Abstract
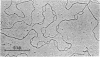
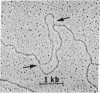
The replicating chromosomal DNA in Drosophila melanogaster cleavage nuclei has been visualized in the electron microscope as a serial array of closely spaced replicated regions created by pairs of diverging replication forks. The fine structure of the forks is very similar to that observed for the replication forks of bidirectionally replicating bacteriophage DNAs. However, the mean length of the single-stranded gaps in Drosophila forks is less than 200 nucleotide residues, much shorter than the gaps in phage forks. This difference in gap length corresponds to the observed difference in the size of Okazaki fragments from Drosophila and phage.
Keywords: electron microscopy, Okazaki fragments, cleavage nuclei, branch migration, fork rate
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blamire J., Cryer D. R., Finkelstein D. B., Marmur J. Sedimentation properties of yeast nuclear and mitochondrial DNA. J Mol Biol. 1972 Jun 14;67(1):11–24. doi: 10.1016/0022-2836(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler D., Wolfson J., Magazin M. Initiation and reinitiation of DNA synthesis during replication of bacteriophage T7. Proc Natl Acad Sci U S A. 1972 Apr;69(4):998–1002. doi: 10.1073/pnas.69.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg B., Hurwitz J. Unbiased synthesis of pulse-labeled DNA framents of bacteriophage lambda and T4. J Mol Biol. 1970 Sep 14;52(2):265–280. doi: 10.1016/0022-2836(70)90030-6. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Inman R. B., Schnös M. Structure of branch points in replicating DNA: presence of single-stranded connections in lambda DNA branch points. J Mol Biol. 1971 Mar 14;56(2):319–325. doi: 10.1016/0022-2836(71)90467-0. [DOI] [PubMed] [Google Scholar]
- Kavenoff R., Zimm B. H. Chromosome-sized DNA molecules from Drosophila. Chromosoma. 1973;41(1):1–27. doi: 10.1007/BF00284071. [DOI] [PubMed] [Google Scholar]
- Klein A., Bonhoeffer F. DNA replication. Annu Rev Biochem. 1972;41(10):301–332. doi: 10.1146/annurev.bi.41.070172.001505. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Manor H., Deutscher M. P., Littauer U. Z. Rates of DNA chain growth in Escherichia coli. J Mol Biol. 1971 Nov 14;61(3):503–524. doi: 10.1016/0022-2836(71)90062-3. [DOI] [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Bonhoeffer F. Discontinuous DNA replication in vitro. I. Two distinct size classes of intermediates. Nat New Biol. 1972 Dec 20;240(103):233–235. doi: 10.1038/newbio240233a0. [DOI] [PubMed] [Google Scholar]
- Petes T. D., Fangman W. L. Sedimentation properties of yeast chromosomal DNA. Proc Natl Acad Sci U S A. 1972 May;69(5):1188–1191. doi: 10.1073/pnas.69.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Sugino A., Okazaki R. Mechanism of DNA chain growth. Vii. Direction and rate of growth of T4 nascent short DNA chains. J Mol Biol. 1972 Feb 28;64(1):61–85. doi: 10.1016/0022-2836(72)90321-x. [DOI] [PubMed] [Google Scholar]
- Werner R. Distribution of growing points in DNa of bacteriophage T4. J Mol Biol. 1968 May 14;33(3):679–692. doi: 10.1016/0022-2836(68)90313-6. [DOI] [PubMed] [Google Scholar]
- Wolfson J., Dressler D., Magazin M. Bacteriophage T7 DNA replication: a linear replicating intermediate (gradient centrifugation-electron microscopy-E. coli-DNA partial denaturation). Proc Natl Acad Sci U S A. 1972 Feb;69(2):499–504. doi: 10.1073/pnas.69.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J., Dressler D. Regions of single-stranded DNA in the growing points of replicating bacteriophage T7 chromosomes. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2682–2686. doi: 10.1073/pnas.69.9.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R. Replicating DNA molecules from eggs of Drosophila melanogaster. Chromosoma. 1973 Jul 23;43(1):1–18. doi: 10.1007/BF01256731. [DOI] [PubMed] [Google Scholar]