Abstract
This study investigated whether KMUP-1, a xanthine-derivative K+ channel opener, could prevent serotonin-induced hypertrophy in H9c2 cardiomyocytes via L-type Ca2+ channels (LTCCs). Rat heart-derived H9c2 cells were incubated with serotonin (10 μM) for 4 days. The cell size increased by 155.5%, and this was reversed by KMUP-1 (≥1 μM), and attenuated by the LTCC blocker verapamil (1 μM) and the 5-HT2A antagonist ketanserin (0.1 μM), but unaffected by the 5-HT2B antagonist SB206553. A perforated whole-cell patch-clamp technique was used to investigate Ca2+ currents through LTCCs in serotonin-induced H9c2 hypertrophy, in which cell capacitance and current density were increased. The LTCC current (ICa,L) increased ~2.9-fold in serotonin-elicited H9c2 hypertrophy, which was attenuated by verapamil and ketanserin, but not affected by SB206553 (0.1 μM). Serotonin-increased ICa,L was reduced by KMUP-1, PKA and PKC inhibitors (H-89, 1 μM and chelerythrine, 1 μM) while the current was enhanced by the PKC activator PMA, (1 μM) but not the PKA activator 8-Br-cAMP (100 μM), and was abolished by KMUP-1. In contrast, serotonin-increased ICa,L was blunted by the PKG activator 8-Br-cGMP (100 μM), but unaffected by the PKG inhibitor KT5823 (1 μM). Notably, KMUP-1 blocked serotonin-increased ICa,L but this was partially reversed by KT5823. In conclusion, serotonin-increased ICa,L could be due to activated 5-HT2A receptor-mediated PKA and PKC cascades, and/or indirect interaction with PKG. KMUP-1 prevents serotonin-induced H9c2 cardiomyocyte hypertrophy, which can be attributed to its PKA and PKC inhibition, and/or PKG stimulation.
Keywords: Serotonin, H9c2 cardiomyocyte-like cell line, perforated whole-cell patch-clamp technique, L-type Ca2+ channels, protein kinases, cardiac hypertrophy
Introduction
Cardiac ventricular hypertrophy is a hallmark of progressive heart failure. Increased myocardial mass characteristic of the compensatory ventricular remodeling process is mainly attributed to hypertrophy of individual cardiomyocytes 1. This process is initiated by a variety of stimuli, including paracrine and endocrine factors such as catecholamines, angiotensin II, endothelin, growth factors and inflammatory cytokines 2. Additionally, serotonin (5-hydroxytryptamine, 5-HT) is believed to play a role in the regulation of cardiac growth in pathological conditions. Previous reports showed higher blood 5-HT levels in heart failure patients, which may be due to either clearance defect or enhanced secretion 3, 4. Moreover, chronic treatment of 5-HT in rodents causes cardiac hypertrophy as well 5, 6.
5-HT receptors are widely expressed in the cardiovascular system. In the heart, converging evidence shows that 5-HT2A receptor expression and function are greatly altered during cardiac remodeling, supporting its role in the development of cardiac hypertrophy and failure. In rodent models of heart failure, a novel ventricular inotropic responsiveness to 5-HT appears, which is mediated in part through the 5-HT2A receptor 7. In addition, the 5-HT2A receptor is upregulated in human heart failure 7 and 5-HT2A receptor blockade reverses hypertrophy during pressure overload in a mouse model of increased 5-HT levels 8. 5-HT2A receptors trigger a hypertrophic response by 5-HT stimulation, through the involvement of transient receptor potential canonical 1 (TRPC1) channels 9 and calcineurin/NFAT activation 1.
5-HT has even been shown to increase the magnitude of the L-type Ca2+ current (ICa,L) in human atrial myocytes via 5-HT4 receptors 10, which may contribute to intracellular calcium overload and arrhythmic activity. The 5-HT4 receptor is functionally present in the human atrium but not in the ventricle 11. The present study used the H9c2 cell line, initiated by Kimes and Brandt (1976), established from spontaneous beating embryonic rat cardiac ventricle. Kimes and Brandt addressed that the beating activity ceased after the third “selective serial passage” 12. This H9c2 cell line has properties similar to neonatal and adult cardiomyocytes 13, 14. After undergoing differentiation, these cells can functionally express L-type Ca2+ channels (LTCCs) and ATP-sensitive K+ channels 13, 15. Until now, the possible mechanism of serotonin-increased ICa,L has never been well described in H9c2 cells. We designed this study to determine whether protein kinases (i.e. PKA, PKC and PKG) are involved in the cascade of serotonin-induced rat heart-derived H9c2 cell hypertrophy model, using functional and electrophysiological studies.
A xanthine-derivative KMUP-1 has been demonstrated to increase cyclic nucleotides and stimulate K+ channels, resulting in relaxation in aortic, corporeal cavernosa and tracheal smooth muscles 16-18, and to activate BKCa channels 19 and inhibit LTCCs 20 in rat basilar artery myocytes. KMUP-1 was found to inhibit monocrotaline-induced chronic pulmonary arterial hypertension via cGMP-dependent inhibition of ROCK and modulation of K+ channels 21. It also attenuates isoprenaline-induced cardiac hypertrophy through the NO/cGMP/PKG pathway 23. We have proved that it might have value in the management of cerebral vasospasm after subarachnoid hemorrhage 24. However, it is not known whether KMUP-1 can modulate the influx of Ca2+ through LTCCs in H9c2 cardiomyocytes. The main objectives of this study were to characterize the role of protein kinases in serotonin-induced H9c2 cell hypertrophy and to elucidate the mechanisms by which KMUP-1 prevents 5-HT-induced ventricular myocyte hypertrophy via LTCCs mediated signaling pathways.
Materials and methods
Cell culture
The H9c2 cardiomyocyte-like cell line (ATCC CLR-1446; Rockville, MD) derived from rat embryonic myoblasts is commonly used as an in vitro model of cardiomyocyte biology 12, 14 since it shows similar hypertrophic and apoptotic responses as those seen in primary adult and neonatal cardiomyocytes 25. Cells were plated onto collagen-coated culture dishes and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a humidified atmosphere of 5% CO2 and 95% air at 37oC. Cells were used under the 20th passage.
Serotonin-induced cell hypertrophy
H9c2 cells were performed under serum-free medium (DMEM with 0.1% FBS) and plated onto 6-cm dishes and stimulated with serotonin (1, 10 μM) for 4 days to measure their changes in cell surface area. Cell images were viewed with a digital camera fixed to a microscope (Nikon). The H9c2 cell surface area was analyzed for at least 30 cells per condition using the public ImageJ 1.47 software. To understand the mechanism of serotonin-induced H9c2 hypertrophy, the cells were exposed to the LTCC blocker verapamil (1 μM), the 5-HT2A antagonist ketanserin (0.1 μM) and the 5-HT2B antagonist SB206553 (0.1 μM) for 4 days. KMUP-1 was also applied in this experiment to observe its preventive effects on serotonin-induced H9c2 hypertrophy.
Patch-clamp electrophysiology
To measure the ICa through L-type Ca2+ channels 20, 26, perforated whole-cell patch-clamp electrophysiology was used in rat heart-derived H9c2 cells. In brief, H9c2 cells treated with or without serotonin were detached with 0.25% trypsin-0.02% EDTA solution, the supernatant removed by centrifugation, and the pellets resuspended in 1 ml of bath solution containing (in mM): 135 tetraethylammonium (TEA)-Cl, 1.8 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES, (pH 7.4, Tris) using a fire polished glass pipette. The cells were put in a recording dish and then perfused with a bath solution. To minimize outward potassium currents, Cs+ rather than K+ was used in the pipette solution. A recording electrode was pulled from borosilicate glass (resistance: 3-5 MΩ), and the pipette was coated with sticky wax close to the tip to reduce capacitance and backfilled with pipette solution containing (in mM) 140 CsCl, 1 EGTA, 1 MgCl2, 5 Na2ATP, and 5 HEPES (pH 7.2, Tris). Nystatin (300 μg/ml) was included in the pipette solution for the perforated patch-clamp recordings. Serotonin-treated cells were clamped at -80 mV and depolarized to 0 mV for 300 ms to evoke whole-cell ICa. Voltage clamped cells were equilibrated for 15 min prior to experimentation. Membrane currents were recorded on the MultiClamp 700B amplifier (Molecular Devices Corporation, Sunnyvale, CA), filtered at 1 kHz using a low-pass Bessel filter, digitized at 5 kHz and stored on a computer for subsequent analysis with Clampfit 10.2. A 1 M NaCl-agar salt bridge between the bath and the Ag-AgCl reference electrode was used to minimize offset potentials. All electrical recordings were performed at room temperature. Following equilibration, serotonin-increased ICa was monitored in the presence and absence of KMUP-1 (0.1, 1, 10 μM). To ascertain whether PKA, PKC or PKG signaling was involved in the KMUP-1-inhibited ICa in serotonin (10 μM)-treated H9c2 cells, the activators and inhibitors of PKA (8-Br-cAMP, 100 μM and H89, 1 μM), PKC (phorbol 12-myristate 13-acetate (PMA), 100 μM and chelerythrine, 1 μM) and PKG (8-Br-cGMP and 100 μM, KT5823, 1 μM) were added in perfusate for this purpose.
Chemicals
Buffer reagents, chelerythrine, H-89, PMA, serotonin, TEA-Cl and verapamil were obtained from Sigma Aldrich Chemical Co. (St. Louis, MO). 8-Br-cAMP, 8-Br-cGMP, KT5823, ketanserin and SB206553 were obtained from Tocris Bioscience (Bristol, UK). All drugs and reagents were dissolved in distilled water unless otherwise noted. Chelerythrine, KT5823 and PMA were dissolved in DMSO at 10 mM. Serial dilutions were made in phosphate buffer solution with a final solvent concentration <0.01%.
Data analysis and statistics
Data were expressed as means ± SE; n indicates the number of cells. A repeated measure ANOVA was used to compare values at a given voltage. When appropriate, a Tukey-Kramer pairwise comparison was used for post hoc analysis. p values ≤0.05 were considered statistically significant.
Results
Effects on serotonin-induced H9c2 cells hypertrophy
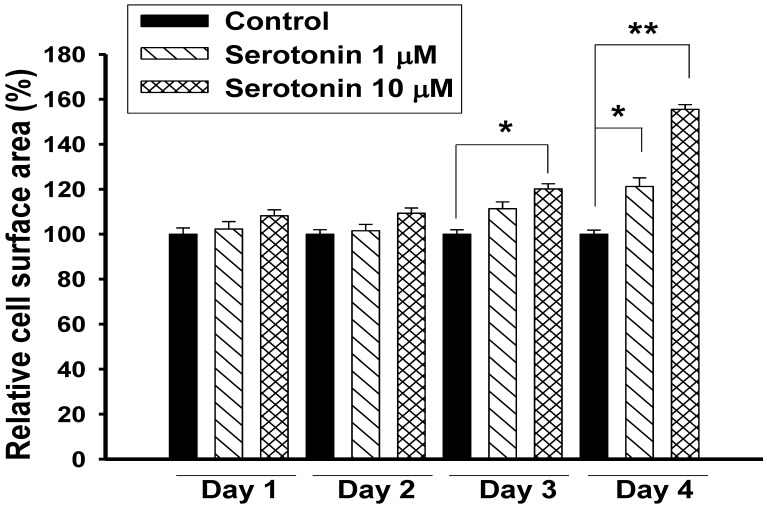
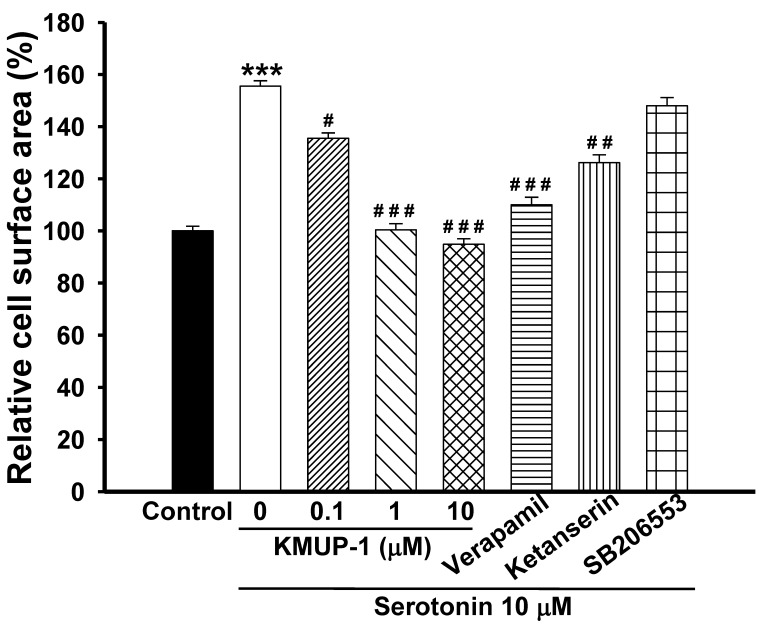
Exposure of H9c2 cardiomyocytes to serotonin (1, 10 μM) caused time-dependent increases in cell surface area, an indicator of cell hypertrophy, and produced a marked hypertrophic response at day 4 (Fig. 1). Serotonin-induced H9c2 cell hypertrophy was significantly reversed by co-incubation with KMUP-1 (1-10 μM). Under the same situation, the LTCC blocker verapamil (1 μM) and the 5-HT2A antagonist ketanserin (0.1 μM) also significantly attenuated the hypertrophic responses induced by serotonin, but not the 5-HT2B antagonist SB206553 (0.1 μM) (Fig. 2).
Figure 1.
Effects of serotonin elicited H9c2 cardiomyocytes hypertrophy. H9c2 cells were cultured in serum-free medium and treated with 1 or 10 μM serotonin for 1-4 days. The cell surface areas (≥30 cells) were analyzed in each condition using ImageJ 1.47 software. Data are means ± SE, n=6. *P<0.05, **P<0.01 compared with control.
Figure 2.
KMUP-1 prevented serotonin-induced H9c2 cardiomyocyte hypertrophy. H9c2 cells were cultured in serum-free medium and treated with 10 μM serotonin, 10 μM serotonin+0.1-10 μM KMUP-1, 10 μM serotonin+1 μM verapamil, 10 μM serotonin+0.1 μM ketanserin or10 μM serotonin+0.1 μM SB206553 for 4 days. The cell surface areas (≥30 cells) were analyzed in each condition using ImageJ 1.47 software. Data are means ± SE, n=6. ** P<0.01 compared with control. ## P<0.01 compared with serotonin group.
Cell capacitance and current density in serotonin-induced hypertrophic cells
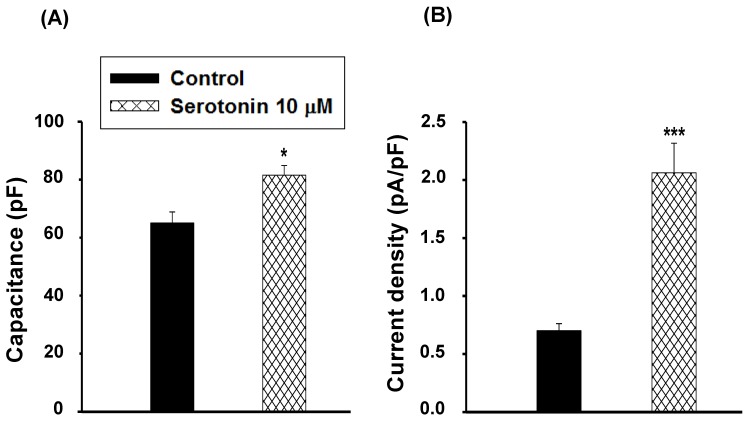
The cell capacitance in serotonin-induced H9c2 cell hypertrophy (81.6±3.3 pF, n≥30) was larger than that of the control cells (serotonin-free; 65.0±3.8 pF, n≥30, p<0.05, Fig. 3A). Data also showed significant increases in LTCC current density in hypertrophic H9c2 cells (2.1±0.3 pA/pF, n≥30) compared to the control cells (0.7±0.1 pA/pF, n≥30, p<0.001, Fig. 3B).
Figure 3.
Cell capacitance and current density in serotonin induced H9c2 cardiomyocytes. (A) Average cell capacitance and (B) ICa,L current density obtained from serum-free conditions as a control and 10 μM serotonin-treated H9c2 cells under serum-free for 4 days as a hypertrophic model. Data are means ± SE, n=10. * P<0.05, *** P<0.001 compared with control.
Effects on ICa,L in serotonin-induced hypertrophic cells
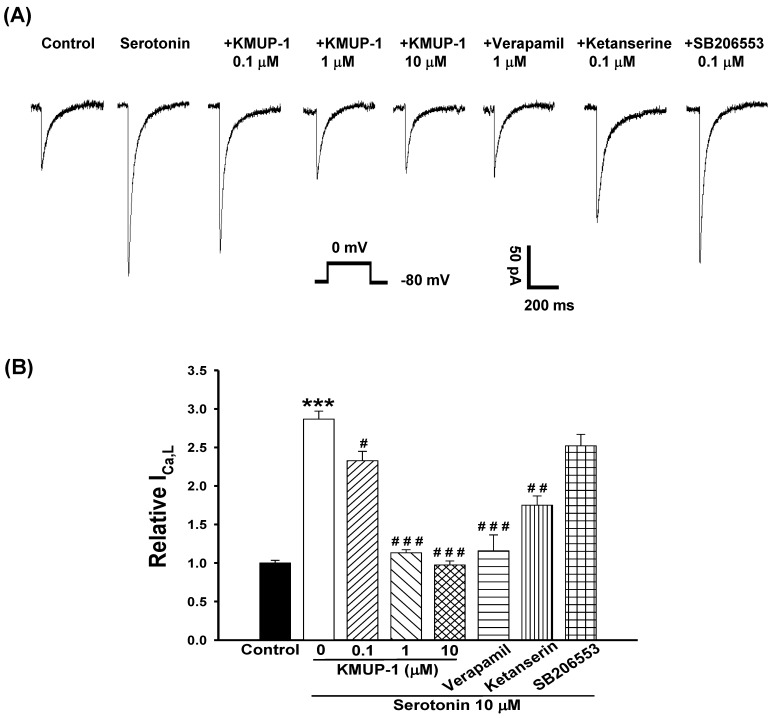
Perforated patch-clamp was used to study the effect of ICa,L increases in serotonin (10 μM)-induced H9c2 cell hypertrophy. Depolarizing single pulse (0 mV for 300 ms) was applied from a hold potential of -80 mV to record the inward ICa,L current. Serotonin induced significant H9c2 cell hypertrophy and enhanced the ICa,L ~2.9-fold control at day 4. The increased ICa,L of hypertrophic H9c2 cells was inhibited by co-incubation with KMUP-1 (0.1-10 μM) in a dose-dependent manner. This current was also inhibited by co-treatment with verapamil (1 μM, ~60%) or ketanserin (0.1 μM, ~40%), but unaffected by SB206553 (0.1 μM) in hypertrophic H9c2 cells (Fig. 4).
Figure 4.
Effects of KMUP-1, verapamil, ketanserin and SB206553 on serotonin-increased ICa,L in hypertrophic H9c2 cells. (A) Representative recordings of ICa,L evoked by test pulse to 0 mV from a holding potential of -80 mV in H9c2 cells. Cells were treated with 10 μM serotonin, 10 μM serotonin+0.1-10 μM KMUP-1, 10 μM serotonin+1 μM verapamil, 10 μM serotonin+0.1 μM ketanserin or10 μM serotonin+0.1 μM SB206553 for 4 days. (B) Bar graph showing the relative ICa,L averaged from the recordings of trace (A). Data are means ± SE, n=6. *** P<0.001 compared with control. # P<0.05, ## P<0.01, ### P<0.001 compared with serotonin group.
Increased ICa,L involved PKC activation in serotonin-induced hypertrophic cells
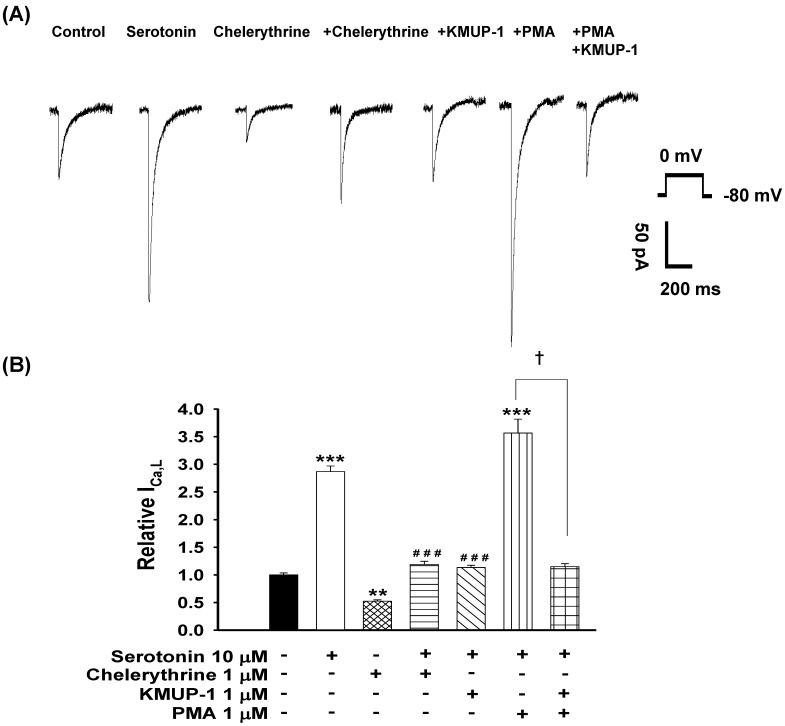
To determine whether PKC plays a role in serotonin-increased ICa,L, the PKC activator PMA and inhibitor chelerythrine were used in this experiment. The possible mechanisms of KMUP-1 protection against serotonin-increased ICa,L were also examined. We monitored the ICa,L of serotonin (10 μM)-induced H9c2 hypertrophic cells in the presence of 1 μM chelerythrine, 1 μM KMUP-1, 1 μM PMA or 1 μM PMA plus 1 μM KMUP-1 for 4 days. As shown in Fig. 5, the PKC inhibitor chelerythrine abolished the increased ICa,L and the PKC activator PMA significantly enhanced this current in hypertrophic H9c2 cells. Enhanced ICa,L from serotonin plus PMA was also abolished by KMUP-1 co-incubation.
Figure 5.
Effects of KMUP-1 on serotonin-increased ICa,L via the PKC pathway in hypertrophic H9c2 cells. (A) Representative recordings of ICa,L evoked by test pulse to 0 mV from a holding potential of -80 mV in H9c2 cells. Cells were treated with 10 μM serotonin, 1 μM chelerythrine, 10 μM serotonin+1 μM chelerythrine, 10 μM serotonin+1 μM KMUP-1, 10 μM serotonin+1 μM PMA or 10 μM serotonin+1 μM PMA+1 μM KMUP-1 for 4 days. (B) Bar graph showing the relative ICa,L averaged from the recordings of trace (A). Data are means ± SE, n=8. ** P<0.01, *** P <0.001 compared with control. ###P<0.001 compared with the serotonin group. ✝P <0.001 compared with the serotonin+PMA group.
Increased ICa,L involved PKA activation in serotonin-induced hypertrophic cells
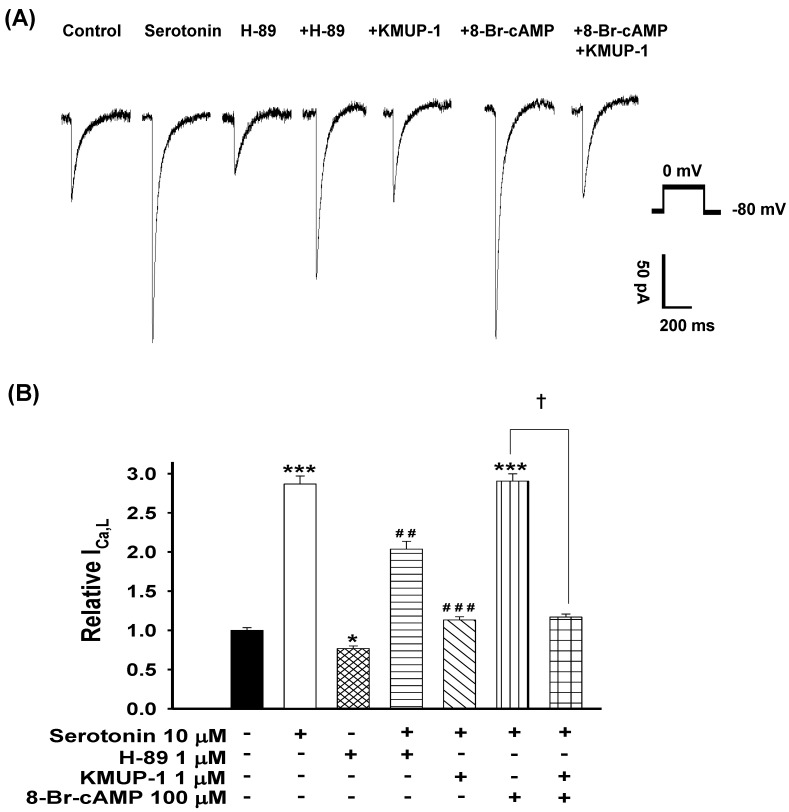
To ascertain the role of PKA in serotonin-increased ICa,L and KMUP-1's effects on this current, the PKA activator 8-Br-cAMP and inhibitor H-89 were used. We monitored the ICa,L of serotonin (10 μM)-induced H9c2 hypertrophic cells in the presence of 1 μM H-89, 1 μM KMUP-1, 100 μM 8-Br-cAMP or 100 μM 8-Br-cAMP plus 1 μM KMUP-1 for 4 days. The PKA inhibitor H-89 alone inhibited the control current ~25%. The PKA activator 8-Br-cAMP combined with serotonin did not further enhance the ICa,L current, and this effect was abolished by KMUP-1. Serotonin-increased ICa,L was attenuated by H-89 or KMUP-1 (Fig. 6).
Figure 6.
Effects of KMUP-1 on Serotonin-increased ICa,L via the PKA pathway in hypertrophic H9c2 cells. (A) Representative recordings of ICa,L evoked by test pulse to 0 mV from a holding potential of -80 mV in H9c2 cells. Cells were treated with 10 μM serotonin, 1 μM H-89, 10 μM serotonin+ 1 μM H-89, 10 μM serotonin+1 μM KMUP-1, 10 μM serotonin+100 μM 8-Br-cAMP or 10 μM serotonin+100 μM 8-Br-cAMP+1 μM KMUP-1 for 4 days. B) Bar graph showing the relative ICa,L averaged from the recordings of trace (A). Data are means ± SE, n=8. * P<0.05, *** P <0.001 compared with control. ## P <0.01, ### P <0.001 compared with the serotonin group. ✝P <0.001 compared with the serotonin+8-Br-cAMP group.
Increased ICa,L involved PKG inhibition in serotonin-induced hypertrophic cells
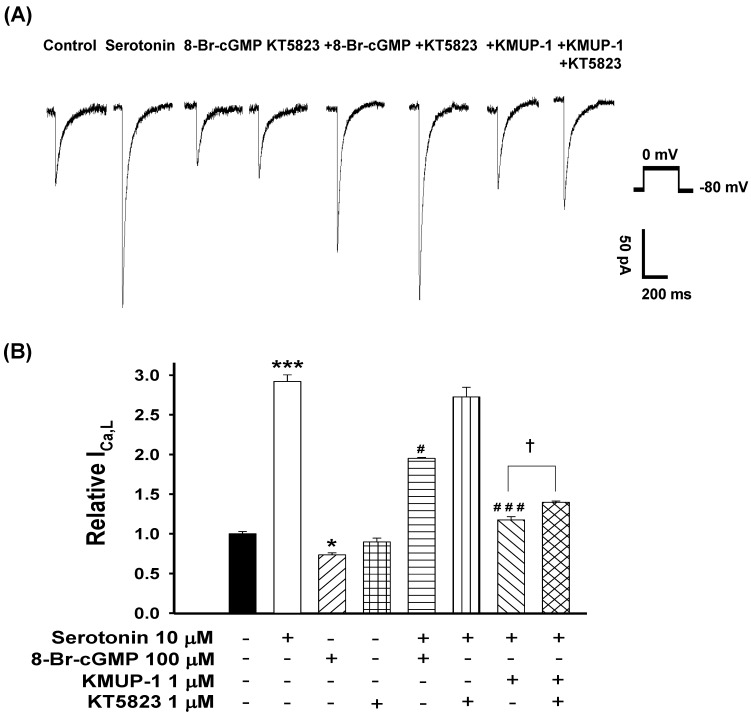
To determine whether PKG is involved in serotonin-increased ICa,L and KMUP-1's effects on this current, the PKG activator 8-Br-cGMP and inhibitor KT5823 were used. We monitored the ICa,L of serotonin (10 μM)-induced H9c2 hypertrophic cells in the presence of 100 μM 8-Br-cGMP, 1 μM KT5823, 1 μM KMUP-1 or 1 μM KT5823 plus 1 μM KMUP-1 for 4 days. The PKG activator 8-Br-cGMP attenuated the ICa,L in hypertrophic H9c2 and control cells, but the PKG inhibitor KT5823 showed no significant effects in both cells. KMUP-1 inhibited serotonin-increased ICa,L which was partly reversed by KT5823 (Fig. 7).
Figure 7.
Effects of KMUP-1 on Serotonin-increased ICa,L via the PKG pathway in hypertrophic H9c2 cells. A) Representative recordings of ICa,L evoked by test pulse to 0 mV from a holding potential of -80 mV in H9c2 cells. Cells were treated with 10 μM serotonin, 100 μM 8-Br-cGMP, 1 μM KT5823, 10 μM serotonin+100 μM 8-Br-cGMP, 10 μM serotonin+1 μM KT5823, 10 μM serotonin+1 μM KMUP-1 or 10 μM serotonin +1 μM KMUP-1+1 μM KT5823 for 4 days. B) Bar graph showing the relative ICa,L averaged from the recordings of trace (A). Data are means ± SE, n=8. * P<0.05, *** P <0.001 compared with control. # P <0.05, ### P <0.001 compared with the serotonin group. ✝P <0.005 compared with the serotonin+KMUP-1 group.
Discussion
This study is the first to use patch-clamp electrophysiology to investigate the role of PKA, PKC and PKG in serotonin-induced H9c2 cardiomyocyte hypertrophy and to examine the protective mechanisms of KMUP-1 against cardiomyocyte hypertrophy. Serotonin induced H9c2 cell hypertrophy, which increased cell size and ICa,L, was reversed by the K+ channel opener KMUP-1, attenuated by the LTCC blocker verapamil and the 5-HT2A antagonist ketanserin, but unaffected by the 5-HT2B antagonist SB206553. We also found that serotonin-increased ICa,L was reduced by the PKA or PKC inhibitor and the PKG activator, and was enhanced by the PKC activator but not affected by the PKG inhibitor. Interestingly, while KMUP-1 blunted serotonin-increased ICa,L, this was partly reversed by the PKG inhibitor. In light of our results, we suggest that serotonin increases ICa,L mainly by activating the 5-HT2A receptor-mediated PKA and PKC cascades, and partly by interacting with PKG. KMUP-1 prevents serotonin-induced H9c2 cardiomyocyte hypertrophy primarily by PKA and PKC inhibition and/or PKG stimulation.
Previous reports have demonstrated that H9c2 cell membranes expressed cardiac and skeletal types of LTCCs. The difference between the Ca2+ current of cardiac and skeletal muscle is related to different mechanisms of depolarization-contraction coupling 14, 27. Hescheler et al. (1991) also pointed out that H9c2 cells show morphological characteristics similar to immature embryonic cardiomyocytes but preserve several elements of the electrical and hormonal signal pathway found in adult cardiac cells. This cell line may be useful as a model for cardiomyocytes in terms of transmembrane signal transduction. To understand H9c2 cardiomyocyte hypertrophy, cardiac ICa,L was our target in this study. Therefore, we suggest that serotonin-induced hypertrophy in H9c2 cells provides advantages to understand the progression of pathogenic myocardial hypertrophy in humans.
A previous report showed that 5-HT2A receptors mediate the receptor-dependent hypertrophic activity of serotonin in cardiac cells. The role of these receptors in cardiac hypertrophy has been supported by various results in vivo. In a mouse model of increased plasma serotonin, 5-HT2A but not 5-HT2B receptors were involved in pressure overload hypertrophy 8. Other studies also showed that 5-HT2A receptors mediated positive inotropic response in ventricular myocytes and were overexpressed in different models of cardiac hypertrophy, along with human heart failure 7, 28. Additionally, 5-HT2A receptor blockade reverses hypertrophy during pressure overload and reduces left ventricular hypertrophy 8, 29. 5-HT2B receptors have also been implicated in ventricular hypertrophy. It has been shown that 5-HT2B receptors on cardiac fibroblasts mediate the release of IL-6 and indirectly induce cardiomyocyte hypertrophy 30. In this study, serotonin-increased cell size and ICa,L were not affected by the pharmacological (SB206553) blockade of 5-HT2B function, which suggested that 5-HT2B receptors could not have an important role in H9c2-mediated hypertrophy.
In the present study we extend previous in vivo findings that KMUP-1 was observed to prevent isoprenaline-induced cardiac hypertrophy via activation of NO/cGMP/PKG and inhibition of ERK1/2/calcineurin A signaling cascades 23. Increased cGMP production prevents cardiac hypertrophy has not been entirely elucidated but it probably involves PKG. PKG inhibits LTCCs thus reducing Ca2+ influx and blocking calcineurin-mediated activation of nuclear factor of activated T cell (NFAT), a transcription factor that is obligatory for hypertrophy 23, 31. This study not only assessed the role of PKG but also PKA and PKC in 5-HT-induced H9c2 cardiomyocyte hypertrophy. Data obtained from patch-clamp recordings, we suggest that PKC plays a major role whereas PKA or PKG plays a relatively minor role in 5-HT-induced H9c2 hypertrophy. This work attempted to explain the connection of 5-HT receptors and intracellular protein kinases in H9c2 hypertrophy induced by serotonin. Another possible hypertrophic mechanism for 5-HT transporter (SERT) needs to be investigated further 1, 32.
To our knowledge, this report is the first to describe the relationship between serotonin-increased ICa,L and the transduction pathway of protein kinases in hypertrophic H9c2 cells using patch-clamp recordings. The novel finding of this report is that serotonin increased the ICa,L currents, a marker of cell hypertrophy, which was attenuated by PKA and PKC inhibitors (H-89 and chelerythrine) and enhanced by the PKC activator PMA. Serotonin-mediated ICa,L effects were also reduced by the PKG activator 8-Br-cGMP, but unaffected by the PKG inhibitor KT5823. Thus, we suggest that serotonin-induced H9c2 hypertrophy can be directly attributed to 5-HT2A receptor-mediated PKA and PKC pathways, indirectly interacting with PKG. The K+-channel opener KMUP-1 reversed or blunted serotonin-mediated cell hypertrophy and ICa,L currents, and therefore its mechanism of action could be due to the modulation of these intracellular cascades of protein kinases (Fig. 8). Previously, we have demonstrated that KMUP-1 inhibits Ca2+ entry via voltage-dependent LTCCs in rat basilar arteries, which is attributed to its modulation of the PKC pathway 20. In this study, the single concentration of protein kinase inhibitors/activators and receptor antagonists used are considered for their selectivity and specificity 33.
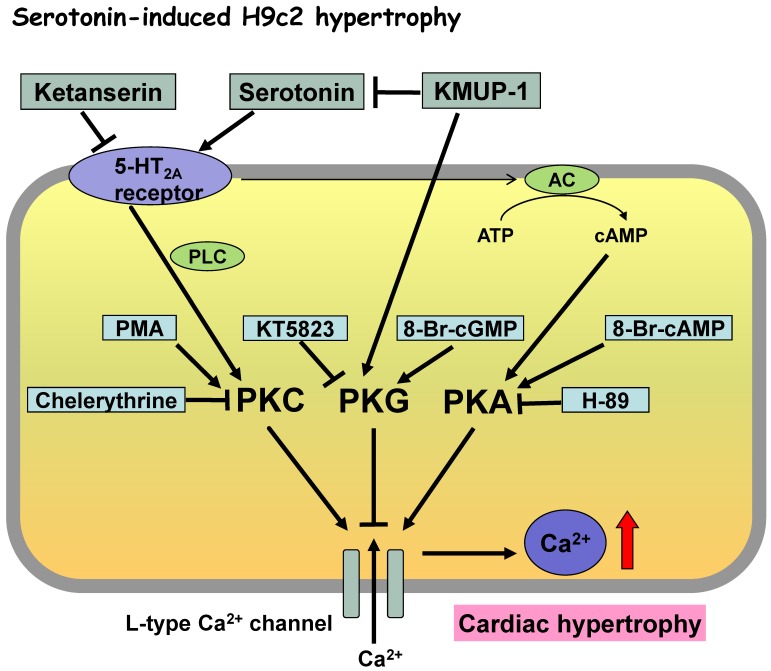
Figure 8.
Proposed mechanisms of KMUP-1 modulation of serotonin-induced hypertrophic H9c2 cardiomyocytes involving the protein kinase cascade. Serotonin-induced H9c2 cell hypertrophy was reversed by the K+ channel opener KMUP-1, attenuated by the LTCC blocker verapamil and the 5-HT2A antagonist ketanserin, but unaffected by the 5-HT2B antagonist SB206553. Collectively, serotonin-increased ICa,L could be mainly due to activated 5-HT2A receptor-mediated PKA and PKC cascades, and partly attributed to interaction with PKG. Accordingly, KMUP-1 blocks serotonin-induced H9c2 hypertrophy, which can be attributed to its PKA and PKC inhibition or PKG stimulation.
In summary, this study presents evidence that PKA and PKC play a key role in regulating serotonin-mediated cell hypertrophy and ICa,L changes via activation of 5-HT2A receptors in H9c2 cardiomyocytes. This investigation is also the first to use patch-clamp electrophysiology to study KMUP-1 prevention of serotonin-induced cardiomyocyte hypertrophy by blocking PKA- and PKC-mediated Ca2+ influx through LTCCs, and by stimulating PKG-inhibited Ca2+ influx. Finally, we suggest that KMUP-1 could be of value in controlling cardiac hypertrophy due to serotonin overstimulation under pathophysiologic conditions.
Acknowledgments
We thank Ms Li-Mei An and MSc I-Hsin Liu for their excellent technical assistance and Dr. Susan Olmstead-Wang at Johns Hopkins University for her editorial assistance with the manuscript. This study was supported by grants NSC 101-2320-B-037-032-MY3 and NSC 101-2632-B-037-001-MY2 from the National Science Council, Taiwan, and PS100023 from Pingtung Christian Hospital, Pingtung, Taiwan.
References
- 1.Villeneuve C, Caudrillier A, Ordener C, Pizzinat N, Parini A, Mialet-Perez J. Dose-dependent activation of distinct hypertrophic pathways by serotonin in cardiac cells. American journal of physiology Heart and circulatory physiology. 2009;297:H821–8. doi: 10.1152/ajpheart.00345.2009. [DOI] [PubMed] [Google Scholar]
- 2.Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008;40:2023–39. doi: 10.1016/j.biocel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Chandra M, Gupta V, Johri AK, Misra R, Kumar A, Gujrati V. et al. Serotonergic mechanisms in heart failure. Indian Heart J. 1994;46:153–6. [PubMed] [Google Scholar]
- 4.Nigmatullina RR, Kirillova VV, Jourjikiya RK, Mukhamedyarov MA, Kudrin VS, Klodt PM. et al. Disrupted serotonergic and sympathoadrenal systems in patients with chronic heart failure may serve as new therapeutic targets and novel biomarkers to assess severity, progression and response to treatment. Cardiology. 2009;113:277–86. doi: 10.1159/000205962. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson BI, Tommeras K, Nordrum I, Loennechen JP, Brunsvik A, Solligard E. et al. Long-term serotonin administration induces heart valve disease in rats. Circulation. 2005;111:1517–22. doi: 10.1161/01.CIR.0000159356.42064.48. [DOI] [PubMed] [Google Scholar]
- 6.Keys JR, Greene EA, Koch WJ, Eckhart AD. Gq-coupled receptor agonists mediate cardiac hypertrophy via the vasculature. Hypertension. 2002;40:660–6. doi: 10.1161/01.hyp.0000035397.73223.ce. [DOI] [PubMed] [Google Scholar]
- 7.Brattelid T, Qvigstad E, Birkeland JA, Swift F, Bekkevold SV, Krobert KA. et al. Serotonin responsiveness through 5-HT2A and 5-HT4 receptors is differentially regulated in hypertrophic and failing rat cardiac ventricle. J Mol Cell Cardiol. 2007;43:767–79. doi: 10.1016/j.yjmcc.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Lairez O, Calise D, Bianchi P, Ordener C, Spreux-Varoquaux O, Guilbeau-Frugier C. et al. Genetic deletion of MAO-A promotes serotonin-dependent ventricular hypertrophy by pressure overload. J Mol Cell Cardiol. 2009;46:587–95. doi: 10.1016/j.yjmcc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 9.Vindis C, D'Angelo R, Mucher E, Negre-Salvayre A, Parini A, Mialet-Perez J. Essential role of TRPC1 channels in cardiomyoblasts hypertrophy mediated by 5-HT2A serotonin receptors. Biochem Biophys Res Commun. 2010;391:979–83. doi: 10.1016/j.bbrc.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Jahnel U, Nawrath H, Rupp J, Ochi R. L-type calcium channel activity in human atrial myocytes as influenced by 5-HT. Naunyn Schmiedebergs Arch Pharmacol. 1993;348:396–402. doi: 10.1007/BF00171339. [DOI] [PubMed] [Google Scholar]
- 11.Pau D, Workman AJ, Kane KA, Rankin AC. Electrophysiological effects of 5-hydroxytryptamine on isolated human atrial myocytes, and the influence of chronic beta-adrenoceptor blockade. Br J Pharmacol. 2003;140:1434–41. doi: 10.1038/sj.bjp.0705553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Experimental cell research. 1976;98:367–81. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 13.Menard C, Pupier S, Mornet D, Kitzmann M, Nargeot J, Lory P. Modulation of L-type calcium channel expression during retinoic acid-induced differentiation of H9C2 cardiac cells. J Biol Chem. 1999;274:29063–70. doi: 10.1074/jbc.274.41.29063. [DOI] [PubMed] [Google Scholar]
- 14.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circulation research. 1991;69:1476–86. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 15.Ranki HJ, Budas GR, Crawford RM, Davies AM, Jovanovic A. 17Beta-estradiol regulates expression of KATP channels in heart-derived H9c2 cells. J Am Coll Cardiol. 2002;40:367–74. doi: 10.1016/s0735-1097(02)01947-2. [DOI] [PubMed] [Google Scholar]
- 16.Wu BN, Lin RJ, Lo YC, Shen KP, Wang CC, Lin YT. et al. KMUP-1, a xanthine derivative, induces relaxation of guinea-pig isolated trachea: the role of the epithelium, cyclic nucleotides and K+ channels. Br J Pharmacol. 2004;142:1105–14. doi: 10.1038/sj.bjp.0705791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu BN, Lin RJ, Lin CY, Shen KP, Chiang LC, Chen IJ. A xanthine-based KMUP-1 with cyclic GMP enhancing and K+ channels opening activities in rat aortic smooth muscle. Br J Pharmacol. 2001;134:265–74. doi: 10.1038/sj.bjp.0704231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin RJ, Wu BN, Lo YC, Shen KP, Lin YT, Huang CH. et al. KMUP-1 relaxes rabbit corpus cavernosum smooth muscle in vitro and in vivo: involvement of cyclic GMP and K+ channels. Br J Pharmacol. 2002;135:1159–66. doi: 10.1038/sj.bjp.0704554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu BN, Tu HF, Welsh DG, Chen IJ. KMUP-1 activates BKCa channels in basilar artery myocytes via cyclic nucleotide-dependent protein kinases. Br J Pharmacol. 2005;146:862–71. doi: 10.1038/sj.bjp.0706387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JY, Jiang MC, Chu LW, Hsieh SL, Chen IJ, Wu BN. KMUP-1 inhibits L-type Ca2+ channels involved the protein kinase C in rat basilar artery myocytes. Kaohsiung J Med Sci. 2011;27:538–43. doi: 10.1016/j.kjms.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Dai ZK, Cheng YJ, Chung HH, Wu JR, Chen IJ, Wu BN. KMUP-1 ameliorates monocrotaline-induced pulmonary arterial hypertension through the modulation of Ca2+ sensitization and K+-channel. Life Sci. 2010;86:747–55. doi: 10.1016/j.lfs.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Chung HH, Dai ZK, Wu BN, Yeh JL, Chai CY, Chu KS. et al. The xanthine derivative KMUP-1 inhibits models of pulmonary artery hypertension via increased NO and cGMP-dependent inhibition of RhoA/Rho kinase. Br J Pharmacol. 2010;160:971–86. doi: 10.1111/j.1476-5381.2010.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh JL, Hsu JH, Wu PJ, Liou SF, Liu CP, Chen IJ. et al. KMUP-1 attenuates isoprenaline-induced cardiac hypertrophy in rats through NO/cGMP/PKG and ERK1/2/calcineurin A pathways. Br J Pharmacol. 2010;159:1151–60. doi: 10.1111/j.1476-5381.2009.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JY, Cheng KI, Tsai YL, Hong YR, Howng SL, Kwan AL. et al. Potassium-channel openers KMUP-1 and pinacidil prevent subarachnoid hemorrhage-induced vasospasm by restoring the BKCa-channel activity. Shock. 2012;38:203–12. doi: 10.1097/SHK.0b013e31825b2d82. [DOI] [PubMed] [Google Scholar]
- 25.Stuck BJ, Lenski M, Bohm M, Laufs U. Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem. 2008;283:32562–9. doi: 10.1074/jbc.M801904200. [DOI] [PubMed] [Google Scholar]
- 26.Wu BN, Chen ML, Dai ZK, Lin YL, Yeh JL, Wu JR. et al. Inhibition of voltage-gated L-type calcium channels by labedipinedilol-A involves protein kinase C in rat cerebrovascular smooth muscle cells. Vascul Pharmacol. 2009;51:65–71. doi: 10.1016/j.vph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Sipido KR, Marban E. L-type calcium channels, potassium channels, and novel nonspecific cation channels in a clonal muscle cell line derived from embryonic rat ventricle. Circulation research. 1991;69:1487–99. doi: 10.1161/01.res.69.6.1487. [DOI] [PubMed] [Google Scholar]
- 28.Qvigstad E, Sjaastad I, Brattelid T, Nunn C, Swift F, Birkeland JA. et al. Dual serotonergic regulation of ventricular contractile force through 5-HT2A and 5-HT4 receptors induced in the acute failing heart. Circulation research. 2005;97:268–76. doi: 10.1161/01.RES.0000176970.22603.8d. [DOI] [PubMed] [Google Scholar]
- 29.Cobo C, Alcocer L, Chavez A. Effects of ketanserin on left ventricular hypertrophy in hypertensive patients. Cardiovasc Drugs Ther. 1990;4(Suppl 1):73–6. doi: 10.1007/BF00053431. [DOI] [PubMed] [Google Scholar]
- 30.Jaffre F, Callebert J, Sarre A, Etienne N, Nebigil CG, Launay JM. et al. Involvement of the serotonin 5-HT2B receptor in cardiac hypertrophy linked to sympathetic stimulation: control of interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha cytokine production by ventricular fibroblasts. Circulation. 2004;110:969–74. doi: 10.1161/01.CIR.0000139856.20505.57. [DOI] [PubMed] [Google Scholar]
- 31.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F. et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11363–8. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White K, Dempsie Y, Nilsen M, Wright AF, Loughlin L, MacLean MR. The serotonin transporter, gender, and 17beta oestradiol in the development of pulmonary arterial hypertension. Cardiovascular research. 2011;90:373–82. doi: 10.1093/cvr/cvq408. [DOI] [PubMed] [Google Scholar]
- 33.Chen IS, Dai ZK, Welsh DG, Chen IJ, Wu BN. Protein kinases modulate store-operated channels in pulmonary artery smooth muscle cells. Journal of biomedical science. 2011;18:2. doi: 10.1186/1423-0127-18-2. [DOI] [PMC free article] [PubMed] [Google Scholar]