particles is induced by the interaction of the concanavalin A and the cell surface only in the variant which is agglutinable. From these results it became apparent that the clustered distribution of the membrane particulate components is an acquired feature of the plasma membrane accompanying cell agglutination.
Keywords: intramembranous particles, binding sites, cell agglutination, murine plasmocytoma cell lines
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti E. L., Dunia I., Diawara A. The organization of the plasma membrane in mammalian cells. Eur J Cancer. 1973 Apr;9(4):263–272. doi: 10.1016/0014-2964(73)90092-3. [DOI] [PubMed] [Google Scholar]
- Betel I., van den Berg K. J. Interaction of concanavalin A with rat lymphocytes. Eur J Biochem. 1972 Nov 7;30(3):571–578. doi: 10.1111/j.1432-1033.1972.tb02128.x. [DOI] [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin C., Prigent B., Moyne M. A., Paraf A. La différenciation du plasmocytome murin MOPC 173: obtention de variants en culture cellulaire. Bull Cancer. 1972 Oct-Dec;59(4):367–379. [PubMed] [Google Scholar]
- Inbar M., Sachs L. Interaction of the carbohydrate-binding protein concanavalin A with normal and transformed cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1418–1425. doi: 10.1073/pnas.63.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Lustig A. The molecular weight of concanavalin A. Biochim Biophys Acta. 1968 Oct 21;168(2):366–367. doi: 10.1016/0005-2795(68)90161-x. [DOI] [PubMed] [Google Scholar]
- Karnovsky M. J., Unanue E. R., Leventhal M. Ligand-induced movement of lymphocyte membrane macromolecules. II. Mapping of surface moieties. J Exp Med. 1972 Oct 1;136(4):907–930. doi: 10.1084/jem.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky M. J., Unanue E. R. Mapping and migration of lymphocyte surface macromolecules. Fed Proc. 1973 Jan;32(1):55–59. [PubMed] [Google Scholar]
- Legrand E., Moyne M. A., Paraf A., Duplan J. F. Studies on mouse plasmocytoma cells MOPC 173 grown "in vitro". General properties of fibroblastic and epithelioid cell lines. Ann Inst Pasteur (Paris) 1972 Nov;123(5):641–660. [PubMed] [Google Scholar]
- Loor F. Lymphocyte membrane particle redistribution induced by a mitogenic-capping dose of the phytohemagglutinin of Phaseolus vulgaris. Eur J Immunol. 1973 Feb;3(2):112–116. doi: 10.1002/eji.1830030212. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Tillack T. W., Jackson R. L., Segrest J. P., Scott R. E. Chemical characterization and surface orientation of the major glycoprotein of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1445–1449. doi: 10.1073/pnas.69.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L. Topography of membrane concanavalin A sites modified by proteolysis. Nat New Biol. 1972 Oct 18;239(94):193–197. doi: 10.1038/newbio239193a0. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Burger M. M. Surface-specific characteristics of a contact-inhibited cell line containing the SV40 viral genome. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1074–1076. doi: 10.1073/pnas.62.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblith J. Z., Ukena T. E., Yin H. H., Berlin R. D., Karnovsky M. J. A comparative evaluation of the distribution of concanavalin A-binding sites on the surfaces of normal, virally-transformed, and protease-treated fibroblasts. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1625–1629. doi: 10.1073/pnas.70.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Marchesi V. T. Structural changes in membranes of transformed lymphocytes demonstrated by freeze-etching. Cell Immunol. 1972 Feb;3(2):301–317. doi: 10.1016/0008-8749(72)90169-4. [DOI] [PubMed] [Google Scholar]
- Shoham J., Inbar M., Sachs L. Differential toxicity on normal and transformed cells in vitro and inhibition of tumour development in vivo by concanavalin A. Nature. 1970 Sep 19;227(5264):1244–1246. doi: 10.1038/2271244a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Tillack T. W., Scott R. E., Marchesi V. T. The structure of erythrocyte membranes studied by freeze-etching. II. Localization of receptors for phytohemagglutinin and influenza virus to the intramembranous particles. J Exp Med. 1972 Jun 1;135(6):1209–1227. doi: 10.1084/jem.135.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Perkins W. D., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972 Oct 1;136(4):885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahara I., Edelman G. M. Restriction of the mobility of lymphocyte immunoglobulin receptors by concanavalin A. Proc Natl Acad Sci U S A. 1972 Mar;69(3):608–612. doi: 10.1073/pnas.69.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]




