Abstract
Background and Aims
There is increasing concern about the development of pancreatitis in patients with diabetes mellitus who received long-term GLP-1 analog treatment. Its pathogenesis is unknown. The effects of GLP-1 agonists on pancreatic endocrine cells is well studied, however there is little information on effects on other pancreatic tissues that might be involved in inflammatory processes. Pancreatic stellate cells (PSCs) can play an important role in pancreatitis, secreting various inflammatory cytokines/chemokines, as well as collagen. In this study, we investigated GLP-1R occurrence in normal pancreas, acute/chronic pancreatitis, and the effects of GLP-1 analog on normal PSCs, their ability to stimulate inflammatory mediator secretion or proliferation.
Methods
GLP-1R expression/localization in normal pancreas and pancreatitis (acute/chronic) tissues were evaluated with histological/immunohistochemical analysis. PSCs were isolated from male Wistar rats. GLP1R expression and effects of GLP-1 analog on activated PSCs was examined with realtime PCR, MTS assays and Western Blotting.
Results
In normal pancreas, pancreatic β cells expressed GLP-1R, with only low expression in acinar cells, whereas in acute or chronic pancreatitis, acinar cells, ductal cells and activated PSCs expressed GLP-1R. With activation of normal PSCs, GLP-1R is markedly increased, as is multiple other incretin-related receptors. The GLP-1 analog, liraglutide, did not induce inflammatory genes expression in activated PSCs, but induced proliferation. Liraglutide activated multiple signaling cascades in PSCs, and the ERK pathway mediated the PSCs proliferation.
Conclusions
GLP-1Rs are expressed in normal pancreas and there is marked enhanced expression in acute/chronic pancreatitis. GLP-1-agonist induced cell proliferation of activated PSCs without increasing release of inflammatory mediators. These results suggest chronic treatment with GLP-1R agonists could lead to proliferation/chronic activation of PSCs, which may lead to important effects in the pancreas.
Keywords: diabetes mellitus, glucagon-like peptide-1, GLP-1 receptor, liraglutide, pancreatic stellate cells, pancreatitis, proliferation
GLP-1 analogs and DPPIV inhibitors are increasingly used long-term in patients with diabetes mellitus worldwide (1–4). These drugs appear to be generally safe, however a concern receiving increasing attention is the development of pancreatitis (5–15). At present, the pathogenesis of this effect is unknown.
Glucagon-like peptide-1 (GLP-1) is released postprandially by intestinal L cells and its activation of GLP-1 receptors (GLP-1R) on β cells stimulates insulin biosynthesis/secretion (16, 17). GLP-1Rs are expressed not only in pancreatic β cells, but also in other organs, such as brain, heart, stomach, muscle and liver (18, 19). Whereas, the effects of GLP-1 on pancreatic endocrine cells have been well studied, there is little information on the effects of GLP-1R in other pancreatic exocrine tissues that might be involved in inflammatory conditions of the pancreas.
Recent studies establish the important role of various resident pancreas cells (particularly acinar cells and PSCs) in various aspects of pancreatitis (acute/chronic), especially leukocyte attraction via secretion of chemokines and cytokines and expression of adhesion molecules (20–28). PSCs, not only can secrete various inflammatory cytokines/chemokines (29–31), which can participate in inflammatory processes, they can also proliferate and secrete collagen(32, 33). Whereas numerous stimulants can affect PSCs including activation of some G-protein-coupled receptors (PGE2, angiotensin, CCK-A, CCK-B) (34–39), it is unknown if GLP-1R occurs on PSC either in normal pancreas or pancreatic disease states or, whether PSCs are activated in acute or chronic pancreatitis, or if it does occur, whether the GLP-1R agonists affect the PSC signaling cascade, growth or ability to release inflammatory mediators. To address these questions, in the present study we have examined the occurrence of the GLP-1R in PSCs in normal pancreas, in acute/chronic pancreatitis models, as well as the ability of the GLP-1R agonist, liraglutide, to alter function of PSCs and effect PSCs behavior.
Materials and Methods
Materials
Liraglutide was from Shoyaku Co. Ltd. (Fukuoka, Japan), U0126 and cerulein from Sigma–Aldrich (St Louis, MO, USA), Recombinant rat PDGF-BB from R&D Systems (Minneapolis, Minn) and rabbit anti- phosphorylated extracellular signal-regulated kinase (ERK) antibody, rabbit anti-phosphorylated JNK antibody, rabbit anti-phosphorylated p38 antibody, rabbit anti-phosphorylated Akt antibody, mouse anti-total IκB antibody anti-rabbit IgG-HRP-conjugated antibody and anti-mouse IgG-HRP-conjugated antibody from Cell Signaling Technology (Beverly, MA, USA). Rabbit anti-GLP-1R antibody, mouse anti-glial fibrillary acidic protein (GFAP) and mouse anti-alpha smooth muscle actin (α-SMA) were from Abcam PLC (Cambridge, UK), Goat anti-type I collagen antibody from Southern Biotechnology (Birmingham, Alabama, USA), rabbit antibody against glyceraldehydes-3-phosphate dehydrogenase (GAPDH) from Trevigen (Gaithersburg, MD, USA) and anti-rabbit IgG Alexa488 conjugated antibody, anti-mouse-IgG-Alexa555-conjugated antibody, anti-goat-IgG-Alexa350 conjugated antibody and Hoechst33342 were from Invitrogen (Carlsbad, CA, USA).
Animals
15-week-old male Wistar rats and Wistar/Bonn-Kobori (KOB) rats (KBT Oriental, Saga, Japan) were used. All animal procedures were performed in accordance with the guidelines of the Committee on Animal Care (Kyushu University). L-arginine induced acute pancreatitis was performed by administrating a single intraperitoneal injection of 4.0g/kg body-weight L-arginine monohydrochloride in 0.9% sodium chloride (pH7.0) as described previously (40). Rats were fed ad libitum after the treatment and killed 72h later. Cerulein induced acute pancreatitis was also performed by injecting intraperitoneally into the right lower quadrant with a 50 μg/kg/body weight of cerulein dissolved in 0.9% saline in a volume of 100 μl. Injections were at hourly intervals up to 7 injections. Animals were sacrificed 8 hours after the first injection of cerulein. To study results in chronic pancreatitis (CP), a Male Wistar/Bonn-Kobori (KOB) rats (41, 42) were used as previously described (31).
Isolation of PSCs and cell culture
PSCs were isolated from the pancreas of male Wistar rats and were maintained as previously described (43). Cell purity was assessed initially by a typical star-like configuration and by detecting vitamin A autofluorescence and was >95%. To further assess the purity, in the 3rd passage (activated PSCs) using immunohistochemistry to assess the co-expression of α –SMA (marker of activated stellate cells) and the presence of a cell (Hoechst33342) using ImageJ (NIH). We found the purity of PSCs was >99%. Furthermore, trypsin mRNA expression as a marker for acinar cell contamination was assessed using PCR (primers: Table. 1, PCR conditions: Tm=60°C, cycles 40). In the 3rd passage of PSCs and was >1 million times lower than in acinar cells, indicating contamination by acinar cells was negligible. Unless otherwise specified, PSCs were incubated in serum-free medium for 24h before the addition of experimental reagents. The ERK specific inhibitor (U0126) was added 30min before the addition of liraglutide.
Table 1.
Sequences of primers used in this study
| Gene | Sequence |
|---|---|
| rat GLP-1R: | |
| sense | 5′-CTGAGGAACAGCTCCTGTCG-3′ |
| anti-sense | 5′-AGCTGACAAGGATGGCTGAA-3′ |
| rat GLP-2R: | |
| sense | 5′-GGGGAAGTGTTCCAAGAAGC-3′ |
| anti-sense | 5′-GTGTGAGCTGCATGTGGAGA-3′ |
| rat GIPR: | |
| sense | 5′-AGATCTTCCCACCTCCCAGA-3′ |
| anti-sense | 5′-TCTGTGTGTCCATCCATCCA-3′ |
| rat GlucagonR: | |
| sense | 5′-CTCTGCGAAGACCTCATTGG-3′ |
| anti-sense | 5′-GAGGCCCTTCTGAATCCAAC-3′ |
| rat proglucagon: | |
| sense | 5′-CGCCATAGCTGAGGAACTTG-3′ |
| anti-sense | 5′-CTCTGGTGGCAAGGTTATCG-3′ |
| rat GIP: | |
| sense | 5′-AGACCTGCTCTCTGCTGCTG-3′ |
| anti-sense | 5′-ATGGGATCGGAACTCAACCT-3′ |
| rat DPPIV: | |
| sense | 5′-CCCAGGTCCAAGCATACAAA-3′ |
| anti-sense | 5′-CTCAGAAAACGGTGCCAGTC-3′ |
| rat α-SMA: | |
| sense | 5′-CCTCAGGGTGCTCGTGGAT-3′ |
| anti-sense | 5′-CAGGACTGCCAGGCTCTCC-3′ |
| rat TNF-α: | |
| sense | 5′-CTGGTGGTACCAGCAGATGG-3′ |
| anti-sense | 5′-GGAGGCTGACTTTCTCCTGG-3′ |
| rat IL-6: | |
| sense | 5′-CCACCAGGAACGAAAGTCAA-3′ |
| anti-sense | 5′-CAGTCCCAAGAAGGCAACTG-3′ |
| rat IL-1β: | |
| sense | 5′-GCACAGTTCCCCAACTGGTA-3′ |
| anti-sense | 5′-CCGACCATTGCTGTTTCCTA-3′ |
| rat MCP-1: | |
| sense | 5′-ACGTGCTGTCTCAGCCAGAT-3′ |
| anti-sense | 5′-GTTCTCCAGCCGACTCATTG-3′ |
| rat CINC-1: | |
| sense | 5′-CCACACTCAAGAATGGTCGCG-3′ |
| anti-sense | 5′-AGACGCCATCGGTGCAATC-3′ |
| rat CX3CL1: | |
| sense | 5′-CACAAGATGACCTCGCCAAT-3′ |
| anti-sense | 5′-GCTGTCTCGTCTCCAGGATG-3′ |
| rat type I collagen: | |
| sense | 5′-AGTTGGTGATGATGCCGTGTT-3′ |
| anti-sense | 5′-ATGGGCCAAAAGGACAGCTAT-3′ |
| rat trypsin: | |
| sense | 5′-AGATTCCTGCCAGGGTGACT-3′ |
| anti-sense | 5′-CCATAGCCCCAGGAGACAAT-3′ |
| rat GAPDH: | |
| sense | 5′-GCTCTCTGCTCCTCCCTGTT-3′ |
| anti-sense | 5′-CACACCGACCTTCACCATCT-3′ |
Expressional changes of incretin-related and pancreatitis-related mRNAs in PSCs: real-time reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from PSCs using an RNeasy Mini Kit (Qiagen, Valencia, CA) as previously described (44). Total RNA was reverse transcribed into first-strand complementary DNA (cDNA) and RT-PCR was performed using a LightCycler Real-Time PCR system (Roche, Switzerland) as previously described (44). To control for variations in the reactions, all PCR data were normalized against GAPDH expression. The PCR products were separated in a 2 % agarose gel and visualized under UV illumination.
Histochemical analysis
Specimens from the body of each pancreas were used for histopathological analysis. The pancreas specimens were fixed in 4% paraformaldehyde in PBS and embedded in paraffin. Tissue sections were stained with Hematoxylin/Eosin (HE) or Masson-Trichrome (MT), which preferentially labels collagen fibrils.
Immunofluorescent staining
Immunofluorescent staining was performed as previously described (43). Briefly, double-immunofluorescent staining for GLP-1R and GFAP (staining quiescent PSCs), type I collagen/α-SMA (staining activated PSCs) was performed as previously described(31). Hoechst33342 was used for nuclear counter staining. The degree of colocalization of GLP-1R and α-SMA was calculated using ImageJ (NIH).
Western Blotting
Western blot analysis was performed as previously described (43). Briefly, cells were lysed in RIPA buffer and cellular proteins were fractionated by electrophoresis on a polyacrylamide gel (Bio-Rad, Hercules, Calif). The proteins were transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, Calif), and it was incubated for 2–6h with primary antibodies (at 1:1000–10000 dilutions). After incubating with HRP-conjugated secondary antibody (at 1:10000 dilution), the proteins were visualized by using an ECL kit and ImageQuant™ LAS 4000mini (GE Healthcare). Levels of phosphorylated ERK, JNK, p38, Akt, total IκB and GAPDH were determined by General-Purpose Analysis Software Multi Gauge.
Cell Proliferation Assay: MTS Assay
Cell proliferation was assessed by the MTS assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay, Madison, WI, USA) as previously described (44).
Statistical analysis
Results are expressed as the means (SEM) of 3–4 separate cell preparations. The two-tailed unpaired Student’s t-test was used for the statistical analyses. P values of <0.05 were considered statistically significant.
Results
Expression of the glucagon-like peptide-1 receptor in normal pancreas
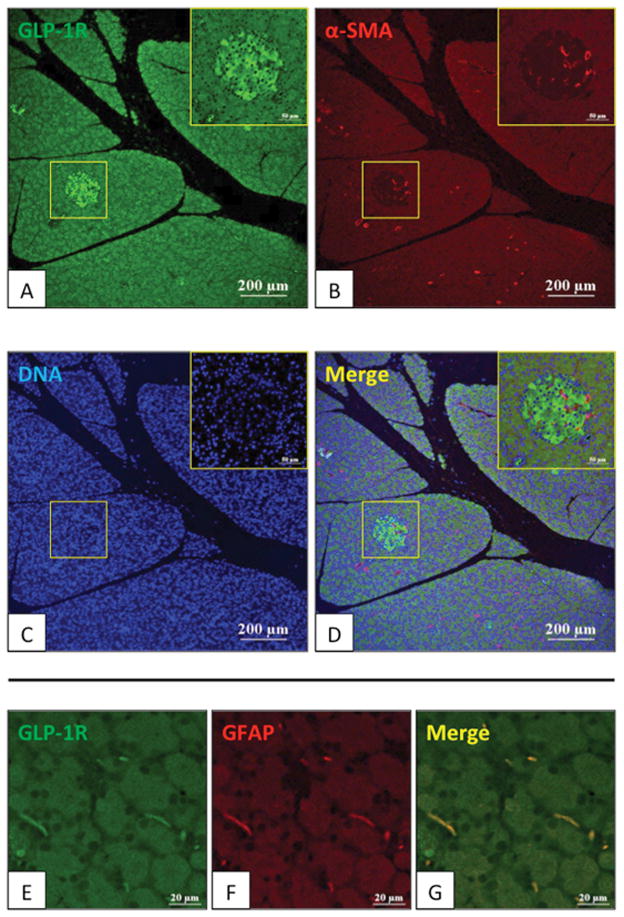
Initially, we performed immunofluorescent staining to evaluate the expression of the GLP-1R in normal pancreas from male Wistar rats (Fig. 1, 2). As previously reported (45), high expression of the GLP-1R occurred in pancreatic islets (Fig. 1A). α-SMA positive capillary vessel cells in the islets did not show GLP-1R stain (Fig. 1B, D). Only weak staining for GLP-1R was seen in acinar cells. In the normal pancreas specimen, no α-SMA-positive PSCs (activated myofibroblast-like cells) were seen, however glial-fibrillary-acidic protein (GFAP) positive quiescent PSCs showed low expression of the GLP-1R (Fig. 1 E–G). To investigate further expression of GLP-1R in normal islets, the GLP-1R distribution was examined in β cells (central islet area) and α cells (islet-periphery for GLP-1R) (Fig. 2A–F). GLP-1R was only present in β cells, a finding similar to some reports (45, 46), however different from others, which reported the GLP-1R also in α cells (47–49).
Figure 1. Expression of glucagon-like peptide-1 receptor (GLP-1R) in normal pancreas of Wistar rats.
Expression of GLP-1R, alpha-smooth muscle actin (α-SMA) [activated pancreatic stellate cells (PSCs)] and Glial fibrillary-acidic-protein (GFAP) [quiescent PSCs] in the pancreas of 15-week-old Wistar rats was examined by immunofluorescence staining. In the top four panels (A–D), figures are 100x and the area in the square is shown at 200x in the insert. α-SMA (red) and GLP-1R expression (green) are shown. In the bottom panels (E–G), figures are 1000x magnification of an area showing two PSCs. GFAP (red) and GLP-1R expression (green) are shown. This figure demonstrates that acinar cells stained faintly for GLP-1R, whereas islets stained strongly, and that quiescent normal PSCs also have tGLP-1R expression. These pictures are representative of four experiments.
Figure 2. Expression of glucagon-like peptide-1 (GLP-1) in pancreatic islet cells of normal pancreas of Wistar rats.
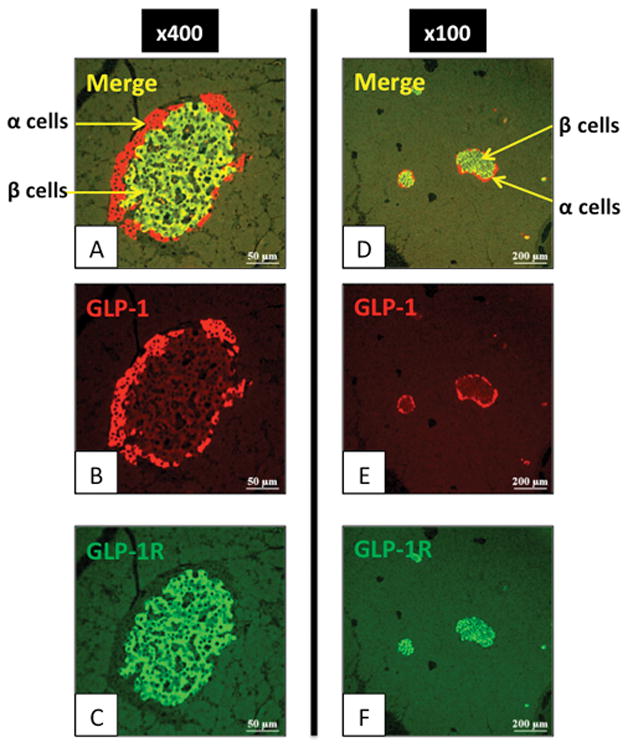
Expression of the GLP-1R in the pancreas of 15-week-old Wistar rats was examined by immunofluorescence staining. In the left panels (A–C), figures are 400x magnification of an area showing a pancreatic islet. GLP-1 (red) [α cells] and GLP-1R expression (green) are shown. In the right panels (D–F), figures are 100x magnification of an area showing expression in other islets (α and β cells). GLP-1 (red) and GLP-1R expression (green) are shown. This slide shows GLP-1R is present in β cells and not α cells. These pictures are representative of four experiments.
Expressions of GLP-1R in activated PSCs in the pancreas of rats with acute pancreatitis (AP)
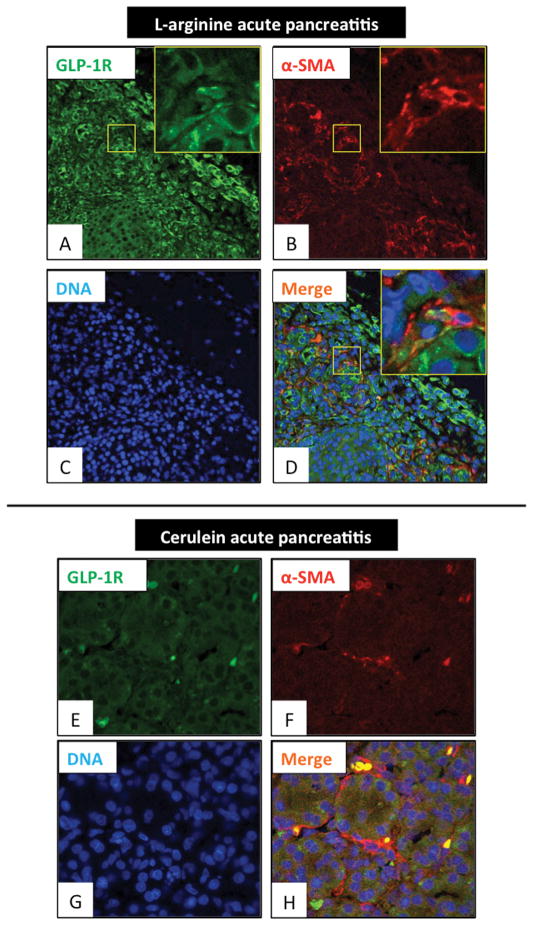
We next investigated whether GLP-1R expression was present in a model of AP [L-arginine pancreatitis-model (72 hours) and cerulein-induced pancreatitis-model (8 hours)]. Double-staining for α-SMA and GLP-1R (Fig. 3) showed a marked increase in GLP-1R expression throughout the inflamed pancreas (Fig. 3A–H) and also α-SMA-positive cells (activated PSCs) were seen throughout the pancreas, especially in the interstitial area (Fig. 3B, F). Double-positive staining cells (yellow) were observed supporting the conclusion that GLP-1R was present on α-SMA-positive cells (activated PSCs) (Fig. 3D, H). In general, the changes were milder in the cerulein-induced acute pancreatitis model (Fig. 3E–H) than in the model of L-arginine-induced acute pancreatitis (Fig. 3A–D). To determine the degree that GLP-1R colocalized in activated PSCs, we performed quantitative immunohistochemistry of GLP-1R and α-SMA positive cells (activated PSCs) (Fig. 3). GLP-1R was found in 83 % of the activated PSCs. We also performed the reverse analysis by determining the percentage of GLP-1R positive cells that were activated PSCs and found that (Fig. 3A) it was 24%. Dilated pancreatic duct cells (DC) also expressed the GLP-1R (Fig. 3D).
Figure 3. Glucagon-like peptide-1 receptor (GLP-1R) expression in activated pancreatic stellate cells (α-SMA positive) in an area of acute inflammation of L-arginine induced acute pancreatitis.
Expression of GLP-1R and alpha-smooth muscle actin (α-SMA) (to identify activated pancreatic stellate cells) in the pancreas of L-arginine induced acute pancreatitis model rats and cerulein induced acute pancreatitis model rats were examined by immunofluorescence staining. Panels A–D are 400x magnification and the area in the square is shown in the insert at 2000x. Panels E–H are 1000x magnification. Shown are GLP-1R expression (in green: A, E), α-SMA (in red: B, F) and nuclei (in blue: C, G). A merged image is shown in Panel D and H. This figure shows acute pancreatitis. There is marked GLP-1R expression in activated PSCs (α-SMA positive cells) and dilated pancreatic duct cells (DC). These pictures are representative images from four experiments. DC: ductal cells.
GLP-1R expression in pancreatic sections from model of chronic pancreatitis (CP)
We next performed similar studies in a model of CP (Wistar Bonn/Kobori rat) (Fig. 4). HE and Masson-Trichrome (for collagen fiber) staining showed destruction of the normal pancreatic structure, as well as fibrosis in peri-islet-areas (P-IA) and stromal fibrotic-areas (FA) (Fig. 4A–E). GLP-1R was markedly increased in both islets and acinar tissue. GLP-1R was also expressed in a type I collagen abundant lesion of the P-IA and FA, especially in spindle-shaped fibroblasts (Fig. 4K–O).
Figure 4. Glucagon-like peptide-1 receptor (GLP-1R) and type I collagen expression in pancreas of Wistar/Bonn-Kobori (WBN/Kob) rats (a chronic pancreatitis model).
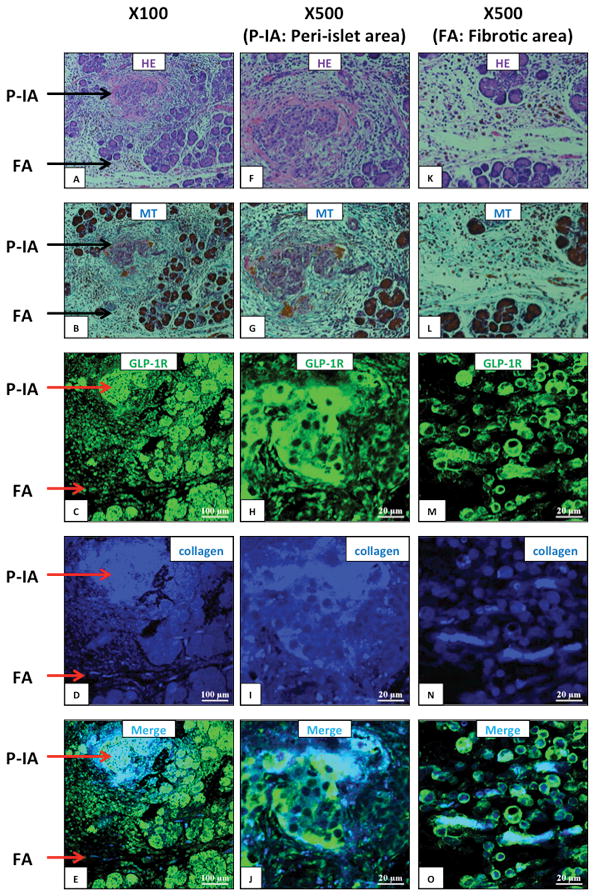
Shown are representative sections showing peri-islet area (P-IA) and a fibrotic area (FA) at the indicated magnifications (100x, 500x). Expression of GLP-1R and type I collagen in the pancreas of 15-week-old WBN/Kob rats was examined by immunofluorescence staining. Panel (A, F, K) shows Hematoxylin-Eosin (HE) staining, Panel (B, G, L) Masson Trichrome (MT) staining for collagen fibrils, Panel (C, H, M) GLP-1R staining (green), Panel (D, I, N) type I collagen staining (blue) and Panel (E, J, O) a merged image of type I collagen and GLP-1R. These pictures are from sequential sections showing the same area. This figure demonstrates in this chronic pancreatitis model increased collagen deposition (MT staining) and increased GLP-1R expression in areas where increased collagen is present (merged picture). These pictures are representative images from four experiments.
Expression of GLP-1R in activated PSCs in the pancreas of rats with CP
The possible expression of GLP-1R in activated PSCs in the CP model was investigated in more detail (Fig. 5). To determine whether the spindle-shaped fibroblasts seen in Fig. 4 were activated PSCs, we performed double-staining of α-SMA and GLP-1R in an area showing fibrosis (Fig. 5A–D). A marked increase in α-SMA-positive cells was seen throughout the pancreas, especially near areas of pancreatic islet cell destruction (Fig. 5B). In this area, double-positive stained cells (yellow) were observed for GLP-1R and α-SMA, demonstrating that GLP-1R was expressed on the most activated PSCs (Fig. 5D). Similar to Fig. 3A, we determined the degree that GLP-1R is colocalized with activated PSCs. GLP-1R is colocalized in 99 % of the activated PSCs (shown by positive α-SMA stain). These data (Fig. 3A, 5) demonstrate that by far the majority of activated PSCs in this chronic model of pancreatitis have GLP-1R. We also performed the reverse analysis by determining the percentage of GLP-1R positive cells that were activated PSCs, and which was 71% in CP models (Fig. 5A). These data support the conclusion that GLP-1R expression in these inflammatory conditions (acute/chronic pancreatitis), can also occur in non-stellate cells (acinar cells, islet cells and inflammatory cells). The proportion of GLP-1R occurring in non-stellate cells is higher when the inflammation is mild (76% acute pancreatitis, Fig. 3A; 29% chronic pancreatitis, Fig. 5). Ductal cells (DC) also expressed GLP-1R (Fig. 5A, D).
Figure 5. Glucagon-like peptide-1 receptor (GLP-1R) expression in activated pancreatic stellate cells (α-SMA positive) and surrounding areas in the pancreas of Wistar/Bonn Kobori (WBN/Kob) rats (a chronic pancreatitis rat model).
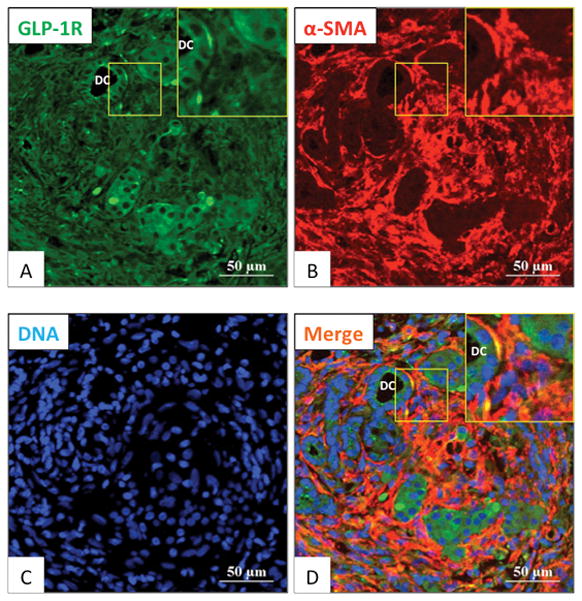
Expression of GLP-1R and alpha-smooth muscle actin (α-SMA: markers for activated PSCs) in the pancreas of 15-week-old WBN/Kob chronic pancreatitis model rats was examined by immunofluorescence staining. Panels A–D are 500x magnification and the area in the square is shown in the insert at 1000x. Shown are GLP-1R expression (in green: A), α-SMA (in red: B) and nuclei (in blue: C). A merged image is shown in Panel D. This figure shows in a chronic pancreatitis model, the marked GLP-1R expression co-localizes with activated PSCs (α-SMA positive). These pictures are representative images from four experiments. DC: ductal cells.
Both quiescent and activated PSCs from normal rat expressed the GLP-1R in vitro and the expression is increased in activated PSCs
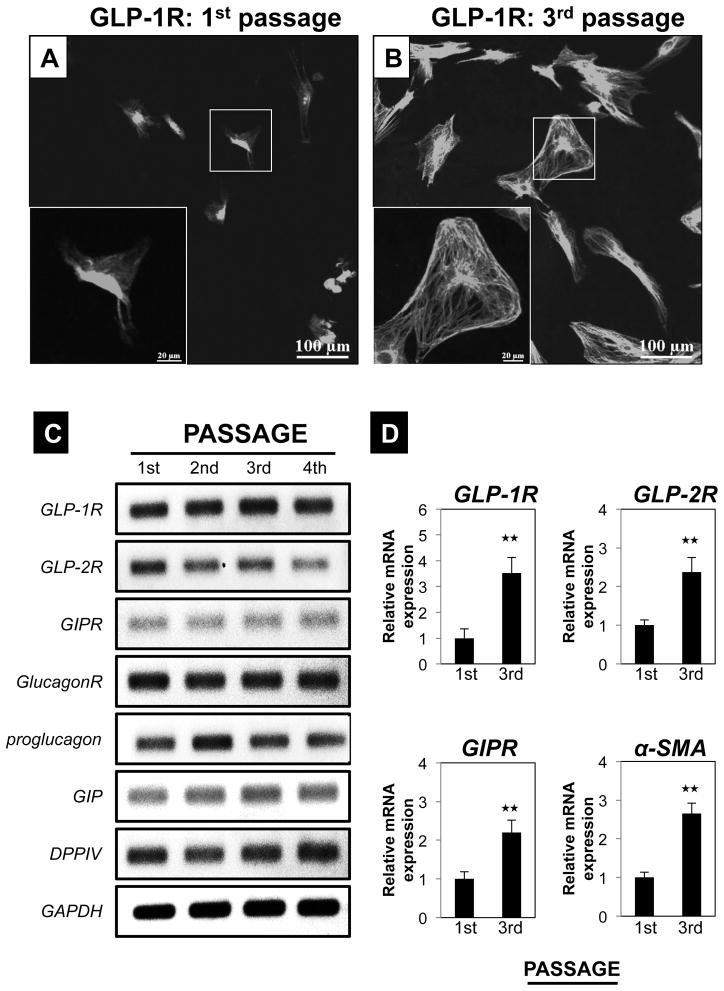
To further investigate the expression of GLP-1R in normal pancreas and the result of its activation, we studied PSCs isolated from normal pancreas in both a quiescent phase (1st passage: 1 day) (Fig. 6A) and an activated phase (3rd passage) in vitro (Fig. 6B). Activation of the PSCs during the 3rd passage was confirmed by demonstrating the presence of immunofluorescent staining for α-SMA (supplemental Fig. S1). GLP-1R expression by immunohistochemical staining suggested that there was a marked increase in the expression in the PSCs, with activation (compare Fig. 6A vs. 6B). We confirmed that all of the PSCs in both quiescent and activated state expressed GLP-1R with nuclear staining (supplemental Fig. S1). Not only GLP-1R, but also other incretin-related mRNAs (GLP-2R, GIPR, GlucagonR, proglucagon, GIP and DPPIV) were expressed in PSCs in sequential 1–4 passages (Fig. 6C). Quantitative realtime PCR revealed greater expression of GLP-1R, GLP-2R and GIP-R mRNA in activated PSCs from the 3rd passage (activated) compared to quiescent PSCs from the 1st passage (quiescent) (Fig. 6D).
Figure 6. Pancreatic stellate cells (PSCs) express incretin related genes as well as GLP-1 receptor (GLP-1R) in vitro and the expression increases with PSC activation.
In the top panel (A–B), expression of GLP-1R protein in both freshly isolated PSCs (1st) and culture-activated PSCs (3rd passage) are shown. Magnification is x200 and the area in the box is shown in the insert at x500. Panel C shows a representative RT-PCR result for different incretin-related mRNAs at passages 1–4. Panel D shows the quantitation of RT-PCR result from 4 experiments for passages 1–3. ★★=P<0.01, comparing passage 1–3. These results were representative of four experiments. Abbreviations GLP-1R=Glucagon-like peptide receptor-1, GLP-2R=Glucagon-like peptide receptor-2, GIPR=Gastric inhibitory peptide receptor, GlucagonR=Glucagon receptor, GIP=Gastric inhibitory peptide, DPPIV=Dipeptidyl peptidase IV, GAPDH=glyceraldehyde-3-phosphate dehydrogenase
The GLP-1 agonist, liraglutide, did not stimulate inflammatory mRNA expression, but stimulated cell proliferation of PSCs (Fig. 7)
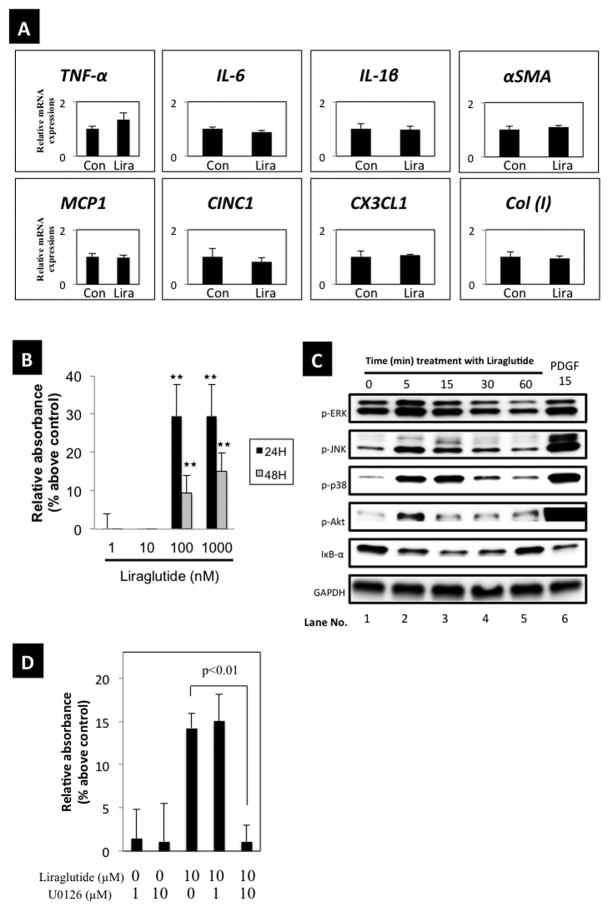
Figure 7. Effects of the Glucagon-like peptide-1 (GLP-1) agonist, liraglutide, on inflammatory mediators, proliferation of PSCs and activation of signaling cascades, and effects of the ERK inhibitor, U0126, on basal or liraglutide stimulated growth.
Panel A shows the RT-PCR results from 4 experiments for passages 3–5 of PSCs, comparing non-treatment (Con) with treatment with liraglutide (1000 nM) for 6 hours. Panel B shows the concentration-dependent effect of liraglutide on cell proliferation after 24 and 48 hours. Panel C shows the liraglutide-induced signaling cascade activation. Results for phospho-ERK, phospho-JNK, phospho-p38, and phospho-Akt were determined using specific phospho-antibodies. Results with IκB-α were determined using a specific IκB-α antibody. The results were determined at the indicated times with liraglutide and after a 15 min incubation with PDGF-BB. Panel D shows effects of U0126, a specific inhibitor of ERK. PSCs (passages 3–5) (2000 cells/well) were pre-incubated for 24 hours in serum free medium and then incubated for 24 hours with indicated concentrations of liraglutide alone or with U0126. U0126 was added 30 min prior to liraglutide. Growth was determined by MTS assay using coloriometric microplate reader. Results are the means ± SEM from 4 experiments.
We next examined whether the GLP-1R agonist, liraglutide, stimulated inflammatory responses in PSCs (Fig. 7A). Liraglutide (1 μM) did not increase the mRNA expression of inflammatory cytokines (TNF-α, IL-6, IL-1β) or chemokines (MCP-1, CINC-1, CX3CL1) (Fig. 7A). α-SMA and type I collagen mRNA, which are cell activation markers, were also not increased by liraglutide (Fig. 7A). However, liraglutide stimulated PSCs proliferation at 24 and 48 hours after its addition (Fig. 7B).
The GLP-1 agonist, liraglutide, activated various cell signaling cascades in PSCs (Fig. 7C)
Phosphorylation of MAPKs (ERK, JNK, p38) and Akt was rapidly stimulated by liraglutide (by 5 min) with a maximal response between 5–15 min (Lanes 2, 3). Degradation of total IκB also occurred rapidly with liraglutide treatment indicating NF-κB activation. PDGF-BB was used as a positive control and activated each of the cell signaling pathways (Lane 6).
ERK activation plays an essential role in liraglutide-induced cell proliferation of PSCs
ERK activation in PSCs is important in stimulating the growth effects of numerous stimuli (43, 50). To assess this, U0126, an ERK inhibitor, at the indicated concentrations (1–10 μM) was added 30 min before liraglutide (10 μM). Liraglutide induced cell proliferation was completely abolished by U0126, demonstrating ERK activation is important in mediating liraglutide-induced proliferation of PSCs (Fig. 7D).
Discussion
GLP-1 analogs and DPPIV inhibitors are now one of the main drugs used for treatment in patients with diabetes mellitus (1–3). The use of these drugs has generally been associated with few side-effects, however, a concern receiving increasing attention is the possible development of pancreatitis (7, 8). A number of studies report an association of GLP-1-agonist use and pancreatitis (10–15), however others either do not show this association or dispute it(51–53). At present, the pathogenesis of this effect is unknown. PSCs are increasingly recognized as playing an important role in various aspects of pancreatitis, especially chronic pancreatitis (26, 27). PSCs secrete various inflammatory cytokines/chemokines (29, 30), which can participate in inflammatory processes and can also secrete collagen and play an essential role in fibrosis (32, 33). Numerous stimulants activate PSCs, including activation of some G-protein coupled receptors (PGE2, angiotensin, CCK-A, CCK-B) (34–39). However, it is unknown if GLP-1R occurs on PSCs in normal pancreas, on activated PSCs in acute or chronic pancreatitis or if this does occur, whether the GLP-1R agonists affect the PSC signaling cascade, PSC growth or ability to release inflammatory mediators. To address these questions, in the present study we examined the occurrence of the GLP-1R in PSCs in normal pancreas, in acute/chronic pancreatitis models, as well as the ability of the GLP-1R agonist, liraglutide, to alter function of PSCs and effect PSCs behavior.
We first examined GLP-1R expression in normal pancreas, followed by its expression in PSCs in the pancreas of various pancreatitis models (acute/chronic). Our results show GLP-1R is present predominantly in islets of normal tissue, although it is also weakly present in acinar cells. In the normal islets we found it present in β cells, not α cells, which is consistent with findings in some studies (45, 54), but differ from others(47). GLP-1R has been identified by various methods on islets of numerous species (rat, human, hamsters) (55–57) and GLP-1R activation has also been shown to alter islet cell function (insulin release) (56). Hörsch et al. reported that the GLP-1R had a high density in the β cell zone and were not detected in the α cell zone (45), similar to our results. This distribution pattern is in accordance with the numerous studies reporting GLP-1 binding sites on β cell lines (58, 59). In contrast, the possibility of GLP-1R expression in α cells is controversial, with some reporting it is present (47, 48), whereas others report no GLP-1R in α cells, similar to our results (45, 54).
Our findings that normal pancreatic acinar cells have a low level of expression of GLP-1R is similar to those from some reports (60, 61) but differ from others, which report no GLP-1R in normal pancreatic acinar cells by assessing GLP-1R mRNA levels (45, 57). Furthermore, the pancreatic acinar cell line, AR42J cells responds to GLP-1R agonist (increased cAMP) (60) raising the possibility that acinar cells might potentially contribute to liraglutide-induced pancreatitis. Further investigation focusing on acinar cell responses to liraglutide treatment would be required in the future. We did not find GLP-1R on duct cells in normal pancreas. This result is similar to the findings in two other studies (45, 57). Lastly, in normal pancreas, we did not find GLP-1R in normal pancreatic arteries. This result is consistent with another study (62).
A number of our results, in both acute and chronic pancreatitis models, are consistent with the conclusion that GLP-1Rs are expressed in activated PSCs and expression is increased over normal pancreas. First, we observed an increased number of activated PSCs in L-arginine induced acute pancreatitis, which is consistent with findings from a previous report (63, 64), which demonstrated increased α-SMA expression(a marker for activated PSCs) coincided with TGF-β1 activation in this model. Second, in the chronic pancreatitis model (WBN/KOB-rat), we found increased numbers of PSCs, which is consistent with findings in other studies (65, 66). Third, we found in both the pancreatitis models a marked overexpression of GLP-1R, which generally co-localized with activated PSCs or with areas of marked increased in PSC activity in the chronic pancreatitis model with enhanced fibrosis. However, in our study, the localization of PSCs (peri-acinar and peri-islet) in pancreatitis differed in some aspects from other reports. In some studies, activated PSCs are reported in vascular walls (37, 66), whereas we could not find this. We did, however, find that dilated vascular structures in our acute pancreatitis model stained positive for α-SMA. We found activated PSCs not only in peri-acinar lesions, but also in peri-islet lesions in the chronic pancreatitis model which differs from that reported in another pancreatitis study which showed only a peri-acinar location of PSCs (65, 66).
In quiescent PSCs, we found the expression of GLP-1R, as well as numerous other incretin-related receptors including the receptors for glucagon, gastric inhibitory peptide (GIP) receptor and GLP-2R. This result is similar to others in pancreatic β cells, which are reported to express glucagon receptors and GIP receptors as well as GLP-1R, whereas α cells express glucagon and GIP receptors, but no GLP-1Rs (54). In culture-activated PSCs from normal pancreas, our results demonstrate there is a marked increase in the expression of GLP-1R with activation, as well as an increase in numerous other incretin-related receptors. Similar to our results with GLP-1R, an increased expression in activated PSCs of the AT1 receptor (36), ET receptor (67) and PAR2 (68) have also been reported. These results suggest the possibility that these GPCRs, including GLP-1R may play an important role in conditions where PSCs are activated such as acute/chronic pancreatitis, and may contribute to the various changes seen with these diseases.
With liraglutide treatment, we did not find an increased expression of mRNA’s of inflammatory cytokines (TNF-α, IL-1β) or chemokines (MCP-1, CX3CL1) in activated PSCs. These results are consistent with numerous findings in various studies in other tissues. Specifically, liraglutide did not induce ROS or increase PTX3 (an inflammatory signal) expression in endothelial cells in one study (69), and in fact, it attenuated the increases in both ROS and PTX3 induced by TNF-α, suggesting it was not increasing inflammatory activity. Exendin-4, a GLP-1R agonist, also suppressed basal expression of several inflammatory mediators and in combination with phosphodiesterase inhibitors, decreased CXCL10 expression by IFN-γ (a type II-interferon) in human islets and in MIN6 cells (a mouse β cell line) (70). Lastly, GLP-1 attenuated IL-1β-mRNA expression induced by lipopolysaccharide (a component of gram-negative bacteria) in rat astrocytes (71). These results are consistent with the conclusion that in PSCs and other cells with GLP-1Rs, GLP-1R agonists do not stimulate inflammatory responses, and may, in fact, attenuate their development by other inflammatory stimulants. From our data, we can not establish whether the prolonged GLP-1R agonist treatment effects quiescent PSCs and induces them to proliferate and stay activated. Therefore, at present we can not address the events of induction of the pancreatitis.
GLP-1 has not only insulinotropic effects in β cells, but also has cell-proliferative and anti-apoptotic effects (72, 73). A proliferative effect by GLP-1 agonists has also been reported in mouse skin cells and the pancreatic acinar cell line, AR42J (74, 75). Our results demonstrate that the GLP-1R agonist, liraglutide stimulated proliferation of PSCs. These results are similar to the effects of activation of two other GPCRs on PSCs, the AT1R and PAR2-R, which also result in PSC cell proliferation (35, 36, 68). In contrast, our results differ from activation of ET-1 receptors on PSCs, which does not stimulate proliferation (67, 76). The role of the proliferative effect on PSCs of liraglutide and the other GPCRs in acute or chronic pancreatitis at present is unknown, as is the possibility that prolonged treatment with GLP-1R agonists for diabetes or other diseases, may lead to increased fibrosis due to enhanced PSC growth and activation.
In other cells, various signaling cascades, including activation of MAPK, PI3K, adenylate cyclase and PKCζ by GLP-1 agonists, mediate the effects of GLP-1R activation on various cellular functions including insulin secretion and biosynthesis, β cell proliferation and neogenesis (16, 77, 78). We found several signaling cascades are activated by liraglutide in PSCs including ERK, JNK, p38, Akt and IκBα. Furthermore, the ERK activation was essential for GLP-1R-mediated PSC proliferation. These results have similarities and differences from GLP-1R-mediated growth reported in other cells. GLP-1-induced cell proliferation of β cells is inhibited by a ERK inhibitor, suggesting the involvement of ERK (73). Furthermore, ERK activation by GLP-1 in mouse skin cells (74) mediates its proliferative effect. In contrast, in other studies (72, 73), protein kinase A, phospho-inositol-3-phosphate kinase (PI3K) and PKC-zeta activation are needed for GLP-1 induced cell proliferation of β cells. Our finding of high basal ERK level and a rapid increase in ERK activation with liraglutide that return to normal by 30 min is similar to reports by others in PSCs with other stimuli and in other cells with ERK activation (79, 80). Our results with GLP-1R-mediated PSC proliferation also have similarities and differences with the ability of other GPCRs to stimulate growth in PSCs. Similar to our findings, PSC cell proliferation induced by activation of ERK signaling pathway is needed for growth mediated by PAR2-receptor or angiotensin-receptor activation, however the latter effect requires EGFR-transactivation (35, 68). These results show that ERK is one of the important signal cascades mediating cell proliferation of PSCs.
In conclusion, we demonstrated the GLP-1R expression in normal pancreas occurs primarily in β cells and only weakly in acinar cells or quiescent PSCs. However, in acute/chronic pancreatitis there is marked increase in GLP-1R, primarily in PSCs. Furthermore, with activation of normal PSCs, there is marked increase in the GLP-1R level. However, in activated PSCs, liraglutide, a GLP-1R agonist, did not stimulate release of inflammatory cytokines/chemokines, however, it stimulated PSC proliferation which was dependent on ERK activation. Chronic GLP-1 use in humans has recently been reported to cause expansion of both exocrine and endocrine pancreas and led to concerns about its unattended proliferative effects in these pancreatic tissues (81). Our study raises a similar concern for its long-term proliferative effect on PSCs. At present, it remains unclear whether these changes in PSCs contribute to the development of pancreatitis seen in patients with chronic use of GLP-1R agonists, or whether they could lead to complications of the increased PSCs proliferation due to long-term use of GLP-1 agonists.
Supplementary Material
In the top panel (1st passage), expression of GLP-1R protein in freshly isolated PSCs (1st) is shown, and in the bottom panel (3rd passage), expression of GLP-1R protein in activated PSCs is shown. All cells were identified using nuclear staining and the percentage of the cells expression GLP-1R was assessed. Magnification is x200. These results were representative of four experiments. Abbreviations GLP-1R=Glucagon-like peptide receptor-1.
Acknowledgments
Grant support: This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (20590808, Ito T) and the Research Committee provided by the Ministry of Health, Labour, and Welfare Japan (50253448, Ito T). Also partially supported by intramural research funds of NIDDK, NIH.
We appreciate the technical support from the Research Support Center, Graduate School of Medical Sciences, Kyushu University and partial support from intramural funds from NIH/ /DIDDK funds.
Abbreviations
- AP
acute pancreatitis
- CP
chronic pancreatitis
- GIP
gastric inhibitory peptide
- GLP-1
glucagon-like peptide-1
- GLP-1R
glucagon-like peptide-1 receptor
- GLP-2R
glucagon-like peptide-2 receptor
- PSCs
pancreatic stellate cells
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
All of the authors declare no conflict of interest.
Competing interests None
References
- 1.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. J Am Med Assoc. 2007;298(2):194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ, Nauck MA. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 3.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. doi: 10.1124/pr.108.000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seino Y, Rasmussen MF, Nishida T, et al. Efficacy and safety of the once-daily human GLP-1 analogue, liraglutide, vs glibenclamide monotherapy in japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26(5):1013–22. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 5.Butler PC, Dry S, Elashoff R. GLP-1-based therapy for diabetes: What you do not know can hurt you. Diabetes Care. 2010;33(2):453–5. doi: 10.2337/dc09-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler PC, Matveyenko AV, Dry S, et al. Glucagon-like peptide-1 therapy and the exocrine pancreas: Innocent bystander or friendly fire? Diabetologia. 2010;53(1):1–6. doi: 10.1007/s00125-009-1591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1based therapies. Gastroenterology. 2011;141(1):150–6. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knezevich E, Crnic T, Kershaw S, et al. Liraglutide-associated acute pancreatitis. Am J Health Syst Pharm. 2012;69(5):386–9. doi: 10.2146/ajhp110221. [DOI] [PubMed] [Google Scholar]
- 9.Butler AE, Galasso R, Matveyenko A, et al. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia. 2010;53(1):21–6. doi: 10.1007/s00125-009-1556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gier B, Matveyenko AV, Kirakossian D, et al. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the kras(G12D) mouse model. Diabetes. 2012;61(5):1250–62. doi: 10.2337/db11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer SN, Drake AJ, 3rd, West RL, et al. Case report of acute necrotizing pancreatitis associated with combination treatment of sitagliptin and exenatide. Endocr Pract. 2012;18(1):e10–3. doi: 10.4158/EP11264.CR. [DOI] [PubMed] [Google Scholar]
- 12.Famularo G, Gasbarrone L, Minisola G. Pancreatitis during treatment with liraglutide. JOP. 2012;13(5):540–1. doi: 10.6092/1590-8577/1107. [DOI] [PubMed] [Google Scholar]
- 13.Lee PH, Stockton MD, Franks AS. Acute pancreatitis associated with liraglutide. Ann Pharmacother. 2011;45(4):e22. doi: 10.1345/aph.1P714. [DOI] [PubMed] [Google Scholar]
- 14.Franks AS, Lee PH, George CM. Pancreatitis: A potential complication of liraglutide? Ann Pharmacother. 2012;46(11):1547–53. doi: 10.1345/aph.1Q789. [DOI] [PubMed] [Google Scholar]
- 15.Anderson SL, Trujillo JM. Association of pancreatitis with glucagon-like peptide-1 agonist use. Ann Pharmacother. 2010;44(5):904–9. doi: 10.1345/aph.1M676. [DOI] [PubMed] [Google Scholar]
- 16.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 17.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 18.Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379(6560):69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 19.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137(7):2968–78. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 20.Gorelick FS. Pancreas cell physiology and pancreatitis cell biology: Summary of a symposium held at the joint meeting of the EPC and the IAP, heidelberg 2002. Pancreatology. 2003;3(3):207–8. doi: 10.1159/000070730. [DOI] [PubMed] [Google Scholar]
- 21.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141(1):358–69. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoque R, Malik AF, Gorelick F, et al. Sterile inflammatory response in acute pancreatitis. Pancreas. 2012;41(3):353–7. doi: 10.1097/MPA.0b013e3182321500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- 24.Vonlaufen A, Apte MV, Imhof BA, et al. The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol. 2007;213(3):239–48. doi: 10.1002/path.2231. [DOI] [PubMed] [Google Scholar]
- 25.Pandol SJ, Raraty M. Pathobiology of alcoholic pancreatitis. Pancreatology. 2007;7(2–3):105–14. doi: 10.1159/000104235. [DOI] [PubMed] [Google Scholar]
- 26.Erkan M, Adler G, Apte MV, et al. StellaTUM: Current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61(2):172–8. doi: 10.1136/gutjnl-2011-301220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omary MB, Lugea A, Lowe AW, et al. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J Clin Invest. 2007;117(1):50–9. doi: 10.1172/JCI30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandol S, Gukovskaya A, Edderkoui M, et al. Epidemiology, risk factors, and the promotion of pancreatic cancer: Role of the stellate cell. J Gastroenterol Hepatol. 2012;27(SUPPL 2):127–34. doi: 10.1111/j.1440-1746.2011.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andoh A, Takaya H, Saotome T, et al. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology. 2000;119(1):211–9. doi: 10.1053/gast.2000.8538. [DOI] [PubMed] [Google Scholar]
- 30.Masamune A, Kikuta K, Watanabe T, et al. Pancreatic stellate cells express toll-like receptors. J Gastroenterol. 2008;43(5):352–62. doi: 10.1007/s00535-008-2162-0. [DOI] [PubMed] [Google Scholar]
- 31.Uchida M, Ito T, Nakamura T, et al. ERK pathway and sheddases play an essential role in ethanol-induced CX3CL1 release in pancreatic stellate cells. Lab Invest. 2013;93(1):41–53. doi: 10.1038/labinvest.2012.156. [DOI] [PubMed] [Google Scholar]
- 32.Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: Identification, isolation, and culture. Gut. 1998;43(1):128–33. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115(2):421, 432, 491, 492. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 34.Charo C, Holla V, Arumugam T, et al. Prostaglandin E2 regulates pancreatic stellate cell activity via the EP4 receptor. Pancreas. 2012 doi: 10.1097/MPA.0b013e318264d0f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hama K, Ohnishi H, Yasuda H, et al. Angiotensin II stimulates DNA synthesis of rat pancreatic stellate cells by activating ERK through EGF receptor transactivation. Biochem Biophys Res Commun. 2004;315(4):905–11. doi: 10.1016/j.bbrc.2004.01.155. [DOI] [PubMed] [Google Scholar]
- 36.Reinehr R, Zoller S, Klonowski-Stumpe H, et al. Effects of angiotensin II on rat pancreatic stellate cells. Pancreas. 2004;28(2):129–37. doi: 10.1097/00006676-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, Kuno A, Masuda K, et al. Candesartan, an angiotensin II receptor antagonist, suppresses pancreatic inflammation and fibrosis in rats. J Pharmacol Exp Ther. 2003;307(1):17–23. doi: 10.1124/jpet.103.053322. [DOI] [PubMed] [Google Scholar]
- 38.Berna MJ, Seiz O, Nast JF, et al. CCK1 and CCK2 receptors are expressed on pancreatic stellate cells and induce collagen production. J Biol Chem. 2010;285(50):38905–14. doi: 10.1074/jbc.M110.125534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips PA, Yang L, Shulkes A, et al. Pancreatic stellate cells produce acetylcholine and may play a role in pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 2010;107(40):17397–402. doi: 10.1073/pnas.1000359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tashiro M, Ernst SA, Edwards J, et al. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65(2):118–26. doi: 10.1159/000057713. [DOI] [PubMed] [Google Scholar]
- 41.Akimoto T, Nakama K, Katsuta Y, et al. Characterization of a novel congenic strain of diabetic fatty (WBN/Kob-lepr(fa)) rat. Biochem Biophys Res Commun. 2008;366(2):556–62. doi: 10.1016/j.bbrc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi K, Kim JH, Hara H, et al. WBN/Kob rats. A new spontaneously occurring model of chronic pancreatitis. Int J Pancreatol. 1990;6(4):231–47. [PubMed] [Google Scholar]
- 43.Nakamura T, Ito T, Oono T, et al. Bacterial DNA promotes proliferation of rat pancreatic stellate cells thorough toll-like receptor 9: Potential mechanisms for bacterially induced fibrosis. Pancreas. 2011;40(6):823–31. doi: 10.1097/MPA.0b013e318224a501. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura T, Ito T, Igarashi H, et al. Cytosolic double-stranded DNA as a damage-associated molecular pattern induces the inflammatory response in rat pancreatic stellate cells: A plausible mechanism for tissue injury-associated pancreatitis. Int J Inflam. 2012;2012:504128. doi: 10.1155/2012/504128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hörsch D, Göke R, Eissele R, et al. Reciprocal cellular distribution of glucagon-like peptide-1 (GLP-1) immunoreactivity and GLP-1 receptor mRNA in pancreatic islets of rat. Pancreas. 1997;14(3):290–4. doi: 10.1097/00006676-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Goke R, Oltmer B, Sheikh SP, et al. Solubilization of active GLP-1 (7–36)amide receptors from RINm5F plasma membranes. FEBS Lett. 1992;300(3):232–6. doi: 10.1016/0014-5793(92)80852-8. [DOI] [PubMed] [Google Scholar]
- 47.Heller RS, Kieffer TJ, Habener JF. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. Diabetes. 1997;46(5):785–91. doi: 10.2337/diab.46.5.785. [DOI] [PubMed] [Google Scholar]
- 48.Dillon JS, Lu M, Bowen S, et al. The recombinant rat glucagon-like peptide-1 receptor, expressed in an alpha-cell line, is coupled to adenylyl cyclase activation and intracellular calcium release. Exp Clin Endocrinol Diabetes. 2005;113(3):182–9. doi: 10.1055/s-2005-837526. [DOI] [PubMed] [Google Scholar]
- 49.Kedees MH, Grigoryan M, Guz Y, et al. Differential expression of glucagon and glucagon-like peptide 1 receptors in mouse pancreatic alpha and beta cells in two models of alpha cell hyperplasia. Mol Cell Endocrinol. 2009;311(1–2):69–76. doi: 10.1016/j.mce.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masamune A, Kikuta K, Satoh M, et al. Differential roles of signaling pathways for proliferation and migration of rat pancreatic stellate cells. Tohoku J Exp Med. 2003;199(2):69–84. doi: 10.1620/tjem.199.69. [DOI] [PubMed] [Google Scholar]
- 51.Koehler JA, Baggio LL, Lamont BJ, et al. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes. 2009;58(9):2148–61. doi: 10.2337/db09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tatarkiewicz K, Smith PA, Sablan EJ, et al. Exenatide does not evoke pancreatitis and attenuates chemically induced pancreatitis in normal and diabetic rodents. Am J Physiol Endocrinol Metab. 2010;299(6):E1076–86. doi: 10.1152/ajpendo.00479.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: Acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98(2):271–84. doi: 10.1016/j.diabres.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Moens K, Heimberg H, Flamez D, et al. Expression and functional activity of glucagon, glucagon-like peptide I, and glucose-dependent insulinotropic peptide receptors in rat pancreatic islet cells. Diabetes. 1996;45(2):257–61. doi: 10.2337/diab.45.2.257. [DOI] [PubMed] [Google Scholar]
- 55.Abrahamsen N, Nishimura E. Regulation of glucagon and glucagon-like peptide-1 receptor messenger ribonucleic acid expression in cultured rat pancreatic islets by glucose, cyclic adenosine 3′,5′-monophosphate, and glucocorticoids. Endocrinology. 1995;136(4):1572–8. doi: 10.1210/endo.136.4.7534705. [DOI] [PubMed] [Google Scholar]
- 56.Rosselin G, Leclercq-Meyer V, Boissart C, et al. GLP-1 receptors in golden syrian hamster islets: Identification and functional characterization. Endocrine. 1998;8(3):323–30. doi: 10.1385/ENDO:8:3:323. [DOI] [PubMed] [Google Scholar]
- 57.Huypens P, Ling Z, Pipeleers D, et al. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43(8):1012–9. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- 58.Goke R, Conlon JM. Receptors for glucagon-like peptide-1(7–36) amide on rat insulinoma-derived cells. J Endocrinol. 1988;116(3):357–62. doi: 10.1677/joe.0.1160357. [DOI] [PubMed] [Google Scholar]
- 59.Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A. 1992;89(18):8641–5. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raufman JP, Singh L, Singh G, et al. Truncated glucagon-like peptide-1 interacts with exendin receptors on dispersed acini from guinea pig pancreas. identification of a mammalian analogue of the reptilian peptide exendin-4. J Biol Chem. 1992;267(30):21432–7. [PubMed] [Google Scholar]
- 61.Zhou J, Montrose-Rafizadeh C, Janczewski AM, et al. Glucagon-like peptide-1 does not mediate amylase release from AR42J cells. J Cell Physiol. 1999;181(3):470–8. doi: 10.1002/(SICI)1097-4652(199912)181:3<470::AID-JCP11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 62.Hirata Y, Kurobe H, Nishio C, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, attenuates neointimal hyperplasia after vascular injury. Eur J Pharmacol. 2012;699(1–3):106–11. doi: 10.1016/j.ejphar.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 63.Neuschwander-Tetri BA, Bridle KR, Wells LD, et al. Repetitive acute pancreatic injury in the mouse induces procollagen alpha1(I) expression colocalized to pancreatic stellate cells. Lab Invest. 2000;80(2):143–50. doi: 10.1038/labinvest.3780018. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez AM, Garcia T, Samper E, et al. Assessment of the protective effects of oral tocotrienols in arginine chronic-like pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G846–55. doi: 10.1152/ajpgi.00485.2010. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu K, Kobayashi M, Tahara J, et al. Cytokines and peroxisome proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterology. 2005;128(7):2105–18. doi: 10.1053/j.gastro.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 66.Kuno A, Yamada T, Masuda K, et al. Angiotensin-converting enzyme inhibitor attenuates pancreatic inflammation and fibrosis in male wistar Bonn/Kobori rats. Gastroenterology. 2003;124(4):1010–9. doi: 10.1053/gast.2003.50147. [DOI] [PubMed] [Google Scholar]
- 67.Klonowski-Stumpe H, Reinehr R, Fischer R, et al. Production and effects of endothelin-1 in rat pancreatic stellate cells. Pancreas. 2003;27(1):67–74. doi: 10.1097/00006676-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Masamune A, Kikuta K, Satoh M, et al. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2005;312(2):651–8. doi: 10.1124/jpet.104.076232. [DOI] [PubMed] [Google Scholar]
- 69.Shiraki A, Oyama J, Komoda H, et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-alpha-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis. 2012;221(2):375–82. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 70.Pugazhenthi U, Velmurugan K, Tran A, et al. Anti-inflammatory action of exendin-4 in human islets is enhanced by phosphodiesterase inhibitors: Potential therapeutic benefits in diabetic patients. Diabetologia. 2010;53(11):2357–68. doi: 10.1007/s00125-010-1849-y. [DOI] [PubMed] [Google Scholar]
- 71.Iwai T, Ito S, Tanimitsu K, et al. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55(4):352–60. doi: 10.1016/j.neures.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 72.Buteau J, Foisy S, Rhodes CJ, et al. Protein kinase czeta activation mediates glucagon-like peptide-1-induced pancreatic beta-cell proliferation. Diabetes. 2001;50(10):2237–43. doi: 10.2337/diabetes.50.10.2237. [DOI] [PubMed] [Google Scholar]
- 73.Friedrichsen BN, Neubauer N, Lee YC, et al. Stimulation of pancreatic β-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol. 2006;188(3):481–92. doi: 10.1677/joe.1.06160. [DOI] [PubMed] [Google Scholar]
- 74.List JF, He H, Habener JF. Glucagon-like peptide-1 receptor and proglucagon expression in mouse skin. Regul Pept. 2006;134(2–3):149–57. doi: 10.1016/j.regpep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 75.Zhou J, Wang X, Pineyro MA, et al. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes. 1999;48(12):2358–66. doi: 10.2337/diabetes.48.12.2358. [DOI] [PubMed] [Google Scholar]
- 76.Masamune A, Satoh M, Kikuta K, et al. Endothelin-1 stimulates contraction and migration of rat pancreatic stellate cells. World J Gastroenterol. 2005;11(39):6144–51. doi: 10.3748/wjg.v11.i39.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drucker DJ. Minireview: The glucagon-like peptides. Endocrinology. 2001;142(2):521–7. doi: 10.1210/endo.142.2.7983. [DOI] [PubMed] [Google Scholar]
- 78.Xu G, Kaneto H, Lopez-Avalos MD, et al. GLP-1/exendin-4 facilitates β-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract. 2006;73(1):107–10. doi: 10.1016/j.diabres.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 79.Kikuta K, Masamune A, Satoh M, et al. 4-hydroxy-2, 3-nonenal activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. World J Gastroenterol. 2004;10(16):2344–51. doi: 10.3748/wjg.v10.i16.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masamune A, Kikuta K, Satoh M, et al. Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J Pharmacol Exp Ther. 2002;302(1):36–42. doi: 10.1124/jpet.302.1.36. [DOI] [PubMed] [Google Scholar]
- 81.Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013 doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In the top panel (1st passage), expression of GLP-1R protein in freshly isolated PSCs (1st) is shown, and in the bottom panel (3rd passage), expression of GLP-1R protein in activated PSCs is shown. All cells were identified using nuclear staining and the percentage of the cells expression GLP-1R was assessed. Magnification is x200. These results were representative of four experiments. Abbreviations GLP-1R=Glucagon-like peptide receptor-1.