Abstract
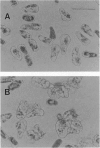
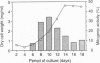
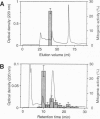
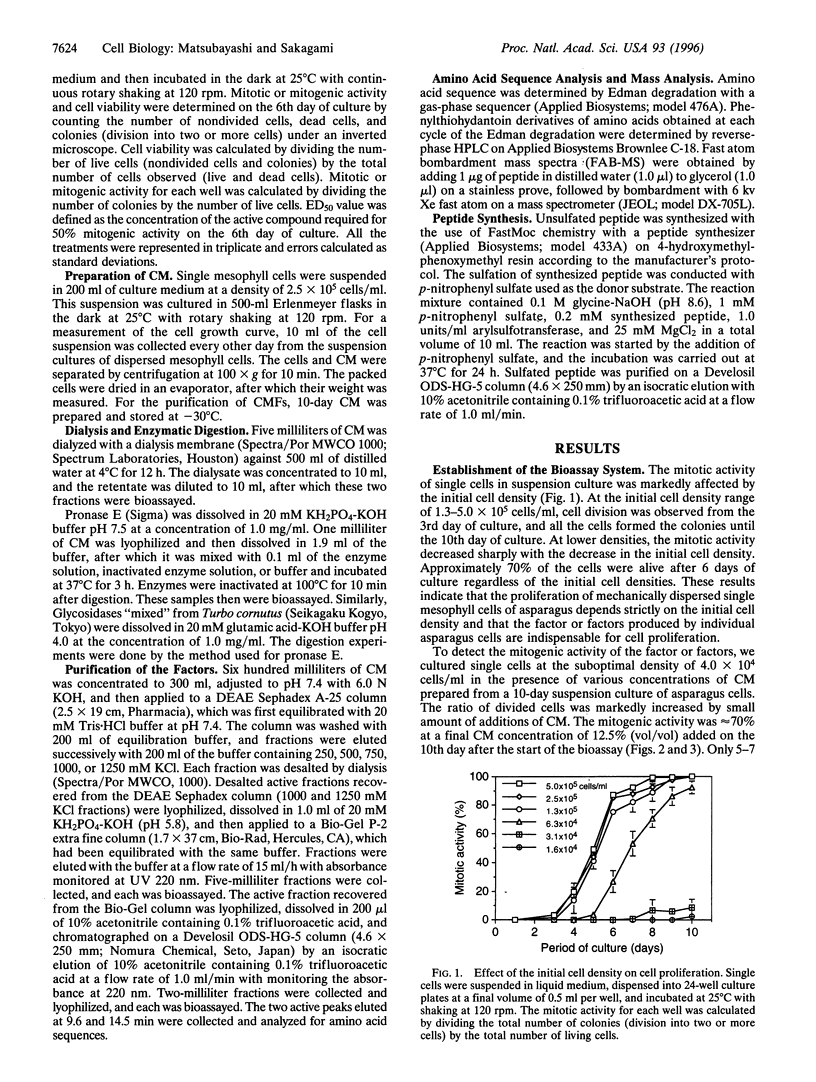
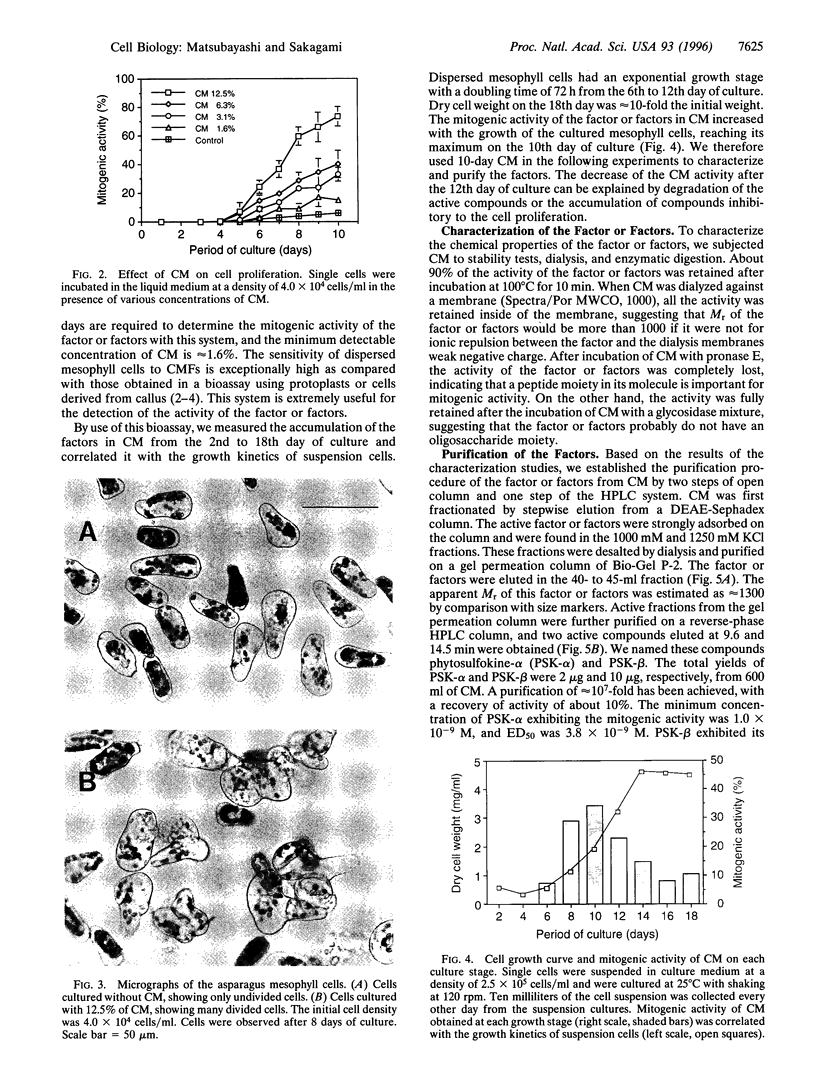
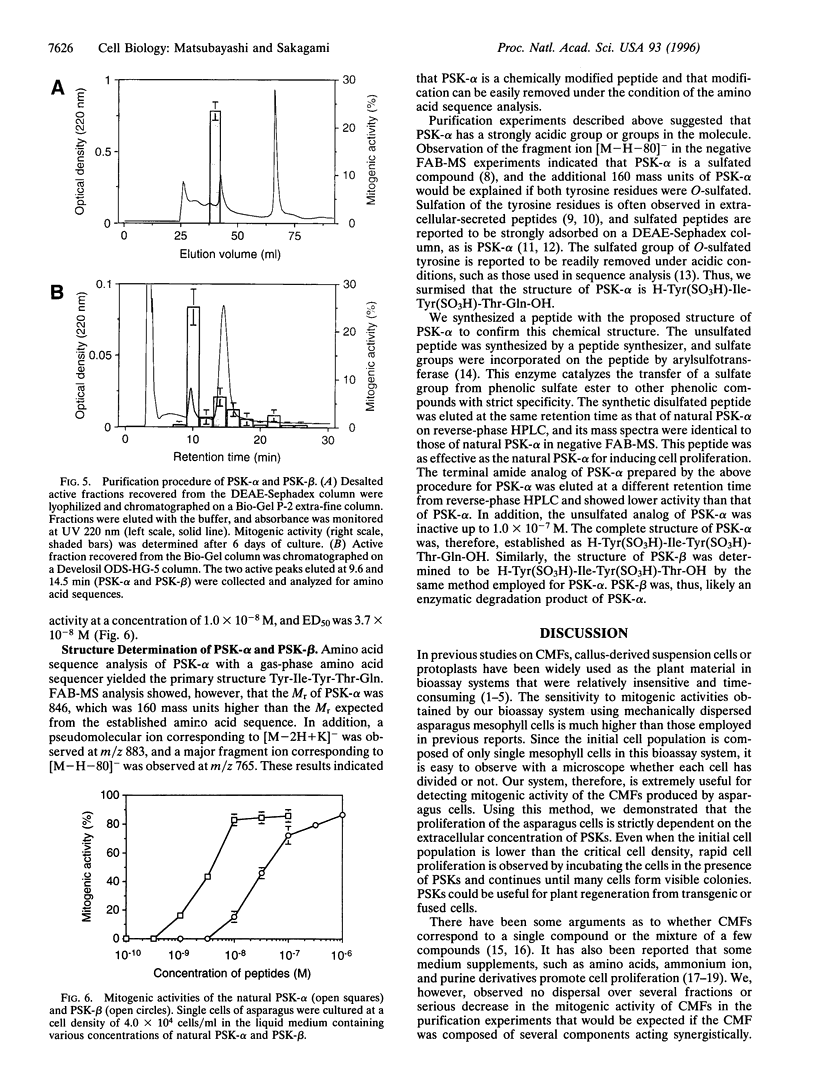
Proliferation of dispersed plant cells in culture is strictly dependent on cell density, and cells in a low-density culture can only grow in the presence of conditioned medium (CM). No known plant hormones have been able to substitute for CM. To quantify the mitogenic activity of CM, we examined conditions for the assay system using mechanically dispersed mesophyll cells of Asparagus officinalis L. and established a highly sensitive bioassay method. By use of this method, the mitogenic activity of CM prepared from asparagus cells was characterized: it was heat-stable, susceptible to pronase digestion, and resistant to glycosidase treatment. On the basis of these results, the mitogenic activity in CM was purified 10(7)-fold by column chromatography, and two factors named phytosulfokine-alpha and -beta (PSK-alpha and PSK-beta) were obtained. By amino acid sequence analysis and mass spectrometry, the structures of these two factors were determined to be sulfated pentapeptide (H-Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln-OH) and sulfated tetrapeptide (H-Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-OH). PSK-alpha and PSK-beta were prepared by chemical synthesis and enzymatic sulfation. The synthetic peptides exhibited the same activity as the natural factors, confirming the structure for PSK-alpha and PSK-beta mentioned above. This is the first elucidation of the structure of a conditioned medium factor required for the growth of low-density plant cell cultures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Erspamer V., Endean R. Isolation and amino acid sequence of caerulein, the active decapeptide of the skin of hyla caerulea. Arch Biochem Biophys. 1968 Apr;125(1):57–68. doi: 10.1016/0003-9861(68)90638-3. [DOI] [PubMed] [Google Scholar]
- GREGORY H., HARDY P. M., JONES D. S., KENNER G. W., SHEPPARD R. C. THE ANTRAL HORMONE GASTRIN. STRUCTURE OF GASTRIN. Nature. 1964 Dec 5;204:931–933. doi: 10.1038/204931a0. [DOI] [PubMed] [Google Scholar]
- Huttner W. B. Sulphation of tyrosine residues-a widespread modification of proteins. Nature. 1982 Sep 16;299(5880):273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Lemp G. F., Gardner J. D. Interactions of COOH-terminal fragments of cholecystokinin with receptors on dispersed acini from guinea pig pancreas. J Biol Chem. 1982 May 25;257(10):5554–5559. [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu R., Nukui E., Sukesada A., Misawa S., Komatsu Y., Okayama T., Wada K., Morikawa T., Hayashi H., Kobashi K. Enzymic O-sulfation of tyrosine residues in hirudins by sulfotransferase from Eubacterium A-44. Eur J Biochem. 1994 Jul 1;223(1):243–248. doi: 10.1111/j.1432-1033.1994.tb18988.x. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E. Structure of porcine cholecystokinin-pancreozymin. 1. Cleavage with thrombin and with trypsin. Eur J Biochem. 1968 Oct 17;6(1):156–162. doi: 10.1111/j.1432-1033.1968.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Nachman R. J., Holman G. M., Haddon W. F., Ling N. Leucosulfakinin, a sulfated insect neuropeptide with homology to gastrin and cholecystokinin. Science. 1986 Oct 3;234(4772):71–73. doi: 10.1126/science.3749893. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Pluscec J., Sabo E. F., Sheehan J. T., Williams N. Synthesis of cholecystokinin-pancreozymin. I. The C-terminal dodecapeptide. J Am Chem Soc. 1970 Jan 14;92(1):195–199. doi: 10.1021/ja00704a033. [DOI] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]