Abstract
The purpose of this study was to evaluate the relationship between the cardiac and sympathetic baroreflex sensitivities within healthy, young humans. The sensitivities of the cardiac and sympathetic baroreflex were compared in 53 normotensive individuals (28 men and 25 women; age 24 yrs ± 0.9; body mass index 24 ± 0.3 cm/kg2, mean ±SEM). Heart rate, arterial blood pressure and peroneal muscle sympathetic nerve activity (MSNA) were recorded under resting conditions (heart rate 58 ± 1 beats/min; systolic blood pressure 126 ± 2 mmHg; diastolic blood pressure 72 ± 1 mmHg; mean arterial blood pressure 89 ± 1 mmHg; MSNA 18 ± 1 bursts/min) and during rapid changes in blood pressure induced by sequential boluses of nitroprusside and phenylephrine. Cardiac and sympathetic baroreflex sensitivities were analyzed using the slopes of the linear portions of the MSNA-diastolic blood pressure and RRI-systolic blood pressure relationships, respectively. When individual cardiac baroreflex sensitivity was compared to sympathetic baroreflex sensitivity, no correlation (RRI: r = −0.13, HR: r =0.21) was observed when studied as a group. Analysis by sex unveiled a correlation in women between the cardiac and sympathetic baroreflex sensitivities (RRI: r = −0.54, P=0.01, no correlation with HR: r =0.29). No relationship was found in men (RRI: r = 0.17; HR: r = 0.12). These results indicate that although both cardiac and sympathetic efferents function in baroreflex control of arterial pressure, there is no correlation in their sensitivities within healthy normotensive humans. However, sex-stratified data indicate that sex-based differential correlations might exist.
Summary words: baroreflex sensitivity, sympathetic, cardiac, sex differences
INTRODUCTION
A primary mechanism through which the autonomic nervous system regulates blood pressure is through the arterial baroreflexes1. Measurements of baroreflex sensitivity, whether spontaneous or via vasoactive drug injection, evaluate the responsiveness or gain in the reflex during transient changes in arterial pressure. Changes in the activity of the cardiac efferent arm are estimated by alterations in heart rate or R-R interval (RRI) whereas responses of the sympathetic component of the baroreflex are reflected by changes in muscle sympathetic nerve activity (MSNA).
Studies show that certain stimuli evoke changes in cardiac baroreflex sensitivity while others alter sympathetic baroreflex sensitivity2–5. Furthermore, the sensitivity of the cardiac baroreflex appears to be diminished with aging, whereas the sensitivity of the sympathetic baroreflex appears to be preserved6 . With evidence of this dissociation between cardiac and sympathetic baroreflexes, it cannot be assumed that either measurement on its own is a complete reflection of how effectively the baroreflex controls arterial pressure.
To our knowledge, a direct comparison of the relationship between individual cardiac and sympathetic baroreflex sensitivities has not been performed previously in humans or animals. There are reports in which simultaneous measurements of the cardiac and sympathetic baroreflex sensitivities have been performed but there are minimal references on the inter-individual correlation of these values5. Furthermore, given the recent evidence of fundamental sex differences in autonomic mechanisms regulating blood pressure7, 8, there may be sex differences in the relationship between the two baroreflex sensitivities.
To better understand how these two components of the baroreflex interact in the regulation arterial blood pressure within individuals, we evaluated the correlation of cardiac and sympathetic baroreflexes sensitivities in individual subjects at rest. We hypothesized that within individuals, an inverse correlation would exist between cardiac and sympathetic baroreflex sensitivities. Furthermore, the female sex hormones might affect blood regulation since evidence demonstrates that A) estrogen in the central nuclei of the brainstem alters baroreflex responsiveness9 and B) sympathetic nerve activity is not related to resistance of the peripheral vessels in women as it is men8. Therefore, we further hypothesized that the relationship between sympathetic and heart rate baroreflex sensitivity might be affected by sex, such that there may be no correlation between the two reflexes in women.
METHODS
Subjects
All records used in this study were retrospectively analyzed from previous10, 11 and on-going trials completed in our laboratory. The investigations from which the data were obtained had ethical approval from the institutional review board of the Mayo Clinic. Fifty-three healthy normotensive non-smokers (28 men, 25 women) gave their informed consent to participate in the specific studies (age 24 yrs ±0.8; body mass index 24 cm/kg2 ±0.3; mean ±SEM). To minimize the effects of the reproductive hormones on autonomic control or cardiovascular function, all women were studied in the early follicular phase of the menstrual cycle or in the low hormone phase of oral contraceptive use12.
Measurements
A 3-lead electrocardiogram was used for continuous monitoring of heart rate. Arterial blood pressure (ABP) monitoring was performed via 5 cm, 20 gauge brachial artery catheter. MSNA was measured with microneurography from the right peroneal nerve at the fibular head using tungsten microelectrodes as described by Sundlöf and Wallin13. The recorded signal was amplified 80,000 fold, band passed filtered (700 to 2000 Hz), rectified and integrated (time constant 0.1 s) by a nerve traffic analyzer.
Protocol
All studies were performed in a clinical research laboratory in the Clinical Research Unit of the Mayo CTSA where ambient temperature was controlled between 22 and 24°C. Subjects were asked to not consume anything except small volumes of water within 2 h of the experiment and were asked to abstain from caffeine or alcohol consumption 24 h before the study. On arrival to the laboratory, subjects rested in the supine positions during instrumentation. Baseline heart rate, arterial pressures and MSNA were measured over a 5 min period of quiet supine rest. This was followed by two modified Oxford trials to estimate baroreflex sensitivities as described previously14. Briefly, sodium nitroprusside (NTP, 100 µg) was infused through a venous catheter inserted into an antecubital vein in the dominant arm, which was followed by phenylephrine (PE, 150 µg) 60 s later. Data were collected for a further 2 min.
Data analyses
Data were analyzed only from control periods i.e. prior to any manipulations associated with the specific study. Data were sampled at 240 Hz using data acquisition software (Windaq, Dataq Instruments; Akron, OH) and stored on a personal computer for offline analysis. Heart rate, RRI, MSNA, systolic, diastolic, and mean arterial pressures, were taken from the 5 min period immediately preceding the nitroprusside administration. Sympathetic bursts in the integrated neurogram were identified using a custom-manufactured automated analysis program15; burst identification was then corrected by inspection by a single observer (ECH). The program then compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle.
Assessment of baroreflex sensitivity
Cardiac baroreflex
The sensitivities of baroreflex control of the heart were assessed using the relationship between R-R interval (RRI) and systolic blood pressure during the vasoactive drug boluses16, 17. The slope of the linear portion of this relation was used as an index of baroreflex sensitivity (12–15 points per regression line). Values for RRI from baroreflex trials were pooled over 2 mmHg ranges for analysis to minimize variability due to non-baroreflex influences such as respiration16. Analysis of baroreflex sensitivity in terms of RRI gives results that are directly related to efferent vagal activity to the heart18. However, the reciprocal relationship between heart rate and RRI can result in a decrease in the slope of the RRI pressure relation with changes in baseline heart rate due to the mathematical effect that a given change in heart rate results in less of a change in RRI when baseline heart rate is higher19. This effect is minimized when data are expressed in terms of heart rate. Data in terms of both RRI and heart rate are presented to be comprehensive in the analysis.
Sympathetic baroreflex
An index of baroreflex control of sympathetic outflow was provided by the relationship between MSNA and diastolic blood pressure during the drug boluses5, 10, 11. To perform a linear regression between the total MSNA activity and pressure, values for MSNA from baroreflex trials were first signal-averaged over 3 mmHg pressure ranges (“bins”) via custom software20. This pooling procedure reduces the statistical impact of the inherent beat-by-beat variability in nerve activity due to non-baroreflex influences. A window of nerve activity that was 1.0 s in length and synchronized by the R wave of the electrocardiogram was signal averaged. The window was time shifted to account for the latency between R waves and sympathetic bursts. The duration of the shift was varied as needed from subject to subject. Any cardiac cycle not followed by a burst was assigned a total integrated activity of zero. Diastolic blood pressure was used because MSNA correlates more closely with diastolic blood pressure than with systolic pressure21. The sensitivity of the baroreflex measured by the modified Oxford method was defined as the slope of the linear regression between MSNA and the means of the diastolic blood pressure bins. Assessment of cardiac and sympathetic baroreflex sensitivities were performed by a single observer (ECH).
Statistics
Baseline demographic data are presented as means ± SEM. Linear regression analysis was used to evaluate the correlation between values for cardiac and sympathetic baroreflex sensitivity. The alpha level was set at 0.05. Subgroup analyses were performed to evaluate correlation between cardiac and sympathetic baroreflex sensitivity based on sex differences.
RESULTS
Baseline demographics and cardiovascular hemodynamics
There was no difference in baseline demographics with the exception of greater height and weight in men (Table 1, P<0.001). Table 2 outlines baseline cardiovascular variables at rest which were not different between men and women.
Table 1.
Average baseline demographic variables for patients ± SEM.
| Variables | Combined (n=53) |
Men (n=28) |
Women (n=25) |
P value |
|---|---|---|---|---|
| Age (y) | 24±0.9 | 24±1.3 | 25±1.0 | 0.54 |
| Height (cm) | 173.7±1.2 | 179.7±1.2 | 161.3±1.3 | <0.001 |
| Weight (kg) | 71.8±1.5 | 77.0±1.9 | 66.1±2.0 | <0.001 |
| BMI (m/kg2) | 23.7±0.32 | 24.0±0.40 | 23.4±0.48 | 0.40 |
SEM = Standard error of the mean; BMI = body mass index
Table 2.
Average baseline cardiovascular and neural variables ±SEM.
| Variables | Combined (n=353) |
Men (n=28) |
Women (n=25) |
P value |
|---|---|---|---|---|
| HR (beats/min) | 58±1.1 | 58±1.4 | 57±1.7 | 0.56 |
| SBP (mmHg) | 126±2.0 | 127±2.4 | 124±3.4 | 0.50 |
| DBP (mmHg) | 72±1.2 | 73±1.4 | 70±2.2 | 0.31 |
| MAP (mmHg) | 89±1.3 | 89±1.5 | 89±2.5 | 0.98 |
| MSNA (bursts/min) | 18.1±1.2 | 19.1±1.6 | 16.9±2.0 | 0.40 |
SEM = standard error of mean; HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; MSNA = muscle sympathetic nerve activity
Averaged data for cardiac and sympathetic baroreflex sensitivities (including RRI, heart rate and MSNA) are shown in Table 3. There are no significant differences in the average baroreflex sensitivities between men and women.
Table 3.
Averaged values ±SEM for cardiac baroreflex sensitivity measured by RRI or HR slope and sympathetic baroreflex sensitivity measured by MSNA slope.
| Baroreflex sensitivity |
Combined (n=53) |
Men (n=28) |
Women (n=25) |
P value |
|---|---|---|---|---|
| RRI slope (ms/mmHg) | 20.5±1.3 | 22.1±2.1 | 18.7±1.5 | 0.21 |
| HR slope (beats/min/mmHg) | −1.3±0.07 | −1.3±0.09 | −1.2±0.10 | 0.67 |
| MSNA slope (AU/beat/mmHg) | −6.31±0.40 | −6.6±0.51 | −6.0±0.64 | 0.46 |
SEM = standard error of mean; RRI = R-R interval; HR = heart rate; MSNA = muscle sympathetic nerve activity; AU = arbitrary units
Relationship between MSNA and cardiac baroreflex sensitivity
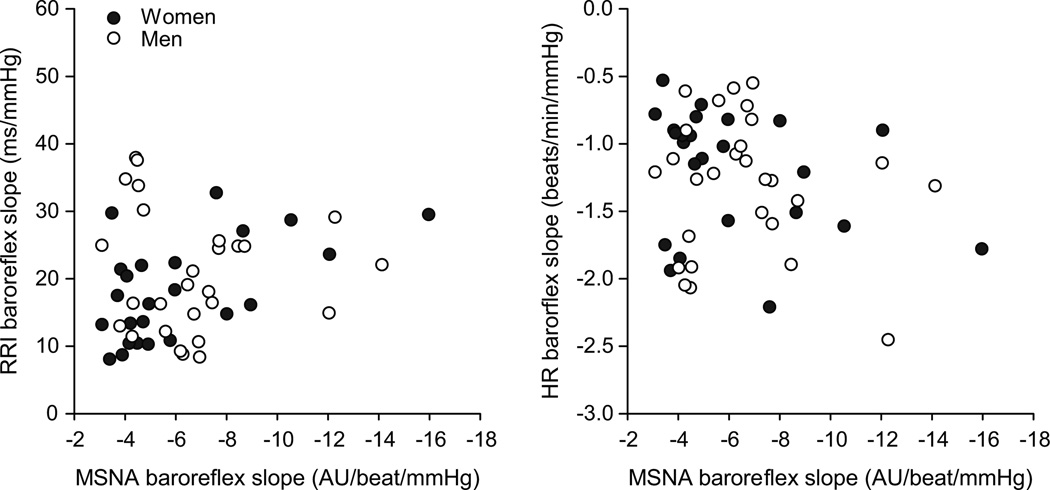
The relationship between individual cardiac baroreflex sensitivity and sympathetic baroreflex sensitivity for the group is shown in Figure 1. There was no correlation between either of the cardiac measures of baroreflex sensitivity and sympathetic baroreflex sensitivity (based on MSNA).
Figure 1.
Regression analysis shows no relationship between sympathetic (MSNA) and cardiac baroreflex sensitivity within individuals when studied as a group including men (open circle) and women (closed circle). This absence of a relationship is evident whether cardiac baroreflex sensitivity is represented by (a) R-R Interval slope or (b) heart rate (HR) slope. AU; arbitrary units.
Sex based differences
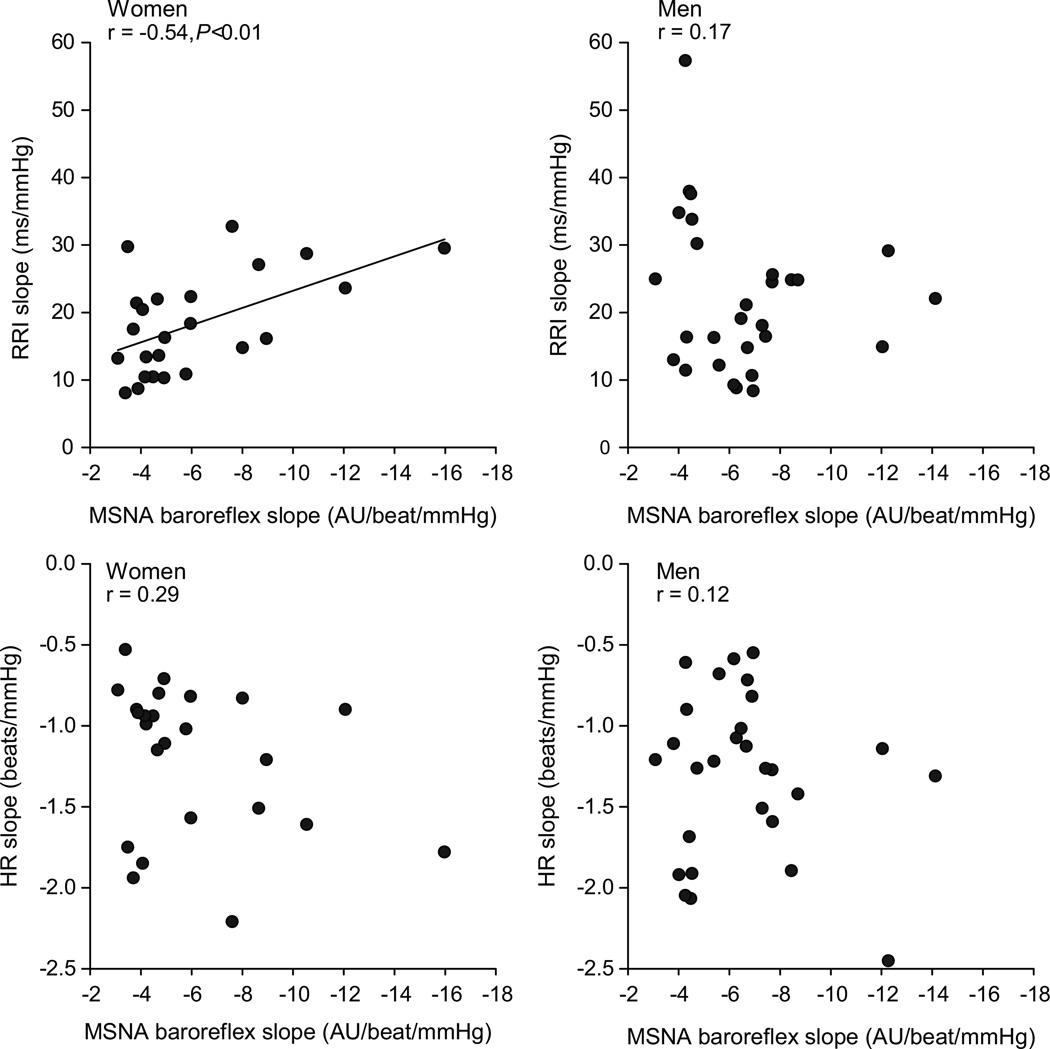
Subgroup analysis based on sex demonstrated some differences in the relationships between sympathetic and cardiac baroreflex sensitivity when men and women were analyzed separately. In women, there was a positive relationship of MSNA baroreflex sensitivity to cardiac baroreflex sensitivity (Figure 2, RRI: r = −0.54, P<0.01). Data using HR to estimate cardiac baroreflex sensitivity failed to show this relationship in women (Figure 2, HR: r = 0.29). In men, there was no correlation between MSNA baroreflex sensitivity and cardiac baroreflex sensitivity based on either RRI or HR (Figure 2, RRI: r = 0.17, HR: r = 0.12).
Figure 2.
Linear regression analysis of the relationship between individual baroreflex sensitivity when separated on the basis of sex. In women there was a positive relationship between sympathetic and cardiac BRS shown by (a) RRI: r = −0.54, P<0.01. This relationship was not significant when HR was used to estimate cardiac BRS in (c) HR: r = 0.29. In men, there was no correlation between sympathetic and cardiac baroreflex sensitivity whether RRI (b) or HR (d) was used. AU; arbitrary units.
DISCUSSION
The major new finding of this study is that within individuals, there does not appear to be any direct correlation between the sensitivities of the cardiac and sympathetic efferent arms of the baroreflex mechanism. These results emphasize the distinct pathways associated with cardiac and sympathetic baroreflex control, and the need to use caution when drawing conclusions about the baroreflex as a whole if only one or the other arm of the reflex is studied.
Relationship between cardiac and sympathetic baroreflexes
The essential pathways of the arterial baroreflex are well established22. Mechanosensitive afferent inputs from carotid and aortic arterial baroreceptors related to alterations in arterial blood pressure are relayed to the nucleus of the solitary tract. Multiple parallel pathways handle information centrally including a sympathoinhibitory pathway, processed through the ventrolateral medulla and intermediolateral cell column, and a cardioinhibitory pathway, processed through vagal preganglionic neurons in the nucleus ambiguus23. While the management of the information is multifaceted, cardiac and sympathetic baroreflex responses are results of the same input and therefore it seems reasonable to postulate that there should be some relationship between the magnitudes and sensitivities of their reactions.
Several relationships might exist between these two elements of the baroreflex. On one hand, individuals with high parasympathetic (vagal) responsiveness in control of heart rate might also have highly responsive sympathetic neural activity, indicating a direct relationship between the two respective baroreflex sensitivities. An alternative possibility is that individuals with more responsive cardiac baroreflex sensitivity might not “require” as much responsiveness in their sympathetic baroreflex to regulate arterial pressure, and vice versa. With either scenario, a relationship between the cardiac and sympathetic baroreflex sensitivities would exist.
The data presented in the present analysis indicate that when studied as a group, there is no correlation between the cardiac and sympathetic baroreflex sensitivities within individuals (Figure 1). There are several reasons for this lack of agreement between cardiac and sympathetic baroreflex sensitivity. First, the baroreflex could be affected by several factors including alterations in baroreceptor input, alterations in the response of the central nuclei to these baroreceptor inputs, and changes in end organ responsiveness. The heart rate baroreflex may be affected by both changes in central nuclei responses to baroreceptor input (central) and end organ responsiveness (peripheral), whereas, the sympathetic baroreflex is not affected by end-organ responsiveness (when measuring MSNA and not changes in TPR). Future studies could focus on end organ responsiveness (i.e. focusing on changes in vessel diameter or TPR), whilst measuring changes in sympathetic nerve activity. Second, we hypothesized that sex could confound the relationship between sympathetic and cardiac baroreflex sensitivities and thus analyzing the group as a whole might mask any relationship between the two reflexes.
Potential role of sex in correlation of cardiac and sympathetic baroreflex sensitivity
It is important to highlight that the aim of this paper was not to investigate sex differences in baroreflex sensitivity (we did not find any sex differences between cardiac and sympathetic baroreflex sensitivity, which is similar to findings reported by Tank et al. 24). The aim of our investigation was to measure whether sex affected the balance between cardiac and sympathetic baroreflex sensitivity. We hypothesized that the relationship which exists between the cardiac and sympathetic arms of the baroreflex control is potentially different between men and women. When using changes in RRI as a measure of cardiac baroreflex sensitivity, higher sensitivity was associated with higher sympathetic baroreflex sensitivity in women (Figure 2). This relationship was not seen when using HR responses to estimate cardiac baroreflex sensitivity (see discussion below). Unlike women, men did not have a correlation between their cardiac and sympathetic baroreflex sensitivities whether using RRI or HR slope. Figure 2 shows the linear regression graphs of these data.
It is possible that the sex differences observed in this study are related to the previously reported sex-related differences in the sympatho-hemodynamic balance controlling arterial blood pressure 8. In that study, normotensive men (but not women) with a high baseline MSNA were found to have a higher total peripheral resistance and lower cardiac output, suggesting that low cardiac output balances the pressor effects of high MSNA in men but not women. In this context, men with high sympathetic baroreflex sensitivity (responsiveness) may display a larger increase in total peripheral resistance in response to decreases in arterial blood pressure compared to women. This response would minimize the necessity for higher cardiac baroreflex sensitivity (heart rate response) during changes in blood pressure in men. In contrast, in young women there is no relationship between baseline MSNA and total peripheral resistance. Thus, women with high sympathetic baroreflex sensitivity might still require high cardiac baroreflex sensitivity to help maintain arterial blood pressure. These sex differences in arterial blood pressure regulation may be related to whether a sympathetic burst increases arterial pressure (or vascular resistance) differently in men and women.
The measurements used for this study evaluated baroreflex responses to sequential decreases and increases in arterial pressures using the gold standard modified Oxford test. Thus, our data reflects the sensitivity of the sympathetic and cardiac baroreflex pushed over a wide pressure range. It is possible that both sympathetic and cardiac baroreflex sensitivities may be different when responding to only increasing or decreasing arterial pressures, given that recent data indicates some “hysteresis” within the baroreflex. Along these lines, Studinger et al25, indicate that the sympathetic baroreflex is more responsive to increasing rather than decreasing pressures in young healthy subjects. Interestingly, data from the same group suggests that the cardiac sympathetic baroreflex is actually more responsive to increasing rather than decreasing pressures26. Consequently, the responsiveness of the sympathetic and cardiac arms of the baroreflex may offset each other depending on the direction of the change in arterial pressure. Potentially, measuring the baroreflex responses to increases and decreases in arterial pressure might point to sex differences in the relationship between sympathetic and cardiac baroreflex sensitivity. In our study we tried splitting the decreasing and increasing pressures into separate baroreflex curves. However, since MSNA is often completely suppressed during the increasing pressure phase (phenylephrine bolus) we found that we could not construct significant baroreflex curves when this segment was analyzed alone.
Cardiac baroreflex sensitivity: R-R interval versus HR
Previous studies regarding cardiac baroreflex sensitivity have sometimes drawn conflicting conclusions based on whether HR or RRI was used to evaluate the sensitivity of the reflex19. The mathematical issues associated with use of one or the other variable add complexity to this issue, and are most often related to conditions in which baseline heart rate / RRI is different between two conditions. These issues have been discussed in detail by O’Leary19. However, since men and women had similar resting heart rates in the present study, we do not feel the potential confound of differences in baseline occurred here. With regard to physiological interpretation of our present data, previous studies have demonstrated that changes in RRI reflect changes in parasympathetic tone, whereas changes in HR are not as tightly linked to changes in vagal activity27, 28. Thus the correlation between cardiac (RRI) and sympathetic baroreflex sensitivities in women raises the possibility that there is some relationship between the separate autonomic arms of the baroreflex in women.
Clinical implications
Evidence suggests that baroreflex sensitivity is lowered in hypertensive patients29, 30 and has heritable qualities31 including potentially altered baroreflex sensitivity in patients with family histories of hypertension32, 33 . Measurements of baroreflex sensitivity in these studies are often indirect and have evaluated only the cardiac arm of the baroreflex. In contrast, it is controversial whether baroreflex modulation of sympathetic tone remains intact or is altered in hypertensive subjects34. This information has led to a discussion about baroreflex sensitivity as a predictor of those individuals at risk of cardiovascular disease and hypertension. Our findings suggest that quantification of one arm of the arterial baroreflex does not necessarily reflect how well the other efferent arm is functioning, especially in men. Therefore, when using baroreflex sensitivity as a prognostic tool, both arms of the baroreflex should be considered, or, at the very least, one arm of the reflex should not be considered to represent the other. This is especially important with increasing interest in using baroreflex sensitivity measures as a prognostic tool for cardiovascular disease1.
Limitations
There are several important limitations to this study. The relationship between the cardiac and sympathetic baroreflex sensitivities is complex and difficult to quantify by simple linear regression. For example, control of heart rate is mediated by both the sympathetic and parasympathetic nervous systems. Therefore, cardiac baroreflex sensitivity as analyzed by RRI and heart rate changes is a composite measurement of parasympathetic and sympathetic cardiac mediation. In addition, hormonal and other neurochemical signals, including locally released NO, angiotensin II and circulating AVP, exert differential modulation on various components of the baroreflex 23. Clearly all of these factors are potential sites of action and make studies in conscious humans challenging. Additionally, measuring baroreflex sensitivity is complex and our conclusions are based on modest sample sizes with subjects studied one time by one group of investigators. To further test and confirm the conclusions we have drawn, baroreflex sensitivities will need to be repeated within individuals, and data will need to originate from multiple experimental groups. Finally, we could not estimate whether increases in cardiac sympathetic tone during baroreflex activation affected changes in RRI which may confound our measurements of cardiac (vagal) baroreflex sensitivity 35.
PERSPECTIVES
There is no correlation between cardiac and sympathetic baroreflex sensitivities when men and women are combined and studied as a group. However, men and women studied separately might have different relationships between their cardiac and sympathetic baroreflex sensitivities. These findings suggest that when measuring baroreflex sensitivity in humans, both efferent arms of the baroreflex should be considered.
Acknowledgments
We are grateful to Shelly Roberts, Karen Krucker, Shirley Kingsley-Berg, Pamela Engrav, Nancy Meyer and Jessica Sawyer for their assistance in the conduct of the studies. Finally, we thank the subjects for their participation.
Funding
Supported by NIH grants HL83947 and DK082424, AHA grant number 070036Z and by Swedish Medical Research Council Grant 12170. This project was also supported by grant number 1 UL1 RR024150 from the National Center for Research Resources (NCRR) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Additional support came from the Mayo Foundation including a philanthropic gift from the Caywood family and the Mayo Clinic Department of Anesthesia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191–207. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. American Journal of Physiology - Heart & Circulatory Physiology. 2004;287:H1658–H1662. doi: 10.1152/ajpheart.00265.2004. [DOI] [PubMed] [Google Scholar]
- 3.Cui J, Wilson TE, Crandall CG. Baroreflex modulation of muscle sympathetic nerve activity during cold pressor test in humans. Am J Physiol Heart Circ Physiol. 2002;282:H1717–H1723. doi: 10.1152/ajpheart.00899.2001. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol. 2003;88:769–774. doi: 10.1113/eph8802632. [DOI] [PubMed] [Google Scholar]
- 5.Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. American Journal of Physiology. 1999;276:H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 6.Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. J Auton Nerv Syst. 1996;60:209–212. doi: 10.1016/0165-1838(96)00057-4. [DOI] [PubMed] [Google Scholar]
- 7.Christou DD, Jones PP, Jordan J, Diedrich A, Robertson D, Seals DR. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 8.Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach JH, Joyner MJ. Sex differences in sympathetic neural-hemodynamic balance: implications for human blood pressure regulation. Hypertension. 2009;53:571–576. doi: 10.1161/HYPERTENSIONAHA.108.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saleh TM, Connell BJ, Saleh MC. Acute injection of 17beta-estradiol enhances cardiovascular reflexes and autonomic tone in ovariectomized female rats. Auton Neurosci. 2000;84:78–88. doi: 10.1016/s1566-0702(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 10.Charkoudian N, Halliwill JR, Morgan BJ, Eisenach JH, Joyner MJ. Influences of hydration on post-exercise cardiovascular control in humans. Journal of Physiology. 2003;552:635–644. doi: 10.1113/jphysiol.2003.048629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charkoudian N, Eisenach JH, Joyner MJ, Roberts SK, Wick DE. Interactions of plasma osmolality with arterial and central venous pressures in control of sympathetic activity and heart rate in humans. American Journal of Physiology - Heart & Circulatory Physiology. 2005;289(6):H2456–H2460. doi: 10.1152/ajpheart.00601.2005. [DOI] [PubMed] [Google Scholar]
- 12.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 13.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudas L, Crossman AA, Morillo CA, Halliwill JR, Tahvanainen KU, Kuusela TA, Eckberg DL. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am J Physiol. 1999;276:H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 15.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. Journal of Applied Physiology. 2002;93:857–864. doi: 10.1152/japplphysiol.01103.2001. [DOI] [PubMed] [Google Scholar]
- 17.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- 18.Parker P, Celler BG, Potter EK, McCloskey DI. Vagal stimulation and cardiac slowing. Journal of the Autonomic Nervous System. 1984;11:226–231. doi: 10.1016/0165-1838(84)90080-8. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary DS. Heart rate control during exercise by baroreceptors and skeletal muscle afferents. Medicine & Science in Sports & Exercise. 1996;28:210–217. doi: 10.1097/00005768-199602000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol. 2000;88:767–773. doi: 10.1152/jappl.2000.88.2.767. [DOI] [PubMed] [Google Scholar]
- 21.Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Benarroch EE. The arterial baroreflex: functional organization and involvement in neurologic disease. Neurology. 2008;71:1733–1738. doi: 10.1212/01.wnl.0000335246.93495.92. [DOI] [PubMed] [Google Scholar]
- 24.Tank J, Diedrich A, Szczech E, Luft FC, Jordan J. Baroreflex regulation of heart rate and sympathetic vasomotor tone in women and men. Hypertension. 2005;45:1159–1164. doi: 10.1161/01.HYP.0000165695.98915.9a. [DOI] [PubMed] [Google Scholar]
- 25.Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol. 2009;587:2049–2057. doi: 10.1113/jphysiol.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Studinger P, Goldstein R, Taylor JA. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. J Physiol. 2007;583:1041–1048. doi: 10.1113/jphysiol.2007.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol. 1970;218:1030–1037. doi: 10.1152/ajplegacy.1970.218.4.1030. [DOI] [PubMed] [Google Scholar]
- 28.Parker P, Celler BG, Potter EK, McCloskey DI. Vagal stimulation and cardiac slowing. J Auton Nerv Syst. 1984;11:226–231. doi: 10.1016/0165-1838(84)90080-8. [DOI] [PubMed] [Google Scholar]
- 29.Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circulation research. 1971;29:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 30.Parmer RJ, Cervenka JH, Stone RA. Baroreflex sensitivity and heredity in essential hypertension. Circulation. 1992;85:497–503. doi: 10.1161/01.cir.85.2.497. [DOI] [PubMed] [Google Scholar]
- 31.Tank J, Jordan J, Diedrich A, Stoffels M, Franke G, Faulhaber HD, Luft FC, Busjahn A. Genetic influences on baroreflex function in normal twins. Hypertension. 2001;37:907–910. doi: 10.1161/01.hyp.37.3.907. [DOI] [PubMed] [Google Scholar]
- 32.Iwase N, Takata S, Okuwa H, Ogawa J, Ikeda T, Hattori N. Abnormal baroreflex control of heart rate in normotensive young subjects with a family history of essential hypertension. J Hypertens Suppl. 1984;2:S409–S411. [PubMed] [Google Scholar]
- 33.Ookuwa H, Takata S, Ogawa J, Iwase N, Ikeda T, Hattori N. Abnormal cardiopulmonary baroreflexes in normotensive young subjects with a family history of essential hypertension. Journal of clinical hypertension. 1987;3:596–604. [PubMed] [Google Scholar]
- 34.Grassi G, Cattaneo BM, Seravalle G, Lanfranchi A, Mancia G. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;31:68–72. doi: 10.1161/01.hyp.31.1.68. [DOI] [PubMed] [Google Scholar]
- 35.Fritsch JM, Smith ML, Simmons DT, Eckberg DL. Differential baroreflex modulation of human vagal and sympathetic activity. Am J Physiol. 1991;260:R635–R641. doi: 10.1152/ajpregu.1991.260.3.R635. [DOI] [PubMed] [Google Scholar]