Background: Growth factor receptor-bound 14 (Grb14) is a negative regulator of insulin receptor (IR).
Results: Grb14 modulates rod cGMP-gated channels and perhaps also phosphodiesterase 6.
Conclusion: Grb14 may contribute to rod transduction and adaptation.
Significance: Phototransduction can be regulated by proteins involved in the IR signaling pathway.
Keywords: Cyclic GMP (cGMP), Cyclic Nucleotides, Neurobiology, Photoreceptors, Receptor-tyrosine Kinase, Retina
Abstract
Previous experiments have indicated that growth factor receptor-bound protein 14 (Grb14) may modulate rod photoreceptor cGMP-gated channels by decreasing channel affinity for cGMP; however, the function of Grb14 in rod physiology is not known. In this study, we examined the role of Grb14 by recording electrical responses from rods in which the gene for the Grb14 protein had been deleted. Suction-electrode recordings from single mouse rods showed that responses of dark-adapted Grb14−/− mice to brief flashes decayed more rapidly than strain-controlled wild type (WT) rods, with decreased values of both integration time and the exponential time course of decay (τREC). This result is consistent with an increase in channel affinity for cGMP produced by deletion of Grb14. However, Grb14−/− mouse rods also showed little change in dark current and a large and significant decrease in the limiting time constant τD, which are not consistent with an effect on channel affinity but seem rather to indicate modulation of the rate of inactivation of cyclic nucleotide phosphodiesterase 6 (PDE6). Grb14 has been reported to translocate from the inner to the outer segment in bright light, but we saw effects on response time course even in dark-adapted rods, although the effects were somewhat greater after rods had been adapted by exposure to bleaching illumination. Our results indicate that the mechanism of Grb14 action may be more complex than previously realized.
Introduction
Growth factor receptor-bound protein 14 (Grb14)2 is an adaptor protein that plays an important role in receptor-tyrosine kinase signaling pathways and insulin signaling (1, 2). Although there is convincing evidence of a negative role of Grb14 in insulin signaling (3, 4), experiments with Grb14−/− animals have also revealed positive effects of Grb14 on receptor-tyrosine kinase signaling in a tissue-specific manner (5, 6).
We previously identified Grb14 in retinal tissue (7). Grb14 is localized predominantly to the rod inner segment, nuclear layer, and synapse in dark-adapted rods, whereas in light-adapted rods, Grb14 can be found throughout the entire cell including the outer segment (6). Light induces activation of the insulin receptor, and ablation of Grb14 results in the loss of light-dependent activation (6). Grb14 undergoes tyrosine phosphorylation by light-activated non-receptor-tyrosine kinase Src, and phosphorylated Grb14 acts as a positive regulator of the insulin receptor (8). By competing for protein-tyrosine phosphatase 1B (PTP1B), a negative regulator of the insulin receptor (8), phosphorylated Grb14 enhances activation of the insulin receptor (9).
These observations suggest that Grb14, perhaps in concert with the insulin receptor, may have some role in the physiology of photoreceptors. One possible mechanism of Grb14 is the modulation of photoreceptor-specific cyclic nucleotide-gated channels, perhaps by direct binding of the Grb14 Ras-associating domain with the C-terminal region of the CNGA1 channel subunit (10). In vitro kinetic and biochemical assays on rod outer segment membrane vesicles suggest that the channels may be more sensitive to cGMP and open at a lower concentration of cGMP in Grb14−/− mice (10); however, the functional consequence of Grb14 interaction with the cyclic nucleotide-gated channels in rod physiology in vivo has not been previously investigated.
In this study, we explored the function of Grb14 in rod photoreceptors by recording electrical responses from rods in which the gene for the Grb14 protein had been deleted. We discovered that rod responses from Grb14−/− mice recover after illumination more rapidly than responses of wild type (WT) mouse rods, as expected if the channels are more sensitive to cGMP and open at a lower concentration after deletion of the Grb14 gene. Much to our surprise, however, the effect of knocking out Grb14 was observed even in dark-adapted rods and seemed unexpectedly to be the result at least in part of an effect on the rod phosphodiesterase. Preliminary results of this study were reported at a meeting of the Association for Research in Vision and Ophthalmology (11).
EXPERIMENTAL PROCEDURES
Animals
All animal work was in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Vision Research. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Oklahoma Health Sciences Center, Dean A. McGee Eye Institute, and UCLA. Grb14−/− mice were kindly provided Dr. Roger Daly, Garvan Institute of Medical Research, Darlinghurst, Australia (3) and were re-derived on a BALB/c background. Control recordings from WT BALB/c mice were always made from littermates of the Grb14−/− mice used in our experiments.
Immunoblot Analysis
Retinas from wild type and Grb14−/− mice were homogenized in a lysis buffer containing 1% Triton X-100, 137 mm NaCl, 20 mm Tris-HCl (pH 8.0), 10% glycerol, 1 mm EGTA, 1 mm MgCl2, 1 mm phenylmethylsulfonyl fluoride, 0.2 mm Na3VO4, 10 μg/ml leupeptin, and 1 μg/ml aprotinin (12). Insoluble material was removed by centrifugation at 17,000 × g for 20 min at 4 °C, and the protein concentrations of the solubilized proteins were determined by the bicinchoninic acid (BCA) reagent following the manufacturer's instructions (Pierce Biotechnology). Proteins were resolved by 10% or gradient (4–20%) SDS-PAGE and transferred to nitrocellulose membranes. The blots were washed twice for 10 min with TTBS (20 mm Tris-HCl, pH 7.4, 100 mm NaCl, and 0.1% Tween 20) and blocked with either 5% bovine serum albumin or nonfat dry milk powder (Bio-Rad) in TTBS for 1 h at room temperature. Blots were then incubated with anti-Grb14 (1:1000), anti-CNGA1 (1:500), anti-PDEγ (1:1000), anti-RGS9-1 (1:1000), anti-Gβ5L and anti-Gβ5S (1:1000), anti-R9AP (1:1000), anti-PDEβ (1:1000), and anti-actin (1:1000) for 1 h at room temperature. Following primary antibody incubations, immunoblots were incubated with HRP-linked secondary antibodies (mouse or rabbit or goat) and developed by enhanced chemiluminescence according to the manufacturer's instructions. The GAP protein antibodies (RGS9-1, Gβ5L, and R9AP) were kindly provided by Dr. Theodore G. Wensel (Baylor College of Medicine, Houston, TX).
Suction-Electrode Recordings
Suction-electrode recordings were made from single mouse rods by methods previously described (13–15). Grb14−/− and WT littermates between 2 and 6 months of age were dark-adapted typically for 5 h but for at least 3 h in a light-tight box. Rods were perfused at 37–39 °C with DMEM (catalogue number D-2902, Sigma) supplemented with 15 mm NaHCO3, 2 mm sodium succinate, 0.5 mm sodium glutamate, 2 mm sodium gluconate, and 5 mm NaCl, bubbled with 5% CO2, pH 7.4. Data were filtered at 30 Hz (8-pole Bessel filter) and sampled at 100 Hz. Flashes of 500-nm light 20 ms in duration were attenuated to different light levels by absorptive neutral density filters. At dim-flash intensities, 10–20 individual responses presented at 5-s intervals were averaged to obtain mean flash responses. At medium flash intensities, 5–10 responses were averaged, and the interflash interval was increased to 10 s. At bright flash intensities above saturation for the rods, only 3–5 responses were averaged, and the interflash interval was increased to 15–20 s. A 500-nm light was also used for steps of light and for bleaching. The amount of bleaching was determined as in previous experiments (16) from the photosensitivity of mouse rods of 5.7 × 10−9 μm2 (17). Other details of response presentation are given in the text and figure legends.
The values of τD for dark-adapted WT and Grb14−/− rods were measured as in Woodruff et al. (13) from a series of five flashes each at seven intensities, chosen for each rod to fall within one-and-a-half log units above the flash intensity that just produced the saturation of the rod response amplitude. Flash intensities were in the range of 159–3250 photons μm−2. The time in saturation (Tsat) was measured as the time from the beginning of the flash to the time at which the mean circulating current recovered to 25% of its dark-adapted value. The value of τD was then calculated rod by rod (see Table 1) or from mean values (see Fig. 5) as the best fitting slope of Tsat versus the natural logarithm of the flash intensity (18). Unless otherwise stated, errors are given as standard errors of the mean. Curve fitting and plotting of data were done with the program Origin (OriginLab, Northampton, MA).
TABLE 1.
Kinetic and sensitivity parameters of rods
All values are means ± S.E. Numbers in parentheses in the first column give the number of rods recorded. Values of rmax (maximum response amplitude) were determined cell by cell from responses to saturating flashes; values of SfD (dark-adapted flash sensitivity) or SF (flash sensitivity of bleached rods) were determined by dividing the peak amplitude of the mean dim-flash response for each cell by the flash intensity; values of ti (the integration time) were determined from the time integral of the mean dim-flash response for each cell divided by the peak amplitude of the response; and values of τREC (response decay constant) were determined by fitting a single exponential decay function to averaged responses of small amplitude (less than 0.3 rmax); and τD (the limiting time constant) for dark-adapted rods as described under “Experimental Procedures.”
| rmax | SfD or SF | ti | τREC | τD | |
|---|---|---|---|---|---|
| Control (23) | 14.2 ± 0.6 | 0.32 ± 0.05 | 316 ± 25 | 201 ± 15 | 174 ± 11 |
| Control bleached (11) | 5.6 ± 0.7 | 0.005 ± 0.001 | 180 ± 30 | 142 ± 36 | |
| Grb14−/− (17) | 14.7 ± 0.7 | 0.31 ± 0.03 | 215 ± 12 | 160 ± 12 | 118 ± 5 |
| Grb14−/− bleached (11) | 6.4 ± 0.8 | 0.010 ± 0.002 | 102 ± 9 | 66 ± 5 |
FIGURE 5.
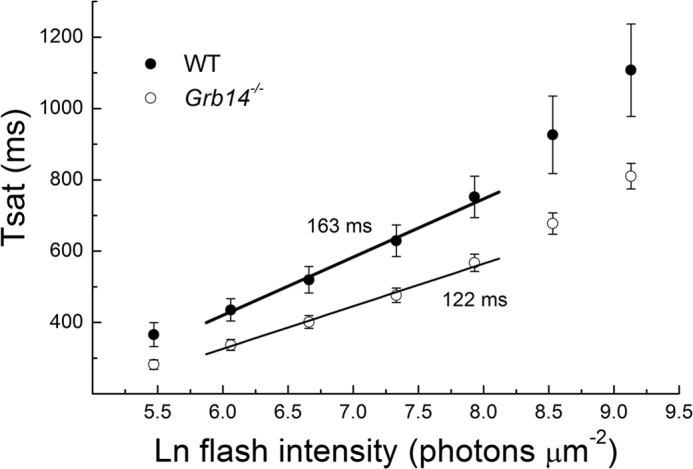
Grb14−/− rods have accelerated limiting time constant, suggesting faster inactivation of PDE6. Time in saturation was measured as time from the beginning of flash to time at which current had recovered to 25% of its dark value (see “Experimental Procedures”). Error bars indicate means and S.E. for 18 WT rods and 15 Grb14−/− rods. The straight lines are best fitting slopes to means, giving values of the limiting time constant τD of 163 ms for WT and 122 ms for Grb14−/− rods. See also Table 1.
RESULTS
In Fig. 1, we show the results of quantitative immunoblot analysis to compare expression levels of Grb14 (panel A), of the rod-channel α subunit (CNGA1, panel B), and of several important transduction proteins for WT rods and rods of Grb14−/− littermates (panels C–G). Densitometric analysis of immunoblots performed in the linear range of detection and normalized to actin (panel H) are plotted in the histograms of Fig. 1I as means with S.E. These results indicate that there were no statistically significant changes (Student's t test) in the expression level of the channels, PDE, or GAP proteins (RGS9-1, Gβ5L, Gβ5S and R9AP) between wild type and Grb1−/− animals.
FIGURE 1.
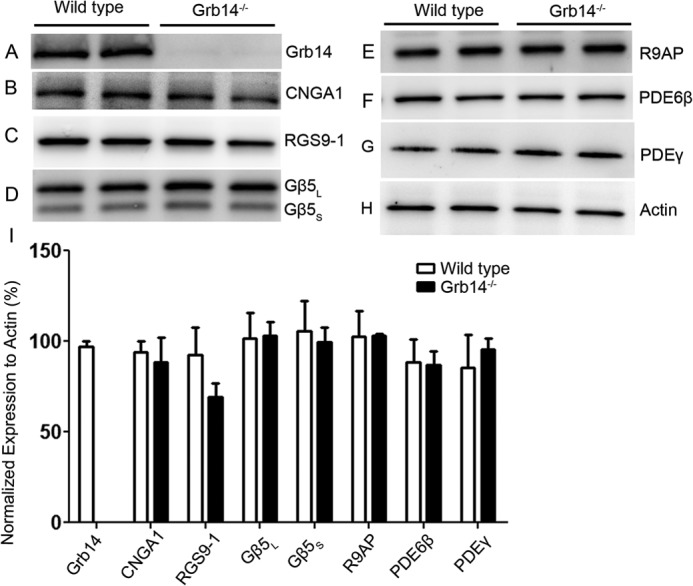
Expression levels of transduction proteins in wild type and Grb14−/− animals. A–H, 10 μg of two independent retinal proteins was subjected to immunoblot analysis with antibodies against Grb14 (A), CNGA1 (B), RGS9-1 (C), Gβ5L (also detects short form Gβ5S) and Gβ5S (D), R9AP (E), PDE6β (F), PDEγ (G), and actin (H). I, densitometric analysis of four independent immunoblots was performed in the linear range of detection, and absolute values were then normalized to actin. Values are mean ± S.E., (n = 4). The wild type was set as 100%. Student's t test was used to show that there were no significant differences in the expression level of transduction proteins between wild type and Grb14−/− animals.
In Fig. 2, we compare responses to a graded series of flashes of dark-adapted WT (Fig. 2A) and Grb14−/− littermates (Fig. 2C). Deletion of the Grb14 gene produced a clear acceleration of the decay of the response. In Table 1, we give mean values with standard errors for the integration time, calculated cell by cell from the time integral of the mean dim-flash response divided by the peak amplitude of the response, and the exponential decay time constant of the dim-flash response, τREC (see for example Ref. 15). Both the integration time and τREC are smaller, and these differences were statistically significant (Student's t test, p < 0.05).
FIGURE 2.
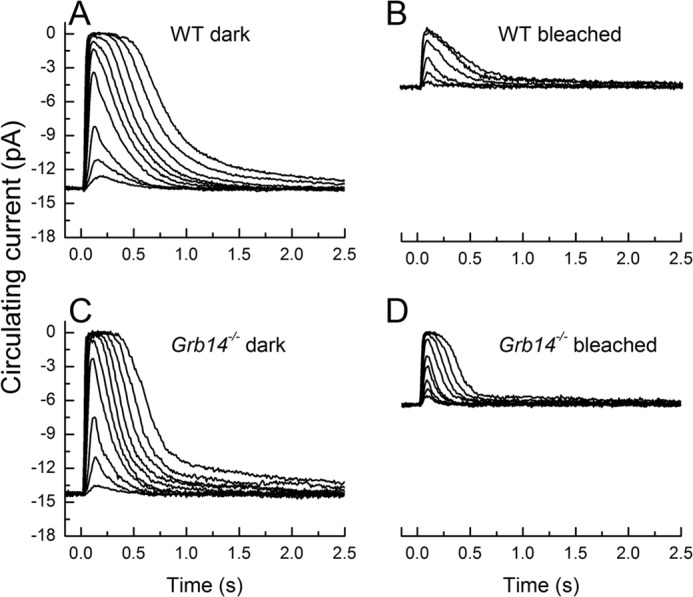
Rod responses to 20-ms flashes of 500-nm light for Grb14−/− and control rods under dark-adapted and bleach-adapted conditions. A, dark-adapted WT strain control at light intensities of 2.4, 8.0, 21, 70, 122, 220, 403, 733, 1430, and 2600 photons μm−2. Traces are mean responses from 22 rods. B, responses of WT rods after 50% bleach. Light intensities were 70, 220, 733, 2600, 8620, and 22,700 photons μm−2. Traces are mean responses from 11 rods. C, flash responses for dark-adapted Grb14−/− at the same light intensities used for the dark-adapted WT rods. The traces are the mean of 10 rods. D, Grb14−/− flash responses after 50% bleach at light intensities of 70, 122, 220, 403, 733, 1430, 2600, 4730, and 8620 photons μm−2. Traces are the mean responses from 10 rods. Data in this and subsequent figures were acquired at 100 Hz and Bessel-filtered at 35 Hz.
The acceleration in the rate of decay of the flash response is consistent with previous experiments indicating that rod cGMP-gated channels may be more sensitive to cGMP or open at a lower concentration of cGMP in Grb14−/− mice (10). Responses would decay faster because, during the recovery of cGMP concentration after illumination, the channels would begin to reopen at a lower cGMP concentration. Previous experiments have also indicated, however, that Grb14 translocates from the inner segment of the rod to the outer segment during bright illumination (6). We were therefore surprised to see such a large effect of deletion of the Grb14 gene on the decay time of the response even in dark-adapted rods.
To investigate the effect of light exposure, we illuminated rods with 500-nm light at an intensity of 3.6 × 105 photons μm2 s−1 for 335 s, sufficient to bleach ∼50% of the rhodopsin (see “Experimental Procedures” and Ref. 16). After waiting 45 min to 1 h for the circulating current and sensitivity of the rod to come to steady state and for any protein translocation to occur, we recorded rod responses from WT (Fig. 2B) and Grb14−/− mice (Fig. 2D). Responses in WT rods decayed more rapidly after bleaching, as we have previously documented (16). A similar effect was observed for Grb14−/− rods. The results in Table 1 verify that the integration time and τREC were shorter for bleached rods than dark-adapted rods in both genotypes (p < 0.05), and both the sensitivity and the maximum response amplitude (rmax) were also significantly smaller. Moreover, deletion of the Grb14 gene produced an acceleration of the integration time constant and τREC in bleached rods similar to the effect of gene deletion on dark-adapted rods, and these differences were again significant (p < 0.05).
In Fig. 3, we show averaged waveforms from WT and Grb14−/− rods in the dark and after bleaching. In Fig. 3, A and B, we show responses to flashes of the same intensity of 730 photons μm−2. Panel A gives actual changes in circulating current, and panel B gives responses normalized rod by rod to the peak amplitude of the response for that rod. A similar comparison is given in panels C and D for flashes of intensity 220 photons μm−2. These results show the progressive change in the time course of decay caused by deletion of Grb14 and light adaptation after pigment bleaching. The decay of responses of bleached Grb14−/− rods is surprisingly rapid; it is nearly as fast as from mouse rods with 4–6 times overexpressed GAP proteins (19, 20).
FIGURE 3.
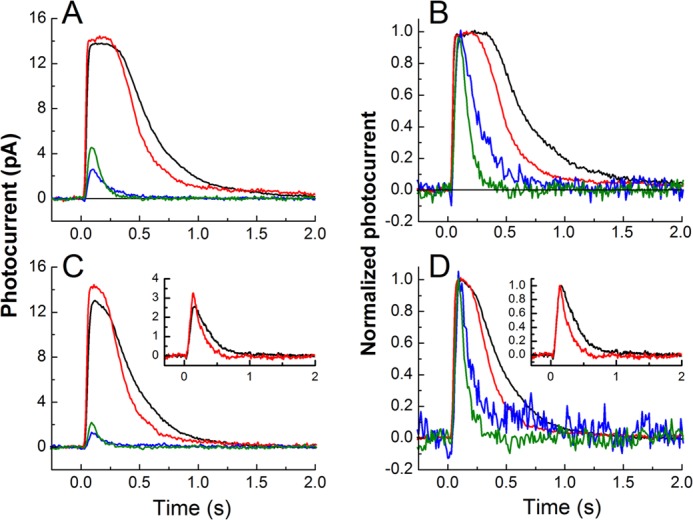
The Grb14−/− and bleached rods have a more rapid rate of response recovery. A, superimposed means of responses to flash intensity of 730 photons μm−2 for dark-adapted WT (black), dark-adapted Grb14−/− (red), bleached-adapted WT (blue), and bleach-adapted Grb14−/− (green). B, the same traces as in A, normalized to show the kinetic difference in the recovery phases of the responses. C, as in A but superimposed means are for flashes of intensity 220 photons μm−2. The inset shows superimposed means for dark-adapted WT (black) and dark-adapted Grb14−/− (red) at flash intensity of 8 photons μm−2. D, same traces as in C, normalized to show the kinetic difference in the recovery phases of the responses.
In the insets of Fig. 3, C and D, we give superimposed responses for dark-adapted WT (black) and Grb14−/− (red) rods at the even dimmer flash intensity of 8 photons μm−2. Responses to bleach-adapted rods at this intensity are not shown because they were too small to be seen above the noise of the recording. The dim-flash responses in the insets to Fig. 3 rise with a similar time course, indicating that deletion of Grb14 had no effect on response activation. To confirm this observation, we calculated the rate of rise by fitting the initial time course of the flash response with a function of the form
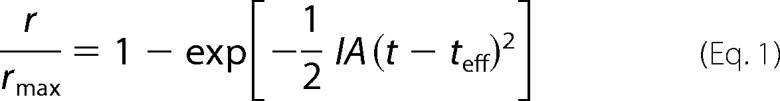 |
where r/rmax is the normalized flash response, I is the flash intensity in photoisomerizations from a collecting area of 0.5 μm2 (21, 22), A is the amplification constant, t is time, and teff is the effective delay time of transduction (23). We estimated best fitting values of the amplification constants with the program Origin for responses to flashes of an intensity of 21 photons μm−2 for WT and Grb14−/− rods and found no significant difference: WT 23.0 ± 3.8 s−2, n = 15; Grb14−/− 23.3 ± 2.8 s−2, n = 10. These values were not significantly different and were similar to those we have previously published for mouse rods (24). The mean values of teff were also similar (WT 29 ms, Grb14−/− 28 ms).
In Fig. 4, we show response-intensity curves for dark-adapted WT rods (●), dark-adapted Grb14−/− rods (■), bleached WT rods (○), and bleached Grb14−/− rods (□). Means and standard errors were calculated and have been plotted for the same cells used for Fig. 2 and Table 1. Bleaching decreased maximum response amplitude and sensitivity in WT and Grb14−/− rods, both by reducing the amount of pigment available to absorb photons and by producing adaptation similar to that produced by background light (16). The effect of bleaching was similar for rods of the two genetic backgrounds. There were small differences in maximum response amplitudes between WT and Grb14−/− rods, indicative of a difference in the circulating currents, but these differences were not statistically significant.
FIGURE 4.
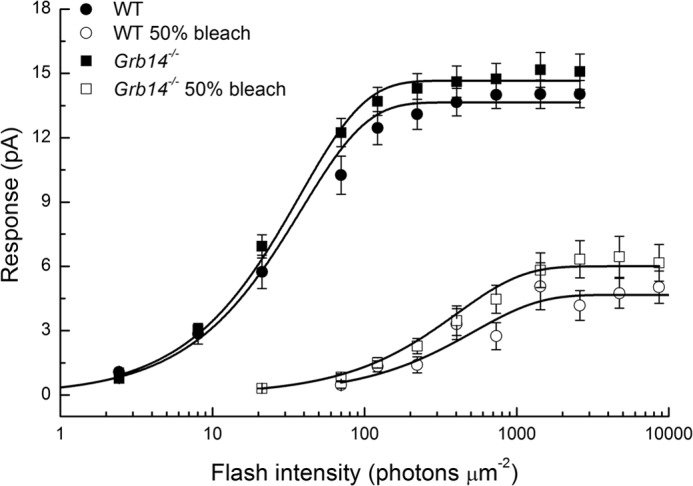
Response as a function of flash intensity Grb14−/− and WT rods under dark-adapted and bleach-adapted conditions. Data are from the rods of Fig. 2. Error bars give standard errors of the mean. Means have been fitted with exponential saturation function, r = rmax (1 − exp(−kI)), where r is the amplitude of the response, rmax is the maximum value of the response, I is the flash intensity, and k is a constant. Best fitting values were as follows: WT dark-adapted, rmax = 13.7, k = 0.026; Grb14−/− dark-adapted, rmax = 14.7, k = 0.027; WT bleach-adapted, rmax = 4.7, k = 0.0019; and Grb14−/− bleach-adapted, rmax = 6.0, k = 0.0025.
Although the small effect on rod circulating current was unexpected, the other results we have so far presented seem consistent at least in part with previous data indicating an effect of Grb14 on cGMP-gated channels (10). Rod cGMP-gated channels are also known to be modulated by Ca2+-calmodulin (25–28), and deletion of the channel Ca2+-calmodulin binding site also accelerates the decay of the rod response (14). The acceleration of decay in Grb14−/− rods was, however, greater than in rods lacking the Ca2+-calmodulin binding site, and we therefore wondered whether the effect of deleting the Grb14 gene could affect some other aspect of the physiology of the photoreceptor. Rod response decay can also be accelerated by an increase in the rate of inactivation of the rod effector enzyme, cyclic nucleotide PDE6. Previous experiments have shown that in mouse rods, the rate of PDE6 inactivation can be estimated from measurements of the limiting time constant τD (15, 19, 20). We therefore compared the values of limiting time constants in WT and Grb14−/− littermates.
In Fig. 5, we show mean values of the time in saturation (Tsat) as a function of the natural logarithm of the flash intensity for both WT rods (●) and Grb14−/− rods (○). The value of Tsat was plotted as the time required for the recovery of 25% of the dark circulating current after the beginning of the flash (see “Experimental Procedures”). The values of Tsat were uniformly smaller for Grb14−/− rods than for WT rods at any given light intensity, reflecting the more rapid recovery of Grb14−/− rods. A similar phenomenon was seen previously for rods whose channel Ca2+-calmodulin binding site had been disrupted (14). The straight lines in the figures give the best fitting slopes, which provide estimates of the limiting time constants τD. We have fitted only those regions of each of the two curves that are most nearly linear, avoiding the change in slope at higher intensities likely due to the finite concentration of PDE6 in each disk compartment (29). The value of τD is about 25% smaller in Grb14−/− rods.
In Table 1, we give values of τD, averaged from determinations made for individual rods. The values are similar to those estimated from the best fits to the means of Tsat in Fig. 4 and are significantly different (Student's t test, p < 0.05). We were unable to make a similar comparison for bleached rods, both because responses were smaller and Tsat measurements accordingly more difficult and because the large desensitization of the rod produced by bleaching (Fig. 3) had the effect that the range of light intensities over which Tsat measurements could be made extended into the range where values of Tsat become nonlinear with light intensity (29).
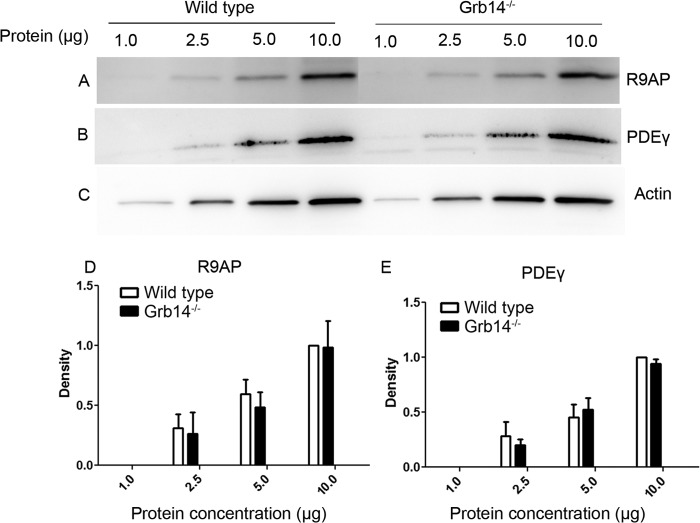
Previous experiments have shown that the rate of decay of mouse rod responses can also be accelerated by increased expression of either PDEγ (30) or GAP proteins (19, 20). Although the data in Fig. 1 indicate that neither PDEγ nor any of the GAP proteins is overexpressed in Grb14−/− rods, we nevertheless reinvestigated this question in the experiment of Fig. 6. Here we present a concentration series of protein expression for R9AP, PDEγ, and actin. These data confirm our view that protein overexpression is not responsible for the kinetic changes we have observed.
FIGURE 6.
Concentration series of R9AP and PDEγ expression in retinal homogenates of wild type and Grb14−/− animals. A–C, for each line, 1.0, 2.5, 5.0, and 10.0 μg of protein was loaded and subjected to immunoblot analysis with antibodies against R9AP (A), PDEγ (B), and actin (C). The blots shown are representative of three retinas examined from wild type and Grb14−/− animals. D and E, representative graphs quantifying the expression of R9AP (D) and PDEγ (E) in retinal homogenates of WT and Grb14−/− animals. For each line, 1.0, 2.5, 5.0, and 10.0 μg of protein was loaded. Densitometric analysis of the R9AP and PDEγ immunoblots was performed in the linear range of detection and plotted as values of band intensity versus protein concentration. Data are mean and S.E. for three wild type and three Grb14−/− animals. Ten microgram protein concentration of wild type was set as 1.0. Student's t test was used to show that there were no significant differences in the expression level of R9AP and PDEγ at different concentrations between wild type and Grb14−/− animals.
DISCUSSION
Our experiments show that deletion of the Grb14 gene produces a clear effect on the response properties of mouse rods. Responses decay more rapidly, consistent with previous experiments indicating an effect of gene deletion on the sensitivity of rod outer segment channels to cGMP (10). As the cGMP concentration in the rod recovers after a flash, channels in Grb14−/− rods open more rapidly and speed the increase in current back to base line.
Some of our findings are, however, at variance with previous results. Our previous experiments (6) found that Grb14 was nearly undetectable in dark-adapted rod outer segments either with immunohistochemistry or with cryosectioning, although subsequent experiments indicated that a small concentration of the protein may be present (10); the protein then translocates and can be found at much higher concentration after bright light exposure. We were therefore surprised to discover a large effect of deletion of the Grb14 gene on response decay even in dark-adapted photoreceptors. One possible explanation of this result is that Grb14 is present in dark-adapted rod outer segments but at too low a concentration to be detected by the methods used in previous experiments (6). Although deletion of the Grb14 gene accelerated response decay in both dark-adapted and bleached rods, the results in Table 1 show that the effect was greater in bleached rods. In the case of τREC, for example, deletion of the Grb14 gene in dark-adapted rods reduced the mean value from 201 to 160 ms, for a decrease of 20%. In bleach-adapted rods, on the other hand, the mean value fell from 142 to 66 ms, a decrease of 54%. The decreases in integration times were 32% for dark-adapted rods and 43% for bleach-adapted rods. It is possible that deletion of the Grb14 gene has a larger effect on bleached rods because the concentration of Grb14 is greater in the outer segments of WT rods that have been exposed to bright light (6).
Our experiments also indicate that the deletion of Grb14 shortens the time of rod response saturation (Tsat) and the limiting time constant τD (Fig. 5). Calculations based on the model of Gross et al. (31) show that an increase in channel affinity should shorten Tsat but should have no effect on τD.3 The effect on τD suggests instead that Grb14 in WT rods may normally prolong the decay of light-activated PDE6. An effect on PDE decay could also explain most of the other effects we have observed in Grb14 deletion rods, including the acceleration of the exponential time constant of decay (τREC) and Tsat. Moreover, an effect on PDE decay might explain why rod responses were accelerated even in dark-adapted rods and why there was little difference in dark current between WT and Grb14−/− rods, a result that seems inconsistent with channel modulation.
We cannot exclude the possibility that deletion of Grb14 is having its principal or even its exclusive effect on PDE kinetics rather than on channel affinity. We have recently demonstrated that phosphorylated Grb14 is a competitive inhibitor of the insulin receptor phosphatase, protein-tyrosine phosphatase 1B (8). It is tempting to speculate that Grb14 may regulate the phosphorylation or dephosphorylation of PDE itself or some other transduction protein, which then alters PDE function. Although there is now considerable evidence for modulation of PDE6 (see Ref.32), this evidence is for acceleration of PDE6 decay and not for its prolongation, and it is unclear how an effect of Grb14 can be reconciled with other evidence for light-dependent regulation of PDE6 recovery. Further studies are required to examine the possible interaction and effect of Grb14 on PDE6 activity.
Previous experiments have indicated that Grb14 interacts with the insulin receptor in rod outer segments (6). The experiments we report in this study do not yet permit us to unravel the various mechanisms of activation of Grb14, the insulin receptor, or the tyrosine phosphatase protein-tyrosine phosphatase 1B in the physiology of the rod. We plan additional experiments with animals whose rods specifically lack these other proteins, as well as from mice with rods having modified cGMP-gated channels, to shed more light on these intriguing phenomena and their role in the physiology of vision.
Acknowledgments
We are grateful to Dustin T. Allen for expert assistance in breeding and maintaining the animals used in this research and to Daniel Tranchina (New York University) for doing model calculations on the effects of increasing the affinity of rod channels to cGMP. Grb14−/− mice were kindly provided Dr. Roger Daly, Garvan Institute of Medical Research, Darlinghurst, Australia. We thank Dr. Robert S. Molday (University of British Columbia, Vancouver, Canada) for providing channel antibodies and Dr. Theodore G. Wensel (Baylor College of Medicine, Houston, TX) for providing antibodies against RGS9-1, Gβ5, and R9AP.
This work was supported, in whole or in part, by National Institutes of Health/NEI Grants EY016507 and EY00871 (to R. V. S. R.) and EY01844 (to G. L. F.).
D. Tranchina, personal communication.
- Grb14
- growth factor receptor-bound protein 14
- PDE
- phosphodiesterase
- GAP
- GTPase-accelerating protein.
REFERENCES
- 1. Cariou B., Bereziat V., Moncoq K., Kasus-Jacobi A., Perdereau D., Le Marcis V., Burnol A. F. (2004) Regulation and functional roles of Grb14. Front Biosci. 9, 1626–1636 [DOI] [PubMed] [Google Scholar]
- 2. Daly R. J. (1998) The Grb7 family of signalling proteins. Cell. Signal. 10, 613–618 [DOI] [PubMed] [Google Scholar]
- 3. Cooney G. J., Lyons R. J., Crew A. J., Jensen T. E., Molero J. C., Mitchell C. J., Biden T. J., Ormandy C. J., James D. E., Daly R. J. (2004) Improved glucose homeostasis and enhanced insulin signalling in Grb14-deficient mice. EMBO J. 23, 582–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desbuquois B., Carré N., Burnol A. F. (2013) Regulation of insulin and type 1 insulin-like growth factor signaling and action by the Grb10/14 and SH2B1/B2 adaptor proteins. FEBS J. 280, 794–816 [DOI] [PubMed] [Google Scholar]
- 5. Lin R. C., Weeks K. L., Gao X. M., Williams R. B., Bernardo B. C., Kiriazis H., Matthews V. B., Woodcock E. A., Bouwman R. D., Mollica J. P., Speirs H. J., Dawes I. W., Daly R. J., Shioi T., Izumo S., Febbraio M. A., Du X. J., McMullen J. R. (2010) PI3K(p110α) protects against myocardial infarction-induced heart failure: identification of PI3K-regulated miRNA and mRNA. Arterioscler. Thromb. Vasc. Biol. 30, 724–732 [DOI] [PubMed] [Google Scholar]
- 6. Rajala A., Daly R. J., Tanito M., Allen D. T., Holt L. J., Lobanova E. S., Arshavsky V. Y., Rajala R. V. (2009) Growth factor receptor-bound protein 14 undergoes light-dependent intracellular translocation in rod photoreceptors: functional role in retinal insulin receptor activation. Biochemistry 48, 5563–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rajala R. V., Chan M. D. (2005) Identification of a NPXY motif in growth factor receptor-bound protein 14 (Grb14) and its interaction with the phosphotyrosine-binding (PTB) domain of IRS-1. Biochemistry 44, 7929–7935 [DOI] [PubMed] [Google Scholar]
- 8. Basavarajappa D. K., Gupta V. K., Dighe R., Rajala A., Rajala R. V. (2011) Phosphorylated Grb14 is an endogenous inhibitor of retinal protein tyrosine phosphatase 1B, and light-dependent activation of Src phosphorylates Grb14. Mol. Cell. Biol. 31, 3975–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajala R. V., Tanito M., Neel B. G., Rajala A. (2010) Enhanced retinal insulin receptor-activated neuroprotective survival signal in mice lacking the protein-tyrosine phosphatase-1B gene. J. Biol. Chem. 285, 8894–8904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta V. K., Rajala A., Daly R. J., Rajala R. V. (2010) Growth factor receptor-bound protein 14: a new modulator of photoreceptor-specific cyclic-nucleotide-gated channel. EMBO Rep. 11, 861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rajala R. V., Woodruff M., Fain G. (2013) Modulation of mouse rod cGMP-gated channels by Grb14. Invest. Ophthalmol. Vis. Sci. 54, E-Abstract 2459 [Google Scholar]
- 12. Li G., Anderson R. E., Tomita H., Adler R., Liu X., Zack D. J., Rajala R. V. (2007) Nonredundant role of Akt2 for neuroprotection of rod photoreceptor cells from light-induced cell death. J. Neurosci. 27, 203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woodruff M. L., Janisch K. M., Peshenko I. V., Dizhoor A. M., Tsang S. H., Fain G. L. (2008) Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J. Neurosci. 28, 2064–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen J., Woodruff M. L., Wang T., Concepcion F. A., Tranchina D., Fain G. L. (2010) Channel modulation and the mechanism of light adaptation in mouse rods. J. Neurosci. 30, 16232–16240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen C. K., Woodruff M. L., Chen F. S., Chen Y., Cilluffo M. C., Tranchina D., Fain G. L. (2012) Modulation of mouse rod response decay by rhodopsin kinase and recoverin. J. Neurosci. 32, 15998–16006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nymark S., Frederiksen R., Woodruff M. L., Cornwall M. C., Fain G. L. (2012) Bleaching of mouse rods: microspectrophotometry and suction-electrode recording. J. Physiol. 590, 2353–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Woodruff M. L., Lem J., Fain G. L. (2004) Early receptor current of wild-type and transducin knockout mice: photosensitivity and light-induced Ca2+ release. J. Physiol. 557, 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pepperberg D. R., Cornwall M. C., Kahlert M., Hofmann K. P., Jin J., Jones G. J., Ripps H. (1992) Light-dependent delay in the falling phase of the retinal rod photoresponse. Vis. Neurosci. 8, 9–18 [DOI] [PubMed] [Google Scholar]
- 19. Chen C. K., Woodruff M. L., Chen F. S., Chen D., Fain G. L. (2010) Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods. J. Neurosci. 30, 1213–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krispel C. M., Chen D., Melling N., Chen Y. J., Martemyanov K. A., Quillinan N., Arshavsky V. Y., Wensel T. G., Chen C. K., Burns M. E. (2006) RGS expression rate-limits recovery of rod photoresponses. Neuron 51, 409–416 [DOI] [PubMed] [Google Scholar]
- 21. Field G. D., Rieke F. (2002) Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron 35, 733–747 [DOI] [PubMed] [Google Scholar]
- 22. Field G. D., Rieke F. (2002) Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron 34, 773–785 [DOI] [PubMed] [Google Scholar]
- 23. Pugh E. N., Jr., Lamb T. D. (1993) Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta 1141, 111–149 [DOI] [PubMed] [Google Scholar]
- 24. Chen C. K., Woodruff M. L., Chen F. S., Shim H., Cilluffo M. C., Fain G. (2010) Replacing the rod with the cone transducin α subunit decreases sensitivity and accelerates response decay. J. Physiol. 588, 3231–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gordon S. E., Downing-Park J., Zimmerman A. L. (1995) Modulation of the cGMP-gated ion channel in frog rods by calmodulin and an endogenous inhibitory factor. J. Physiol. 486, 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsu Y. T., Molday R. S. (1993) Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361, 76–79; Correction (1993) Nature365, 279 [DOI] [PubMed] [Google Scholar]
- 27. Koutalos Y., Nakatani K., Yau K. W. (1995) The cGMP-phosphodiesterase and its contribution to sensitivity regulation in retinal rods. J. Gen. Physiol. 106, 891–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakatani K., Koutalos Y., Yau K. W. (1995) Ca2+ modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J. Physiol. 484, 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martemyanov K. A., Krispel C. M., Lishko P. V., Burns M. E., Arshavsky V. Y. (2008) Functional comparison of RGS9 splice isoforms in a living cell. Proc. Natl. Acad. Sci. U.S.A. 105, 20988–20993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsang S. H., Woodruff M. L., Chen C. K., Yamashita C. Y., Cilluffo M. C., Rao A. L., Farber D. B., Fain G. L. (2006) GAP-independent termination of photoreceptor light response by excess γ subunit of the c-GMP-phosphodiesterase. J. Neurosci. 26, 4472–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gross O. P., Pugh E. N., Jr., Burns M. E. (2012) Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron 76, 370–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fain G. L. (2011) Adaptation of mammalian photoreceptors to background light: putative role for direct modulation of phosphodiesterase. Mol. Neurobiol. 44, 374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]