Background: The dopamine transporter (DAT) regulates the outflow of dopamine from synapses.
Results: We present evidence that the DAT blockers cocaine and methylphenidate increase or reduce the release of DA in the striatum.
Conclusion: The reducing effects on DA release are not dependent on a typical blockade of DAT.
Significance: The paradoxical blunting of dopamine (DA) release could account for differential effects of psychostimulants.
Keywords: Dopamine, Dopamine Transporters, Drug Action, Electrophysiology, Transgenic Mice, Amperometry, Cocaine, Microdialysis, Striatum
Abstract
We combined in vitro amperometric, optical analysis of fluorescent false neurotransmitters and microdialysis techniques to unveil that cocaine and methylphenidate induced a marked depression of the synaptic release of dopamine (DA) in mouse striatum. In contrast to the classical dopamine transporter (DAT)-dependent enhancement of the dopaminergic signal observed at concentrations of cocaine lower than 3 μm, the inhibitory effect of cocaine was found at concentrations higher than 3 μm. The paradoxical inhibitory effect of cocaine and methylphenidate was associated with a decrease in synapsin phosphorylation. Interestingly, a cocaine-induced depression of DA release was only present in cocaine-insensitive animals (DAT-CI). Similar effects of cocaine were produced by methylphenidate in both wild-type and DAT-CI mice. On the other hand, nomifensine only enhanced the dopaminergic signal either in wild-type or in DAT-CI mice. Overall, these results indicate that cocaine and methylphenidate can increase or decrease DA neurotransmission by blocking reuptake and reducing the exocytotic release, respectively. The biphasic reshaping of DA neurotransmission could contribute to different behavioral effects of psychostimulants, including the calming ones, in attention deficit hyperactivity disorder.
Introduction
The abuse potential and psychomotor activation of drugs such as cocaine and methylphenidate are critically regulated by the dopaminergic system. These drugs block the dopamine (DA)2 membrane transporter (DAT), decreasing catecholamine reuptake and, thus, increasing extracellular DA (1–3). For cocaine and other psychostimulants, an enhancement of DAergic transmission is thought to be the “primum movens” for abuse behavior and locomotor activation (4–7). Psychostimulants are also thought to modify synaptic DA release because of synaptic vesicle fusion (8–9), although the mechanisms by which they do so are unclear.
To measure changes in DA synaptic activity caused by drugs affecting the DAT, we have analyzed effects on evoked DA release using electrochemical, two optical, and microdialysis measurements in a striatal slice preparation obtained from wild-type and DAT-modified/cocaine-insensitive (DAT-CI) mice (3). We found that, contrary to the common belief that the dopaminergic signal is always increased by psychostimulants, slightly more elevated concentrations of some of them depress, rather than potentiate, synaptic dopaminergic transmission within the striatum. Therefore, the psychostimulant-induced biphasic shaping of DA release can differently modulate the dopaminergic signal in the brain, giving rise to a diverse susceptibility of individuals to abused drugs.
EXPERIMENTAL PROCEDURES
Corticostriatal Slice Preparation
All experiments were conducted in conformity with the European Communities Council Directive of November 1986 (86/609/ECC). Two 3-weeks old C57BL/6J mice (Harlan, Italy) were anesthetized with halothane and killed by decapitation. The brain was rapidly removed from the skull, and coronal slices (300 μm), including the cortex and the striatum, were cut in cold (8–12 °C) artificial cerebrospinal fluid (ACSF) using a vibratome (Leica) and left to recover at 33 °C for 1 h. Preparation and maintenance of corticostriatal slices in our laboratory have been described previously (10).
Constant Potential Amperometry (CPA)
A bipolar nickel/chromium-insulated stimulating electrode was placed into the striatum. To monitor the electrically evoked dopamine release, we used CPA with carbon fiber electrodes (11). The carbon fiber electrode (active surface 30 μm in diameter and 100 μm long, World Precision Instruments, catalog no. CF10) was gently positioned into the dorsal striatum to a depth of 100–150 μm near the stimulating electrode. It was connected to a potentiostat (MicroC, World Precision Instruments) to apply voltage and measure current. The imposed voltage between the carbon fiber electrode and the silver/AgCl pellet was 0.55 V.
For stimulation, we applied a single rectangular electrical pulse (80–500 μA, 20- to 40-μs duration) every 3–5 min. This protocol was also used to evoke DA release during simultaneous striatal field potential recordings. Noteworthy, stimulation was applied every 20–30 s during the microdialysis experiments. The electrical shock caused a rapid rise in amperometric current that generally decayed back to base line in about 1.5 s. When the extracellular DA response to electrical stimulation was stable for five or six successive stimulations, we superfused drugs to the striatal slice. Signals were digitized using a Digidata acquisition system (Digidata 1440A) coupled to a personal computer running the Clampex 10. In line with a spike-dependent signal depending on DA release, this was completely abolished in the presence of tetrodotoxin (1 μm) or reserpine (1–3 μm) (data not shown).
Field Potential Recordings
Field excitatory postsynaptic potentials from neostriatal medium spiny neurons were recorded with extracellular glass microelectrodes (5–10 MΩ) filled with 2 m NaCl and placed in the dorsal striatum in proximity to the carbon fiber electrode (10). All data were normalized and expressed as a percentage of the control values, the latter representing the mean of responses recorded during a stable period (20 min) before drug applications.
Synaptosome Preparation and Stimulation
Synaptosomes were prepared by homogenizing in sucrose buffer (10 mm Tris-HCl (pH 7.4) at 4 °C, 0.32 m sucrose (pH 7.4)) striatal tissue pooled from five wild-type mice (C57/BL6). Synaptosomal fractions were purified by centrifugation for 5 min at 33,500 × g and isolated from the 10–20% interface of a Percoll-sucrose density gradient (2–6-10−20%, v/v) (12). Synaptosomes were washed in HEPES buffer (140 mm NaCl, 3 mm KCl, 1.2 mm MgSO4, 1.2 mm CaCl2, 1 mm NaH2PO4, 3.5 mm NaHCO3, 10 mm glucose, and 5 mm HEPES, (pH 7.4)).
Synaptosomes were resuspended in 800 μl of HEPES buffer, divided into four aliquots of 200 μl each, and gently agitated at 37 °C. After 20 min of agitation, drugs (5 μm sulpiride, 0.3 or 30 μm cocaine, and 3 or 30 μm methylphenidate) or vehicle (control) were added to the synaptosome suspensions. After a further 30 min, KCl (8 mm) or vehicle was added, and the suspensions were incubated for 2 min. Depolarization stimuli were then stopped by adding 400 μl of 37 °C HEPES buffer to each aliquot, and synaptosomes were left in agitation for another 8 min. After a total stimulation time of 10 min, the suspension was immediately centrifuged at 16,000 × g to collect the synaptosomes.
Immunoblot Analysis of Synapsin I
Synaptosomes were homogenized at 4 °C in Triton X-100 lysis buffer (10 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1% Triton X-100, 1 mm EDTA, 10% glycerol, 1 mm phenylmethylsulphonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). 20 μg of proteins of each sample was then separated by electrophoresis on 10% SDS-polyacrylamide gels. Proteins were transferred to PVDF membranes (Amersham Biosciences). Membranes were incubated overnight at 4 °C in blocking solution with a rabbit polyclonal anti-phospho-synapsin (Ser9) antibody (1:1000, Cell Signaling Technology). Secondary antibody with horseradish peroxidase-conjugated goat anti-rabbit antibodies (1:5000, Amersham Biosciences) was used, and immunoreactivity was assessed by an enhanced chemiluminescence system (Cyanagen). The same membranes were then incubated for 30 min with stripping solution, and then the same protocol was followed for a rabbit polyclonal synapsin I-specific antibody (1:1000, Novus Biologicals). Digitized images of immunoreactive bands were acquired and measured using ImageJ software (National Institute of Health).
Loading and Imaging of FFN102
The probe FFN102 and its use for imaging DA presynaptic terminals and their activity has been described recently (13). All animals used for slice preparation were 2- to 4-month-old male C57BL/6 mice obtained from The Jackson Laboratory (Bar Harbor, ME). All animal protocols were approved by the Institutional Animal Care And Use Committee (IACUC) of Columbia University.
Dopaminergic terminals were visualized at > 25 μm depth in the slice using a Prairie Ultima multiphoton microscopy system (Prairie Technologies, Middleton, WI) with a titanium-sapphire Chameleon laser (Coherent) equipped with a ×60, 0.9 numerical aperture (NA) water immersion objective. FFN102 was excited at 760 nm and visualized using an emission range of 440–500 nm. For striatal slices, images were captured in a 16-bit 37.3 × 37.3 μm field of view at a 512 × 512 pixel resolution and a dwell time of 10 μs/pixel using Prairie View software.
FFN102 (10 μm in ACSF) was loaded into presynaptic terminals during a 30-min incubation at room temperature in oxygenated ACSF. Slices were washed in the perfusion chamber for 10 min before imaging. The tissues were then imaged every minute for 10 min during the ACSF wash, followed by a 10-min wash of ACSF containing the AMPA and NMDA antagonists NBQX (10 μm) and AP-5 (50 μm) with or without cocaine (30 μm) or methylphenidate (30 μm). After the washes, the imaging rate was increased to 19 s for each time point for the next 330 s, during which a 10-Hz stimulation train (pulses of 200 ms at 130–150 mA) was applied locally to the dorsal striatum. Following the stimulus, electrical stimulation was applied locally using stainless steel bipolar electrodes. To compensate for shifts in the z-plane, 5-μm z-stacks comprised of five images, each image acquired at 1-μm intervals, were taken at each time point (before and during stimulation). Slices were continuously perfused with ACSF with or without 30 μm cocaine or methylphenidate during stimulation.
Quantification of activity-dependent changes in fluorescence was determined using Velocity image analysis software version 4.4 (Improvision, PerkinElmer Life Sciences). Puncta were identified by intensity, size, and shape parameters. The same parameters were applied to treated and untreated pairs of slices. The average fluorescence intensity values obtained from the puncta selected at each time point were plotted as a function of time using Microsoft Excel, with the time point prior to stimulation as 100%. A linear line of best fit was determined from the first 20 time points prior to electrical stimulation for each experiment. The data were then subtracted from their respective best fit to estimate changes in fluorescence, excluding any rundown of the dye. The peak maximums after electrical stimulation were then compared using GraphPad Prism 4. Data presented are averages of 1 slice/experiment for each condition from five independent experiments.
Microdialysis
Concentric microdialysis probes (dialyzing portion, 0.5 mm; outer diameter, 0.31 mm) were prepared with AN69 fibers (Hospal Dasco, Bologna, Italy) and inserted into the dorsal striatum of a mouse line with a DAT-CI (3) near the carbon fiber and the stimulating electrodes so that contemporary measurements of electrically evoked DA release were performed by voltammetry and microdialysis. The microdialysis probe was connected to a CMA/100 pump (Carnegie Medicine, Stockholm, Sweden) through PE 20 tubing (Metalant AB), and artificial cerebrospinal fluid (140 mm NaCl, 1 mm MgCl2, 1.2 mm CaCl2 , and 4 mm KCl) was pumped through the dialysis probe at a constant flow rate of 2 μl/min.
Experiments were performed 40 min after probe placement. One base-line sample before drug perfusion was collected. The dialysate was collected every 20 min before, during, and after cocaine perfusion. A volume of 20 μl was analyzed by ultra-performance liquid chromatography to quantify the dopamine levels. Briefly, the ultra-performance liquid chromatography system consisted of an Acquity ultra performance liquid chromatography unit (Waters Corp., Milford, MA) combined with an amperometric detector (model Decade II, Antec Leyden, The Netherlands) equipped with an electrochemical flow cell (VT-03, Antec Leyden) with a 0.7-mm glassy carbon working electrode mounted with a 25-mm spacer and an in situ silver/silver chloride (ISAAC) reference electrode. The electrochemical flow cell was placed immediately after a BEH C18 column (2.1 × 50 mm, 1.7-μm particle size, Waters Corp.), and set at 400 mV of potential. The column was maintained at 37 °C and the flow rate was 0.07 ml/min. The mobile phase was composed of 50 mm phosphoric acid, 8 mm KCl, 0.1 mm EDTA, 2.5 mm 1-octanesulfonic acid sodium salt, and 12% MeOH (pH 6.0) adjusted with NaOH. The peak height produced by oxidation of DA was compared with that produced by a standard (14). The detection limit of the assay was 0.1 picogram.
Data Analysis
Data are presented as the mean ± S.E. The significance level of the results in a statistical analysis was established at p < 0.05. The effects of psychostimulants on the electrically evoked striatal release of DA in constant potential amperometry experiments were analyzed using one-way ANOVA. For the electrophysiological experiments, statistical analysis was performed with one-way ANOVA.
Quantification of phosphorylation of synapsin was expressed as ratio of anti-phospho-synapsin on anti-synapsin I. Values were presented as mean ± S.E. and analyzed by one-way ANOVA followed by Tukey's test. Statistical analysis of data obtained from imaging experiments of DA release was performed by applying one-way ANOVA.
RESULTS
Control of DA Release by Cocaine and Cocaine-like Drugs
DA overflow was evoked by a single electrical pulse delivered in the striatum of wild-type animals and monitored by CPA. When a stable response was obtained, superfusion was switched to cocaine and other DAT blockers. To avoid inhibitory effects on DA release mediated by the stimulation of D2 presynaptic receptors, all experiments were carried out in the presence of the D2 antagonist sulpiride (1–5 μm). To eliminate possible effects of excitatory and inhibitory inputs, most experiments were performed in the presence of a mixture of antagonists, including CNQX (10 μm), D-AP5 (50 μm), bicuculline (30 μm), and CGP54626 (1 μm). Each group was normalized to the first six recordings (15–20 min) of its respective base-line period, and DA levels were measured as percentage change (mean ± S.E.) compared with the control, analyzing both peak amplitude and the half-decay phase of electrochemical signals.
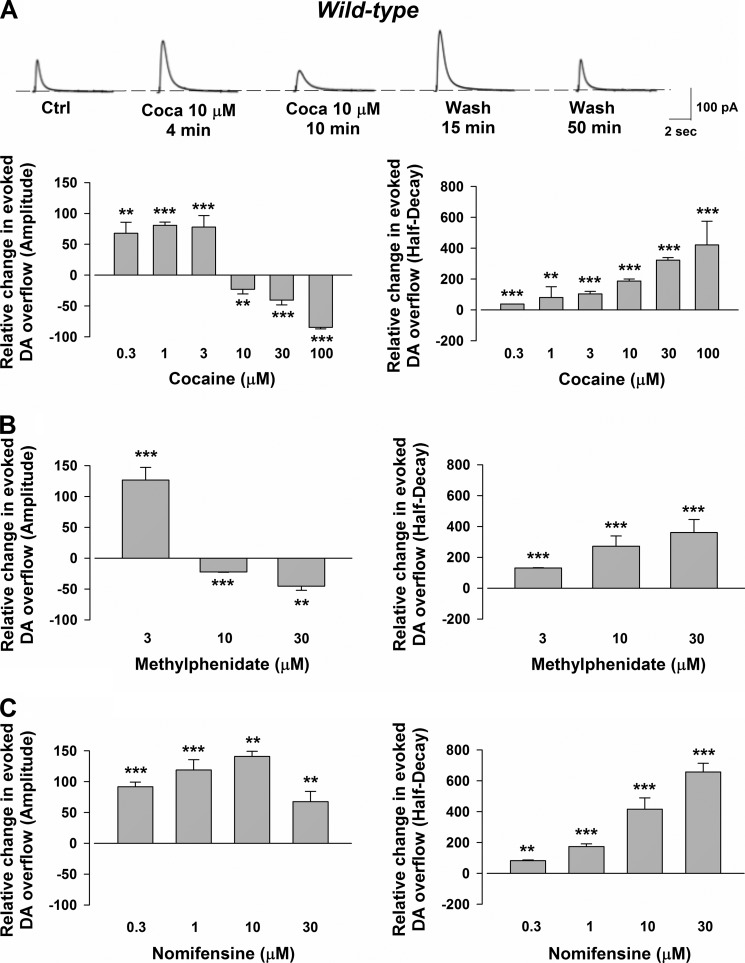
The amperometric measurements (Fig. 1A) showed that, at doses between 0.3–3 μm, cocaine classically produced a concentration-dependent DAT-mediated elevation of the synaptic delivery of DA, increasing the amplitude. Consistently, cocaine prolonged the half-decay phase of the electrochemical signal, which depends on dopamine reuptake. In contrast, increasing the concentrations (10–100 μm) while cocaine further slowed the decaying phase profoundly reduced the amplitude of the signal. The depression usually occurred after a transient potentiation (lasting ≈5 min), peaked in ≈10 min, persisted during the application of the drug, and washed out in 15–50 min. During the washing phase, a transient potentiation of the dopaminergic signal was observed at 15–20 min (raw traces in Fig. 1A).
FIGURE 1.
CPA recordings of the evoked DA efflux and action of cocaine, methylphenidate, and nomifensine in the striata of wild-type mice. A, representative traces showing the effects of cocaine (Coca) at the different time points indicated below. The histogram on the left indicates the effect of cocaine on DA overflow. Note that cocaine (0.3–3 μm) increased the amplitude of the signal by 68 ± 18% with 0.3 μm (n = 4, p = 0.008 F (14.72), one-way ANOVA), by 81 ± 5% with 1 μm (n = 5, p = 2.533E-7 F (250.66), one-way ANOVA), and by 78 ± 19% with 3 μm (n = 11, p = 0.0004 F (17.61), one-way ANOVA). On the other hand, higher concentrations of cocaine (10–100 μm) produced a concentration-dependent reduction of the signal by 23 ± 8% at 10 μm (n = 18, p = 0.005 F (8.77), one-way ANOVA), by 40 ± 8% at 30 μm (n = 28, p = 1.708E-6 F (28.83), one-way ANOVA), and by 85 ± 2% at 100 μm (n = 4, p = 7.967E-6 F (60127), one-way ANOVA). The histogram on the right indicates the effect of cocaine on the decay phase of the DAergic signal. Note that a concentration-dependent increase of the decay phase was seen either with low or high cocaine applications (by 38 ± 1% with 0.3 μm (n = 4, p = 1.596E-13 F (75047), one-way ANOVA), by 81 ± 17% with 1 μm (n = 5, p = 0.001 F (22.63), one-way ANOVA), by 104 ± 16% with 3 μm (n = 9, p = 6.983E-6 F (42.59), one-way ANOVA), by 188 ± 13% with 10 μm (n = 18, p = 1.110E-15 F (195.61), one-way ANOVA), by 322 ± 16% with 30 μm (n = 28, p = 0 F (417.05), one-way ANOVA), and by 421 ± 154% with 100 μm (n = 4, p = 3.685E-14 F (195.70), one-way ANOVA)). Each group of experiments was normalized to the first recordings (15–20 min) of its respective control period. Ctrl, control. **, p < 0.01; ***, p < 0.001. B, the histogram on the left indicates the effect of methylphenidate on DA overflow. Note the increase of the amplitude caused by 3 μm (by 127 ± 25%, n = 6, p = 0.0004 F (26.45), one-way ANOVA)) and the decrease caused by 10 μm (22 ± 1%, n = 4, p = 0.0003 F (2910), one-way ANOVA) and at 30 μm (by 45 ± 7%, n = 4, p = 0.002 F (43.91), one-way ANOVA). The histogram on the right indicates that methylphenidate increases the decay phase of the DAergic signal by 131 ± 1% at 3 μm (n = 6, p = 1.471E-7 F (166.52), one-way ANOVA), by 272 ± 67% at 10 μm (n = 4, p = 0.0001 F (74.74), one-way ANOVA), and by 343 ± 85% at 30 μm (n = 4, p = 0.0003 F (42.36), one-way ANOVA). **, p < 0.01; ***, p < 0.001. C, the histogram on the left indicates the effect of nomifensine on DA overflow. Note that nomifensine increased the amplitude of the signal when superfused at the different concentrations by 92 ± 8% at 0.3 μm (n = 4, p = 0.0002 F (138.95), one-way ANOVA), by 119 ± 17% at 1 μm (n = 11, p = 6.813E-7 F (50.59), one-way ANOVA), by 146 ± 8% at 10 μm (n = 10, p = 0.002 F (11.88), one-way ANOVA), and by 68 ± 17% at 30 μm (n = 7, p = 0.001 F (16.47), one-way ANOVA). The histogram on the right indicates that nomifensine increases in a concentration-dependent manner decay phase of the DAergic signal by 83 ± 4 at 0.3 μm (n = 4, p = 0.00003 F (394.58), one-way ANOVA), by 173 ± 18% at 1 μm (n = 11, p = 3.757E-9 F (97.97), one-way ANOVA), by 415 ± 74% at 10 μm (n = 5, p = 0.0004 F (31.58), one-way ANOVA), and by 657 ± 56% at 30 μm (n = 7, p = 6.140E-8 F (137.95), one-way ANOVA) (**, p < 0.01; ***, p < 0.001).
Similar effects of cocaine on the striatal release of DA were obtained by superfusing the slice with methylphenidate. As expected for a DAT blocker (15), this drug, at concentrations of 3 μm, enhanced the amplitude of the dopaminergic signal, whereas at 10 μm it depressed DA transmission. Methylphenidate slowed the decay of the signal, increasing the half-decay phase (Fig. 1B). In contrast, another DAT inhibitor, nomifensine, always augmented the peak amplitude of evoked DA release. In addition, nomifensine increased the half-decay phase of the amperometric signal (Fig. 1C).
To confirm that the inhibition of evoked synaptic DA release by cocaine and methylphenidate were not linked to the “classical” blocking effects of these drugs on DAT, we took advantage of cocaine-insensitive mice (DAT-CI), which have a three-point mutation in the DA transporter that makes them insensitive to these psychostimulants (3).
In the striatum of DAT-CI animals, the peak amplitude of DA released by a single pulse was similar to control mice (data not shown). The peak latency was, however, longer by ≈93% in DAT-CI compared with wild-type mice, and the half-decay phase of the DA envelope was longer by ≈119% in DAT-CI mice compared with controls. These data indicate that there is a less efficient clearance of released DA by the mutant DAT.
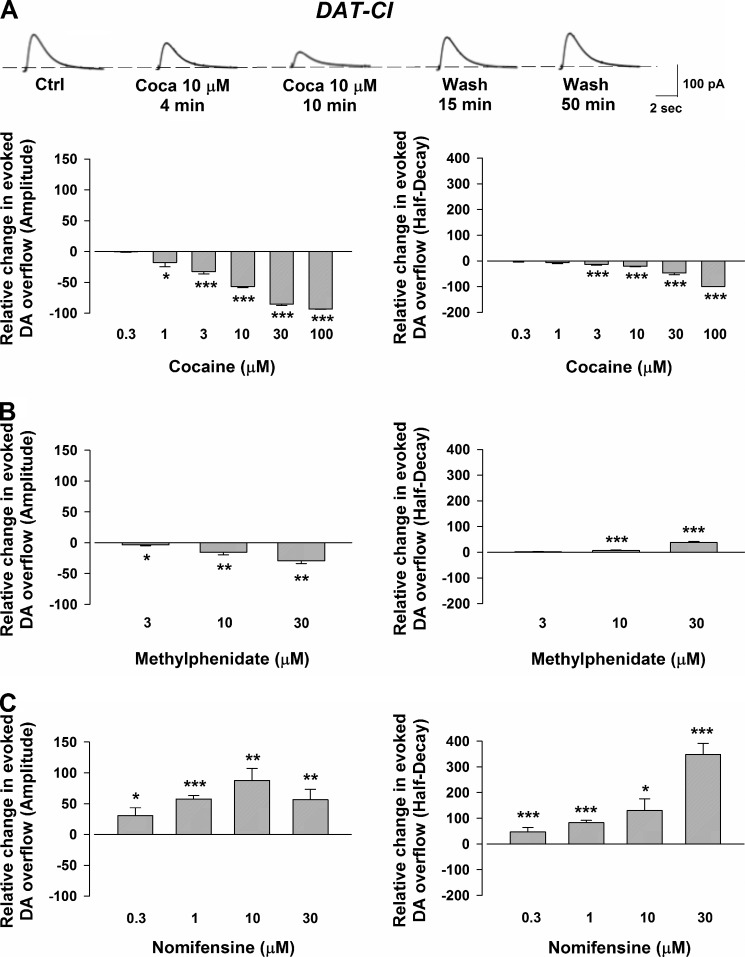
In contrast to wild-type mice, cocaine never augmented DA transients in the DAT-CI animals, but reduced, in a concentration-dependent manner (0.3–100 μm), the amplitude of the signal (Fig. 2A). Moreover, cocaine reduced the half-decay phase of the electrochemical signal (Fig. 2A). Similar depressant effects on the amplitude of the electrically evoked DA transients were caused by methylphenidate (3–30 μm). It also increased the half-decay phase of the amperometric signal (Fig. 2B).
FIGURE 2.
CPA recordings of the evoked DA efflux and action of cocaine, methylphenidate, and nomifensine in the striata of DAT-CI mice. A, traces showing the effects of cocaine (Coca) (10 μm) at the different time points indicated below. The histogram on the left indicates the effect of cocaine on DA overflow. Note that cocaine (0.3 μm) did not change the amplitude of the signal that was instead decreased by 1–100 μm drug (by 1 ± 1% at 0.3 μm (n = 4, p = 0.544 F (0.41), one-way ANOVA), by 17 ± 7% at 1 μm (n = 6, p = 0.029 F (6.39), one-way ANOVA), by 33 ± 2% at 3 μm (n = 14, p = 3.657E-7 F (75.44), one-way ANOVA), by 57 ± 2% at 10 μm (n = 49, p = 0 F (860.53), one-way ANOVA), by 85 ± 2% at 30 μm (n = 19, p = 0 F (1931), one-way ANOVA), and by 93 ± 1% at 100 μm (n = 4, p = 1.659E-9 F (60127), one-way ANOVA)). The histogram on the right indicates that cocaine reduces the decay phase of the DAergic signal by 1 ± 3% with 0.3 μm (n = 4, p = 0.805 F (0.07), one-way ANOVA), by 7 ± 1% with 1 μm (n = 6, p = 0.082 F (3.73), one-way ANOVA), by 13 ± 2% with 3 μm (n = 14, p = 0.0008 F (14.21), one-way ANOVA), by 21 ± 2% with 10 μm (n = 49, p = 0 F (126.25), one-way ANOVA), by 46 ± 8% with 30 μm (n = 19, p = 1.11E-16 F (123.30), one-way ANOVA), and by 99 ± 1% with 100 μm (n = 4 p = 6.991E-9 F (29290), one-way ANOVA). Ctrl, control. (*, p < 0.05; ***, p < 0.001). B, the histogram on the left indicates the effect of methylphenidate on DA overflow. Note the decrease of the amplitude caused by 3 μm (by 4 ± 1%, n = 7, p = 0.014 F (8.19), one-way ANOVA), at 10 μm (by 16 ± 2%, n = 8, p = 0.001 F (15.65), one-way ANOVA), and at 30 μm (by 29 ± 4%, n = 4, p = 0.002 F (47.46), one-way ANOVA). The histogram on the right indicates that methylphenidate decreases the decay phase of the DAergic signal by 1 ± 1% with 3 μm (n = 7, p = 0.298 F (1.17), one-way ANOVA), by 9 ± 1% with 10 μm (n = 8, p = 0.0001 F (27.59), one-way ANOVA), and by 38 ± 4% with 30 μm (n = 4, p = 0.0004 F (116.32), one-way ANOVA) (*, p < 0.05; **, p < 0.01; ***, p < 0.001). C, the histogram on the left indicates the effect of nomifensine on DA overflow. Nomifensine increase the amplitude of the signal when superfused at the different concentrations by 31 ± 13% at 0.3 μm (n = 8, p = 0.029 F (5.90), one-way ANOVA), by 58 ± 5% at 1 μm (n = 20, p = 4.393E-13 F (115.58), one-way ANOVA), by 88 ± 19% at 10 μm (n = 5, p = 0.001 F (20.78), one-way ANOVA), and by 56 ± 17% at 30 μm (n = 8, p = 0.004 F (11.14), one-way ANOVA). The histogram on the right indicates that nomifensine increases the decay phase of the DAergic signal by 46 ± 18% with 0.3 μm (n = 8, p = 0.0002 F (24.35), one-way ANOVA), by 83 ± 9% with 1 μm (n = 20, p = 4.577E-11 F (82.57), one-way ANOVA), by 130 ± 46% with 10 μm (n = 10, p = 0.02 F (11.42), one-way ANOVA), and by 358 ± 43% with 30 μm (n = 8, p = 0.0002 F (24.15), one-way ANOVA). Note that the increase in the amplitude of the DAergic signal in the DAT-CI mice caused by nomifensine is almost half of that evoked in wild-type animals. (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
In contrast to cocaine and methylphenidate, the DAT blocker nomifensine (0.3–30 μm) always augmented the peak amplitude of evoked DA release. In addition, nomifensine increased the half-decay phase of the amperometric signal (Fig. 2C).
Field Potential Recordings and Effects on DA Release by Cocaine
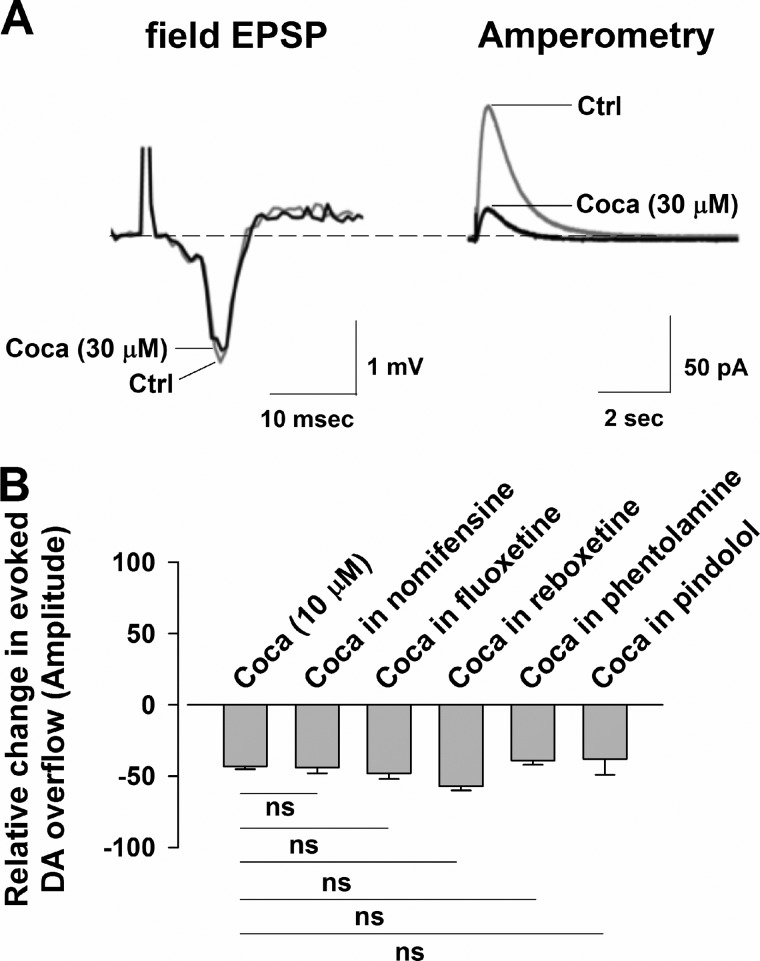
A possible explanation for the decrease of evoked DA release of cocaine is that it results from the anesthetic-like properties of this drug (16). This would be expected to occur at all CNS synapses, and to test this possibility, we conducted electrophysiological recordings of the corticostriatal excitatory field potential. We found that, although evoked release of DA was depressed by cocaine (30 μm), the corticostriatal field potential was not significantly affected, suggesting that an anesthetic-like action of cocaine is not likely to depress evoked DA release (Fig. 3A).
FIGURE 3.
Field potential recordings of the corticostriatal transmission and evoked DA overflow in the presence of cocaine in DAT-CI mice. A, cocaine (Coca) (30 μm) did not change the amplitude of the field potential (97 ± 2% of control (Ctrl), n = 4, p = 0.618 F (0.28), one-way ANOVA), whereas it reduced the evoked DA overflow to 51 ± 2% of control (n = 4, p = 0.0002 F (60.36), one-way ANOVA). B, the reducing effects of cocaine (10 μm) (n = 49) when coapplied with the other DAT inhibitor nomifensine (30 μm, n = 6) (by 50 ± 2%, n = 6, p = 0.179 F (2.12), one-way ANOVA), the SERT blocker fluoxetine (10 μm, n = 8) (by 49 ± 2%, n = 8, p = 0.094 F (3.24), one-way ANOVA), the NET blocker reboxetine (10 μm) (by 56 ± 2% (n = 5, p = 0.944 F (0.005), one-way ANOVA), and the adrenergic antagonists phentolamine (3 μm) (by 40 ± 7%, n = 6, p = 0.560 F (0.344), one-way ANOVA) and pindolol (1 μm) (by 37 ± 11%, n = 4, p = 0.409 F (0.691)). Note that neither of these treatments modified the effects of cocaine. ns, not significant.
Effects of Cocaine, Blockers of Amine Transporters, and DA Antagonists
Further amperometric experiments also revealed that, when an enhanced and stable synaptic DA release was obtained by exposing the striatal slices to nomifensine (30 μm), the subsequent coadministration of cocaine (10 μm) depressed the potentiated dopaminergic signal (Fig. 3B)). This cocaine-induced depression was comparable with that obtained with the same concentration of cocaine alone, further suggesting that nomifensine and cocaine have different effects. In addition, neither the blockade of serotonin transporter (SERT) by fluoxetine (10 μm) nor the blockade of noradrenaline transporter (NET) by reboxetine (10 μm) nor the antagonism of α and β-adrenoreceptors with phentolamine (3 μm) or pindolol (1 μm), respectively, were able to modulate the depression of DA transmission following cocaine (10 μm) superfusion. All of these data suggest that the serotonin and norepinephrine transporters are not involved in the cocaine-induced inhibition of DA release (Fig. 3B). Of note, in the presence of fluoxetine and reboxetine, the amplitude of the DAergic signal was unchanged (96 ± 2% of control, n = 5, p = 0.801 F (0.067) one-way ANOVA, and 95 ± 2% of control, n = 8, p = 0.791 F (0.0726), one-way ANOVA).
To antagonize D1 receptors, experiments were performed superfusing SCH-26390 (10 μm) in addition to sulpiride on striatal slices. Under these conditions we still observed a clear-cut depression of the amperometric signal caused by cocaine (10 μm) (n = 4) (data not shown). This suggests that neither dopamine D1 nor D2 receptors (see above) were involved in the depression of DA release caused by cocaine.
To test whether the depression of DA transmission of cocaine was due to DAT inhibition that blocked synaptic vesicle refilling (17), we measured the evoked DA release following prolonged drug exposure without repeatedly stimulating the striatum. After 20 min of continuous application of cocaine (30 μm), the DA release was reduced, in a reversible manner, by 90 ± 46% (n = 8, p = 6.164E-13 F (608.39), one-way ANOVA) (data not shown). Thus, the inhibition of synaptic DA release of cocaine was not dependent on a depletion of DA in vesicles as a consequence of repeated electrical stimulation.
Microdialysis and Amperometric in Vitro Detections of Changes of DA Release by Cocaine
We next sought to confirm our amperometric results in the DAT-CI animals using in vitro microdialysis detection of DA in conjunction with CPA. A repeated electrical stimulus was applied to striatal slices of DAT-CI mice every 20–30 s, and the levels of DA were measured before, during, and after the application of cocaine (Fig. 4, A and B). Using this protocol, we could assess that the microdialysis technique was able to measure changes in DA concentration above the basal level, consequent to the repeated local stimulation of the tissue. In accordance with the CPA data (Fig. 4B), microdialysis revealed that the change in extracellular content of DA caused by electrical stimulation (128 ± 20%, n = 16) was limited, in a reversible fashion, by 30 μm cocaine at 20 min (C).
FIGURE 4.
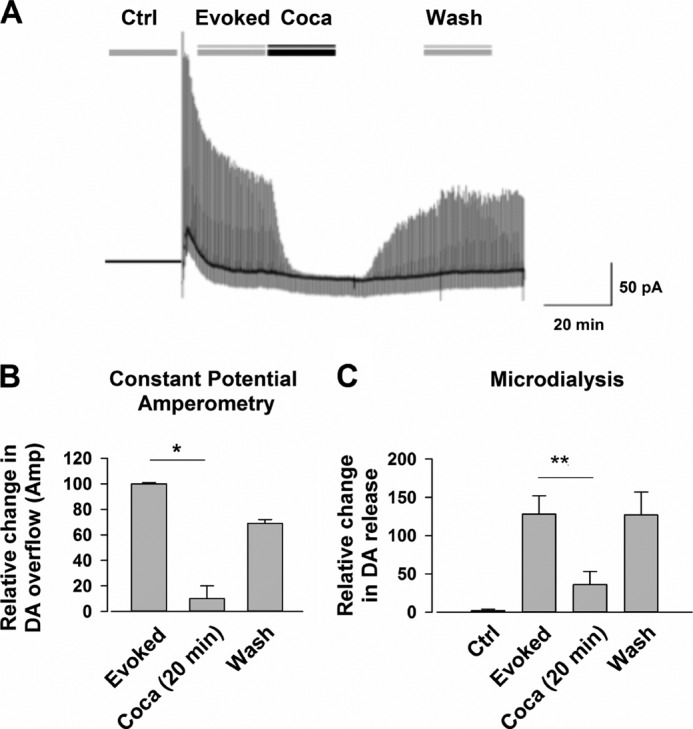
Repetitive tissue stimulation evokes a DA release that is inhibited by cocaine in DAT-CI mice. A, row traces of an experiment. Note that cocaine (Coca) (30 μm) reduced, in a reversible manner, the evoked DA release that was caused by a repetitive electrical stimulation of the tissue. Ctrl, control. B, the reversible inhibition of DA release caused by cocaine (30 μm) in 20-min application (n = 9) (*, p < 0.05). C, an inhibition of the stimulated efflux of DA as detected by constant potential amperometry was detected by in vitro microdialysis after 20 min of cocaine application (by 36 ± 17%, n = 16; p = 0.007; ANOVA (F 2, 37 = 5.624; p < 0.007) **, p < 0.01).
Cocaine Causes a Differential Regulation of Synapsin Phosphorylation in the Striatum
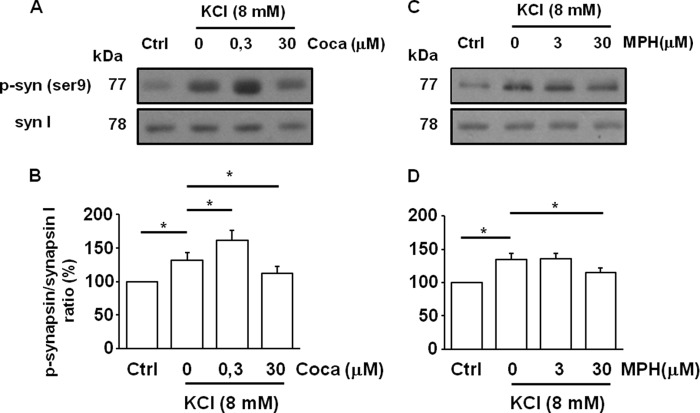
We then examined whether an alteration of vesicular DA exocytosis in the synaptic terminals might underlie the depression of evoked release of cocaine. Recent studies suggest that cocaine can control DA release by affecting synapsin phosphorylation and consequent vesicle mobilization (18). Thus, we analyzed intracellular synapsin phosphorylation in presynaptic striatal isolated terminals. DA release was evoked by KCl (8 mm) applied for 2 min in the presence of 0.3 or 30 μm cocaine. The phosphorylation of synapsin was increased by KCl (132 ± 9% of control, p = 0.020, ANOVA/Tukey's test). Notably, 0.3 μm concentration of cocaine further augmented synapsin phosphorylation (152 ± 8% of control, p = 0.012, ANOVA/Tukey's test), whereas 30 μm cocaine significantly reduced its phosphorylation levels (112 ± 8% of control, p = 0.031, ANOVA/Tukey's test) (Fig. 5, A and B). A reduction in the synapsin phosphorylation level was also observed with methylphenidate at a concentration of 30 μm (115 ± 7% of control, p = 0.044, ANOVA/Tukey's test).
FIGURE 5.
The effect of cocaine and methylphenidate on synapsin I activation in striatal presynaptic terminals. A–C, representative immunoblots of synapsin I (syn I) and synapsin phosphorylation (p-syn) are shown in different experimental conditions. Sulpiride (5 μm) was always present throughout the experiment. Ctrl, control; Coca, cocaine. B–D, quantification of phosphorylation of synapsin was expressed as ratio of anti-phospho-synapsin on anti-synapsin I. Values are mean ± S.E. of five experiments. *, p < 0.05 versus control.
Altered Dopaminergic Synaptic Content in DAT-CI Striatum
To study changes in levels of presynaptic DA and evoked DA release at the level of individual terminals, we used the pH-responsive fluorescent false neurotransmitter FFN102, which detects synaptic vesicle fusion and release of a DA-like optical probe from synaptic terminals with high spatial and temporal resolution (13). FFN102 is a DAT and VMAT2 substrate that becomes brighter when excited at 760 nm following exocytosis from the acidic intravesicular milieu to the neutral extracellular milieu, thus providing a means to observe release during dopaminergic synaptic vesicle fusion.
Incubation of the striatal slice with FFN102 (10 μm, 30 min) led to accumulation of the probe in DA axons and presynaptic terminals (Fig. 6A). The dorsal striatum was imaged in a 5 μm-deep z-stack by two-photon laser scanning microscopy once per minute for 10 min, followed by scanning for a subsequent 10 min with or without 30 μm cocaine. During this period, the signal decayed in a linear fashion (Fig. 6B). We found that the presence of cocaine did not change this decay (−1.17 ± 0.54, −0.98 ± 0.11% change/minute; mean ± S.E. of control and cocaine; n = 5 slices/condition; non-significant, one-way ANOVA).
FIGURE 6.
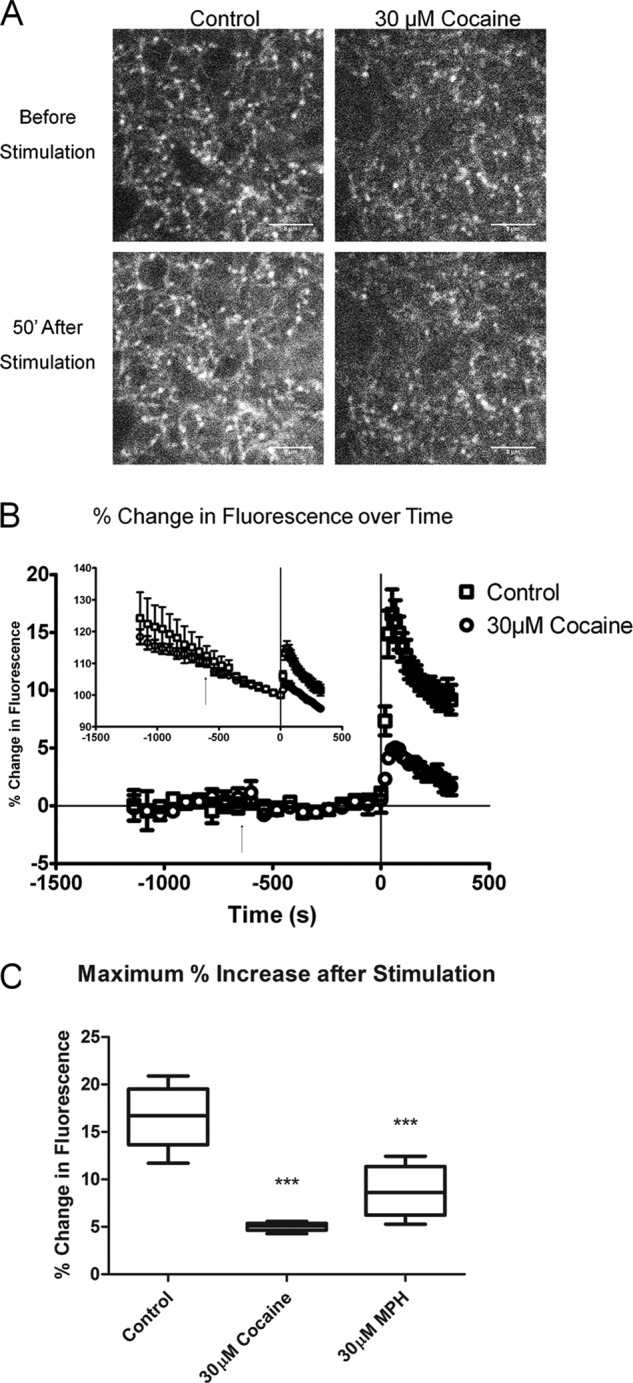
Effect of cocaine and methylphenidate on the release of the fluorescent false neurotransmitter FFN102 in wild-type mice. A, images of FFN102 loaded into dopaminergic terminals of the dorsal striatum before and 51 s after 10-Hz electrical stimulation in the control and with 30 μm cocaine. Scale bars = 8 μm. Both 30 μm cocaine and methylphenidate (not shown) decreased the stimulation-dependent fluorescent signal. B, raw percentage changes in average puncta intensity over time (inset) and then linearly corrected for rundown. The arrow represents the start of cocaine exposure. 10-Hz electrical stimulation starts at t = 0 and continues until the final time point. Error bars represent S.E. The slope of the signal rundown was not affected by cocaine. C, values of maximum fluorescence after 51 minutes of a 10-Hz electrical stimulus. The control signal was increased by 16.5 ± 1.4% in controls (mean ± S.E., n = 6), by 5.0 ± 0.2% (n = 5) in 30 μm cocaine, and 8.8 ± 1.2% in 30 μm methylphenidate (n = 5). Both cocaine and methylphenidate inhibited the evoked increase in fluorescence (p < 0.0001, one-way ANOVA; p < 0.0001 for cocaine versus control; p < 0.0001 for methylphenidate versus control, Tukey-Kramer test).
Alkalinization of the synaptic vesicles leads to an increase in signal. Although cocaine might, in principle, alkalinize the synaptic vesicle interior, we could detect no such alkalinization under these conditions. Thus, even relatively high concentrations of cocaine did not appear to change the presynaptic or the synaptic vesicle content of FFN102.
At 0 s, we applied 10-Hz local electrical stimuli for 330 s, during which the z-stack images were acquired every 19 s. There was an immediate increase in extracellular fluorescent signal, consistent with FFN102 release as described (13). Remarkably, at the peak response at 51 s, the signal was 3.2-fold greater in control than in cocaine-treated slices (Fig. 6C, p < 0.0001, one-way ANOVA), indicating that the DA terminals in control slices released more FFN102. We also examined the effect of 30 μm methylphenidate, which increased the signal 1.9-fold over control tissue (Fig. 6C, p < 0.0001, one-way ANOVA). We conclude that exposure to 30 μm cocaine or methylphenidate decreased the fraction of DA synaptic vesicles that undergo exocytosis, or decreases the synaptic vesicle pore open time, but likely does not alter the presynaptic synaptic vesicle DA stores.
DISCUSSION
Dopamine synthesized in midbrain DAergic neurons is released tonically (in a spike-independent manner) or phasically (under the influence of spike activity) (19–20). Evoked DA release is thought to require a vesicular mobilization and fusion with the plasmalemmal membrane. Accordingly, the release of DA was abolished by tetrodotoxin, a blocker of voltage-dependent sodium channels, or reserpine, a VMAT2 inhibitor that depletes vesicular catecholamine.
In light of these results, the effects of cocaine on evoked release of DA in the striatum can be divided into two contrasting components: a potentiation caused by DAT blockade that elevates extracellular DA and a suppression of exocytosis that inhibits DA release. The inhibition follows a transient increase and requires, to be seen, higher concentrations of the drug. The actions of cocaine were also shared by another DAT blocker, methylphenidate.
Few voltammetric studies have already reported similar changes in DA overflow across psychostimulant concentrations, being the inhibitory effect on DA overflow interpreted as secondary to presynaptic D2 autoreceptor activation (21–22). However, this is not possible because the depression of DA overflow occurs in the presence of the D2 antagonist sulpiride and also in the DAT-CI striatal slices (where an activation of D2 autoreceptors by an enhanced extracellular DA level does not occur).
Noteworthy nomifensine, another DAT blocker (23), only enhanced DA transmission in the striatum of both wild-type or DAT-CI mice. This suggests that it binds on DAT in a different mode. In line with this, it has been shown that nomifensine, differently from cocaine, does not up-regulate DA transport during self-administration (24) and does not protect the specific binding of [3H]cocaine against the effects of pHMBS (25).
We found that the classical binding site of psychostimulants on DAT does not mediate the inhibition, as we observed it when the drugs were superfused in the striatum of DAT-CI mice. From these data, as well as the lack of alteration in the presynaptic fluorescent false neurotransmitter FFN120 with cocaine and methylphenidate, it is doubtful that a classical action on DAT by cocaine and methylphenidate explains the inhibition of the synaptic release of DA. In addition, our experiments using cocaine in the presence of selective concentrations of NET and SERT inhibitors, which, per se, slightly changed the evoked release of DA, exclude a blockade of these transporters that might affect the synaptic release of DA. A binding of cocaine on NET, which could enhances extracellular norepinephrine, to induce the paradoxical blunting of DA release is also excluded by the experiments in the presence of potent adrenergic receptors antagonists such as phentolamine and pindolol. In addition, interference from norepinephrine should be minimal because of its relatively low tissue levels in the striatum.
Cocaine was able to reduce DA release without modifying the field excitatory postsynaptic potentials in the striatum, which is not consistent with an anesthetic-like effect of this compound (16). Moreover, an anesthetic mechanism is not supported by the fact that methylphenidate, which is devoid of anesthetic-like properties, also depressed the release of DA. Another possibility is that vesicular DA depletion is due to the exhaustion of the intracellular content of DA that could be no longer reaccumulated because of the prolonged presence of the DAT blocker. This is very unlikely because it occurred 1) in slices that were not repeatedly stimulated (thus limiting DA depletion), 2) in nomifensine-treated striata (thus, DAT was already blocked), and 3) in DAT-CI mice in whom cocaine does not block DAT activity. Indeed, the depressant effect of the DAergic signal caused by cocaine occurred in the DAT-CI mice at lower concentrations (as low as 1 μm) than that seen in wild-type mice.
This means that, when DAT is working normally, the inhibition is already present with low concentrations of psychostimulants but is masked by the predominant potentiation. Consequently, in case of a “defective” DAT, a depression of DA transmission caused by cocaine and methylphenidate might occur without the potentiation. Of note, we were able to overcome the slowness of the microdialysis technique in detecting rapid changes of DA release by performing repeated electrical stimulation of the tissue so that we measured the fractional stimulated release of this catecholamine in vitro, confirming the CPA results.
These data indicate that the inhibitory effect of cocaine was apparent even in the non-stimulated slice (including in the optical experiments). Another possible explanation of diminished evoked DA release in cocaine-treated striata is a reduction of the synaptic vesicle neurotransmitter. Cocaine, as a lipophilic weak base, might collapse the vesicular pH gradient, similar to “weak base actions” reported for amphetamines, or act on vesicles (26). Work by Erik Floor et al. (27) with isolated synaptic vesicles indicates that they may be quite “leaky,” with ongoing loss of DA into the cytosol. This impression is supported by the actions of reserpine, which can rapidly deplete synaptic vesicles of dopamine.
It is thus possible that, even in the striatal slice, a combination of leaky synaptic vesicles and ongoing, relatively low levels of synaptic vesicle exocytosis requires an ongoing refilling of vesicles with neurotransmitter and that the blockade of reuptake may decrease quantal size. In support of this possibility, a reduction in quantal size of catecholamine release with cocaine and other uptake blockers that is independent of vesicular pH change was observed in cultured PC12 cells (26), which have very little endogenous tonic secretory vesicle fusion. Nevertheless, the observation that cocaine continued to depress evoked release in the DAT-CI mutants indicates that at least some of the inhibitory effect on release is independent of the classical interactions with DAT. Another possible mechanism underlying the inhibitory effects of cocaine and methylphenidate is likely due to actions affecting synaptic protein phosphorylation.
It is thought that phosphorylation of synapsins is required for mobilization of vesicles from the reserve pool during stimulation. As reported before (18), we have seen that 0.3 μm of cocaine further increased the phosphorylation of synapsins in a striatal synaptosomal preparation challenged by high extracellular potassium. Conversely, consistent with the reduction of neurotransmitter release, synapsin phosphorylation levels were reduced by 30 μm cocaine. This occurred even in the presence of a D2 receptor blockade. It is, therefore, possible that a non-DA receptor-mediated function of cocaine leads to decreased synaptic vesicle docking, priming, or fusion. Interestingly, a reduction of synapsin phosphorylation has been also obtained with a high concentration of methylphenidate.
These data support the contention that the clear-cut depression of DA overflow seen with cocaine and methylphenidate is a rather complex phenomenon not entirely dependent on D2 autoreceptor stimulation (18). Thus, the precise mechanism of this depression remains to be determined. In any case, the synapsin phosphorylation/dephosphorylation changes seen with these drugs in the synaptosomal preparation could partially account for their different effects on DA release.
It is worth reminding the reader that the phosphorylation/dephosphorylation data were acquired in isolated synaptosomes. The extent to which the same processes hold sway in the midbrain slices having a preserved neuronal connectivity is currently unknown.
In any case, the FFN102 experiments conducted in the striatal slice suggest that agents such as cocaine and methylphenidate reduce the action potential-mediated DA efflux by restricting mobilization and docking of further vesicles at the presynaptic membrane (28). Interestingly, our findings, reporting dual effects caused by cocaine in the micromolar range, agree with a recent estimation of the concentrations of cocaine in the human brain required to produce subjective effects (29) and to maintain self-administration in rats (30).
It is actually unknown what concentrations of cocaine and methylphenidate reach the synaptic cleft. Certainly active accumulating processes exist. Methylphenidate might accumulate in the central nervous system so that the effective brain concentrations are substantially higher than in plasma (31). With regard to cocaine, most recent data also indicate that striatal levels of this drug are about 6-fold higher than in plasma in animals (32). Although it is not exactly known which concentrations of cocaine are reached in the brain, plasma levels of cocaine users are around 0.2 mg/liter after a single dose and 0.75 mg/liter after multiple doses, which is 3 μm (33). Thus, levels of ∼20 μm (∼6-fold higher than plasma) in the brain are relevant for drug abuse.
More importantly, the experiments obtained with DAT-CI mice also suggest that not only the effective concentrations of psychostimulants but also the scarce expression/turnover/sensitivity of DAT could be key determinants for the positive or negative modulation of the spike-dependent release of DA under various circumstances. Therefore, if DAT is not efficient or is insufficiently sensitive to cocaine/methylphenidate, the paradoxical depressant effects on DA neurotransmission could limit the potentiation, resulting in different behavioral responses (34–37). Therefore, it could be hypothesized that, by providing adjustments to the synaptic level of dopamine, the suppressant effect of cocaine on the phasic release of DA may play a role in the diverse susceptibility to psychostimulants (38–40).
Although the concentration of psychostimulants in the brain is not known, if the inhibitory mechanism on DA release overcomes the classical augmentation of dopaminergic responses caused by DAT inhibition, it might certainly reduce hyperactivity and increase the ability to focus in treated children affected by attention deficit hyperactivity disorder.
This work was supported by a grant from the Italian Minister of Health (Progetto Finalizzato Strategico), by National Institute of Mental Health Grant MH086545, and by the G. Harold and Leila Y. Mathers Charitable Foundation.
- DA
- dopamine
- DAT
- dopamine transporter
- CI
- cocaine-insensitive
- ACSF
- artificial cerebrospinal fluid
- CPA
- constant potential amperometry
- ANOVA
- analysis of variance.
REFERENCES
- 1. Koob G. F., Bloom F. E. (1988) Cellular and molecular mechanisms of drug dependence. Science 242, 715–723 [DOI] [PubMed] [Google Scholar]
- 2. Kuhar M. J., Ritz M. C., Boja J. W. (1991) The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 14, 299–302 [DOI] [PubMed] [Google Scholar]
- 3. Chen R., Han D. D., Gu H. H. (2005) A triple mutation in the second transmembrane domain of mouse dopamine transporter markedly decreases sensitivity to cocaine and methylphenidate. J. Neurochem. 94, 352–359 [DOI] [PubMed] [Google Scholar]
- 4. Penberthy J. K., Ait-Daoud N., Vaughan M., Fanning T. (2010) Review of treatment for cocaine dependence. Curr. Drug Abuse Rev. 3, 49–62 [DOI] [PubMed] [Google Scholar]
- 5. Amalric M., Koob G. F. (1993) Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog. Brain Res. 99, 209–226 [DOI] [PubMed] [Google Scholar]
- 6. Berke J. D., Hyman S. E. (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25, 515–532 [DOI] [PubMed] [Google Scholar]
- 7. Roitman M. F., Wheelerm R. A., Wightman R. M., Carelli R. M. (2008). Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat. Neurosci. 11, 1376–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bradberry C. W. (2008) Comparison of acute and chronic neurochemical effects of cocaine and cocaine cues in rhesus monkeys and rodents. Focus on striatal and cortical dopamine systems. Rev. Neurosci. 19, 113–128 [DOI] [PubMed] [Google Scholar]
- 9. Kalivas P. W., Volkow N. D. (2005) The neural basis of addiction. A pathology of motivation and choice. Am. J. Psychiatry 162, 1403–1413 [DOI] [PubMed] [Google Scholar]
- 10. Geracitano R., Paolucci E., Prisco S., Guatteo E., Zona C., Longone P., Ammassari-Teule M., Bernardi G., Berretta N., Mercuri N. B. (2003) Altered long-term corticostriatal synaptic plasticity in transgenic mice overexpressing human CU/ZN superoxide dismutase (GLY(93)→ALA) mutation. Neuroscience 118, 399–408 [DOI] [PubMed] [Google Scholar]
- 11. Napolitano F., Bonito-Oliva A., Federici M., Carta M., Errico F., Magara S., Martella G., Nisticò R., Centonze D., Pisani A., Gu H. H., Mercuri N. B., Usiello A. (2010) Role of aberrant striatal dopamine D1 receptor/cAMP/protein kinase A/DARPP32 signaling in the paradoxical calming effect of amphetamine. J. Neurosci. 30, 11043–11056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pittaluga A., Feligioni M., Longordo F., Luccini E., Raiteri M. (2006) Trafficking of presynaptic AMPA receptors mediating neurotransmitter release. Neuronal selectivity and relationships with sensitivity to cyclothiazide. Neuropharmacology 50, 286–296 [DOI] [PubMed] [Google Scholar]
- 13. Rodriguez P. C., Pereira D. B., Borgkvist A., Wong M. Y., Barnard C., Sonders M. S., Zhang H., Sames D., Sulzer D. (2013) A fluorescent dopamine tracer resolves individual dopaminergic synapses and their activity in the brain. Proc. Natl. Acad. Sci. U.S.A. 110, 870–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ventura R., Morrone C., Puglisi-Allegra S. (2007) Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc. Natl. Acad. Sci. U.S.A. 104, 5181–5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Federici M., Geracitano R., Bernardi G., Mercuri N. B. (2005) Actions of methylphenidate on dopaminergic neurons of the ventral midbrain. Biol. Psychiatry 57, 361–365 [DOI] [PubMed] [Google Scholar]
- 16. Ruetsch Y. A., Böni T., Borgeat A. (2001) From cocaine to ropivacaine. The history of local anesthetic drugs. Curr. Top. Med. Chem. 1, 175–182 [DOI] [PubMed] [Google Scholar]
- 17. Dackis C. A., Gold M. S. (1985) New concepts in cocaine addiction. The dopamine depletion hypothesis. Neurosci. Biobehav. Rev. 9, 469–477 [DOI] [PubMed] [Google Scholar]
- 18. Venton B. J., Seipel A. T., Phillips P. E., Wetsel W. C., Gitler D., Greengard P., Augustine G. J., Wightman R. M. (2006) Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J. Neurosci. 26, 3206–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grace A. A. (1991) Phasic vs. tonic dopamine release and the modulation of dopamine system responsivity. A hypothesis for the etiology of schizophrenia. Neuroscience 41, 1–24 [DOI] [PubMed] [Google Scholar]
- 20. Grace A. A. (1995) The tonic/phasic model of dopamine system regulation. Its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 37, 111–129 [DOI] [PubMed] [Google Scholar]
- 21. John C. E., Jones S. R. (2007) Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology 52, 1596–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yorgason J. T., España R. A., Jones S. R. (2011) Demon voltammetry and analysis software. Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J. Neurosci. Methods 202, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hunt P., Kannengiesser M., Raynaud J. (1974) Nomifensine. A new potent inhibitor of dopamine uptake into synaptosomes from rat brain corpus striatum. J. Pharm. Pharmacol. 26, 370–371 [DOI] [PubMed] [Google Scholar]
- 24. Tella S. R., Ladenheim B., Cadet J. L. (1997) Differential regulation of dopamine transporter after chronic self-administration of bupropion and nomifensine. J. Pharmacol. Exp. Ther. 281, 508–513 [PubMed] [Google Scholar]
- 25. Refahi-Lyamani F., Saadouni S., Costentin J., Bonnet J. J. (1995) Interaction of two sulfhydryl reagents with a cation recognition site on the neuronal dopamine carrier evidences small differences between [3H]GBR 12783 and [3H]cocaine binding sites. Naunyn-Schmiedeberg's Arch. Pharmacol. 351, 136–145 [DOI] [PubMed] [Google Scholar]
- 26. Sulzer D. (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69, 628–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Floor E., Leventhal P. S., Schaeffer S. F. (1990) Partial purification and characterization of the vacuolar H+-ATPase of mammalian synaptic vesicles. J. Neurochem. 55, 1663–1670 [DOI] [PubMed] [Google Scholar]
- 28. Parsons T. D., Coorssen J. R., Horstmann H., Lee A. K., Tse F. W., Almers W. (1995). The last seconds in the life of a secretory vesicle. Cold Spring Harbor Symp. Quant. Biol. 60, 389–396 [DOI] [PubMed] [Google Scholar]
- 29. Zheng F., Zhan C. G. (2012) Modeling of pharmacokinetics of cocaine in human reveals the feasibility for development of enzyme therapies for drugs of abuse. PLoS Comput. Biol. 8, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zimmer B. A., Dobrin C. V., Roberts D. C. (2011) Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacology 36, 2741–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balcioglu A., Ren J. Q., McCarthy D., Spencer T. J., Biederman J., Bhide P. G. (2009) Plasma and brain concentrations of oral therapeutic doses of methylphenidate and their impact on brain monoamine content in mice. Neuropharmacology 57, 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bystrowska B., Adamczyk P., Moniczewski A., Zaniewska M., Fuxe K., Filip M. (2012) LC/MS/MS evaluation of cocaine and its metabolites in different brain areas, peripheral organs and plasma in cocaine self-administering rats. Pharmacol. Rep. 64, 1337–1349 [DOI] [PubMed] [Google Scholar]
- 33. Couper F. J., Logan B. K. (2004). Drugs and Human Performance Fact Sheets, pp. 20–21, National Highway Traffic Safety Administration, Washington, D.C [Google Scholar]
- 34. George F. R., Ritz M. C. (1990) Cocaine produces locomotor stimulation in SS but not LS mice. Relationship to dopaminergic function. Psychopharmacology 101, 18–22 [DOI] [PubMed] [Google Scholar]
- 35. Marley R. J., Arros D. M., Henricks K. K., Marley M. E., Miner L. L. (1998) Sensitivity to cocaine and amphetamine among mice selectively bred for differential cocaine sensitivity. Psychopharmacology 140, 42–51 [DOI] [PubMed] [Google Scholar]
- 36. Rocha B. A., Scearce-Levie K., Lucas J. J., Hiroi N., Castanon N., Crabbe J. C., Nestler E. J., Hen R. (1998) Increased vulnerability to cocaine in mice lacking the serotonin-1B receptor. Nature 393, 175–178 [DOI] [PubMed] [Google Scholar]
- 37. Bardo M. T., Neisewander J. L., Kelly T. H. (2013) Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol. Rev. 65, 255–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gulley J. M., Hoover B. R., Larson G. A., Zahniser N. R. (2003) Individual differences in cocaine-induced locomotor activity in rats. Behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology 28, 2089–2101 [DOI] [PubMed] [Google Scholar]
- 39. Sabeti J., Gerhardt G. A., Zahniser N. R. (2002) Acute cocaine differentially alters accumbens and striatal dopamine clearance in low and high cocaine locomotor responders. Behavioral and electrochemical recordings in freely moving rats. J. Pharmacol. Exp. Ther. 302, 1201–1211 [DOI] [PubMed] [Google Scholar]
- 40. Briegleb S. K., Gulley J. M., Hoover B. R., Zahniser N. R. (2004) Individual differences in cocaine- and amphetamine-induced activation of male Sprague-Dawley rats. Contribution of the dopamine transporter. Neuropsychopharmacology 29, 2168–2179 [DOI] [PubMed] [Google Scholar]