Abstract
Stem-loop I (SL1) located in the 5′ untranslated region of the hepatitis C virus (HCV) genome initiates binding to miR-122, a microRNA required for hepatitis HCV replication. However, proteins that bind SL1 remain elusive. In this study, we employed a human proteome microarray, comprised of ∼17,000 individually purified human proteins in full-length, and identified 313 proteins that recognize HCV SL1. Eighty-three of the identified proteins were annotated as liver-expressing proteins, and twelve of which were known to be associated with hepatitis virus. siRNA-induced silencing of eight out of 12 candidate genes led to at least 25% decrease in HCV replication efficiency. In particular, knockdown of heterogeneous nuclear ribonucleoprotein K (hnRNP K) reduced HCV replication in a concentration-dependent manner. Ultra-violet-crosslinking assay also showed that hnRNP K, which functions in pre-mRNA processing and transport, showed the strongest binding to the HCV SL1. We observed that hnRNP K, a nuclear protein, is relocated in the cytoplasm in HCV-expressing cells. Immunoprecipitation of the hnRNP K from Huh7.5 cells stably expressing HCV replicon resulted in the co-immunoprecipitation of SL1. This work identifies a cellular protein that could have an important role in the regulation of HCV RNA gene expression and metabolism.
RNA viruses are the cause of numerous human diseases. Because of their relatively simple genomes, successful infection by RNA viruses is intimately linked to host factors that can both contribute to, or antagonize the viral infection process (1–3). Infection by the hepatitis C virus (HCV)1, a positive-sense RNA virus, can lead to liver cirrhosis and hepatocellular carcinoma. Approximately 2–3% of the world's population is chronically infected with HCV, with more than 350,000 annual fatalities in recent years (4). As is typical for viruses, a large number of host factors have been reported to facilitate HCV infection including microRNA-122 (miR-122), CD81, claudin-1, cyclophilins, and lipoproteins, to name a few (5–9). These cellular factors interact with viral proteins or RNA, thus promoting HCV entry, genome translation, and replication.
The 5′-untranslated region (5′-UTR) of the HCV RNA genome contains complex RNA structures that interact with cellular factors. These structures include the internal ribosomal entry site that regulates cap-independent translation of the viral RNA (10–11). The 5′-most stem-loop (SL) structure, namely SL1, has been reported to interact with miR-122 to increase the stability of the genomic RNA and facilitate HCV RNA replication in cells (12–13). However, host proteins that can bind to SL1 remain largely elusive because of a lack of proper tools. Previously, we have shown that functional protein microarrays, comprised of individually purified yeast proteins, are an ideal tool to identify proteins that directly interact with important RNA structures encoded by an RNA virus (14). Here, we took a similar approach using a human proteome microarray to identify human hnRNP K as a specific HCV SL1-binding protein that is required for efficient HCV RNA replication.
EXPERIMENTAL PROCEDURES
RNA-binding Assays on the Human Proteome Microarrays
The human proteome microarrays, comprised of more than 17,000 full-length human proteins with N-terminal GST-fusions, were constructed on the glass-based slides as previously reported (15). Glycerol, BSA, and GST in serial dilutions were spotted as negative controls. The human proteome microarrays were first blocked with 3% BSA and 0.1 mg/ml of salmon sperm DNA (Invitrogen, Carlsbad, CA) in 0.05% diethyl pyrocarbonate-treated phosphate-buffered saline (PBS) for 1 h 30 min at room temperature. The microarrays were then separately probed with 80 μl of 2 μm HCV SL1 and SL3d (stem-loop 3d; used as a control) under a coverslip (LifterSlip™; Thermo Scientific, Waltham, MA). HCV SL1 and SL3d RNAs were chemically synthesized with Cy5 label at the 5′ termini (Bioneer Inc, Korea). The 30-nt RNA probe (nt 1–30) corresponding to SL1 in the HCV genome (genotype 1b) has the following sequence: 5′-GCC AGC CCC CUG AUG GGG GCG ACA CUC CAC-3′ (NCBI RefSeq: NC_004102.1). The HCV RNA probe corresponding to SL3d (nt 251–280) has the following sequence: 5′-UAG CCG AGU AGU GUU GGG UCG CGA AAG GCC-3′. Each human proteome microarray was incubated with a given probe in the dark for 1 h at 37 °C on a shaker rotating at 8 rpm. After incubation, the cover slips were removed and each microarray was washed with 500 ml DEPC-treated PBST (PBS, 0.05% tween-20) for 10 min at 37 °C, on a rotary shaker set at 10 rpm. To monitor the amount of each protein on the proteome chip, 80 μl of 1:1000 diluted DyLight™549-conjugated anti-GST monoclonal antibody (Rockland Immunochemicals Inc., Gilbertsville, PA), in DEPC-PBS, was used to probe the chips. The chips were covered with precleaned cover slips and incubated at 37 °C at 8 rpm for 45 min. After removal of cover slips and two wash steps, the chips were dried and scanned using a LuxScan™ 10K Microarray Scanner (CapitalBio Inc., Beijing, China). Binding signals were acquired and analyzed using GenePix Pro 6.0 software. To identify positive SL1- and SL3d-binding proteins, the cutoff was set to be at least three standard deviations of the mean for all of the signals on the chip.
Protein-RNA Binding Assays
Proteins were expressed and purified as GST fusions from Saccharomyces cerevisiae in a 96-well format (16). Each purified protein was adjusted to 100–400 ng and mixed with 1 pmole of the RNA probe radiolabeled at its 5′ terminus using polynucleotide kinase (17) and [γ−32P]ATP. To determine the binding specificity, 200 ng BSA was added to the same sample to serve as an internal negative control. The reactions were irradiated in a CL-1000 UV cross-linker (Stratalinker, Stratagene, La Jolla, CA) at 1200 mJ/cm2 (254 nm) for 3 min. The protein-RNA mixtures were subjected to SDS-PAGE, and the protein-RNA complex was quantified by autoradiography using a PhosphorImager (GE Healthcare Biosciences, Pittsburgh, PA). The proteins in the gel were visualized by Coomassie brilliant blue staining.
siRNA Knockdown Assays
siRNAs (20 nm per reaction) were transfected into Huh7.5 cells harboring the HCV replicon that expresses the Renilla luciferase (RLuc) (18). The transfection reagent, Lipofectamine™ 2000, was used according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). A scrambled siRNA duplex was used as a negative control. All experiments were conducted in triplicate. The RLuc signals were detected at 48 h post-transfection using the Dual-Glo® Luciferase Assay System (Promega, Madison, WI). The RLuc signals were normalized against the nontransfected HCV replicon cells (mock transfected) to calculate the percentage of luciferase expression.
Quantification of the efficiency of siRNA knockdown was performed 48 h after transfection of 8 × 105 cells. The total RNA was extracted from cells using Trizol and the level of HCV RNA was determined by Real-Time PCR (qRT-PCR) using the iQ™ SYBR Green kit (BioRad, Hercules, CA). The primers used were: HCV 5′ UTR sense (5′-AGC CAT GGC GTT AGT ATG AGT GTC-3′) and HCV 5′ UTR antisense (5′-ACA AGG CCT TTC GCG ACC CAA C-3′). Levels of the GAPDH mRNA were quantified in the same samples using the sense primer (5′-GAG TCA ACG GAT TTG GTC GT-3′) and the antisense primer (5′-TGG GAT TTC CAT TGA TGA CA-3′). The level of β-actin was quantified using the sense primer (5′-AGA GGG AAA TCG TGC GTG AC-3′) and the antisense primer (5′-CAA TAG TGA TGA CCT GGC CGT-3′). qRT-PCR amplifications included the following procedures: 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 20 s at 55 °C, and 30 s at 72 °C. The change in HCV RNA and β-actin mRNA levels in siRNA-treated cells was compared with the mock-transfected control as previously reported (19). The abundance of hnRNP K and the human β-actin proteins were analyzed by Western blotting using hnRNP K- and β-actin-specific monoclonal antibodies (Abcam Inc., Cambridge, MA).
Immunofluorescence Staining of hnRNP K in the HCV Replicon Cells
Human hepatoma cells, Huh7.5, and Huh7.5 cells harboring HCV replicon with GFP-fused nonstructural protein 5A (NS5A-GFP replicon) (20) were cultured overnight on coverslips and with 3.7% formaldehyde in PBS for 20 min on ice. The cells were washed with PBS three times and permeabilized with 0.1% Triton X-100 in PBS for 10 min at room temperature. After incubation with 5% BSA in PBS for 2 h at 4 °C, the cells were sequentially incubated with mouse anti-hnRNP K monoclonal antibody (Abcam, Cambridge, MA) and AlexaFluor™594 goat anti-mouse IgG (H+L) antibody (Invitrogen Inc.) for 1 h. Fluorescent images were acquired using the Leica SP5 Scanning Confocal microscope (Leica Inc.) and processed using the Image J plug-in tool, JACoP (21).
Crosslinking-immunoprecipitation Assay
Cross-linking and immunoprecipitation (CLIP) assay to examine hnRNP K interaction with HCV replicon was performed with Huh7.5 and HCV replicon cells. 5 × 106 cells were grown in 10 cm plate, washed twice with ice-cold PBS, and irradiated on ice with 150 mJ/cm2 at 254 nm (Stratalinker, Strtagene, La Jolla, CA). The cells were scraped, pelleted by centrifugation at 5000 × g for 5 min and lysed with Nonidet P-40 lysis buffer on ice for 1 h. Total RNA in the lysate was fragmented by adding ZnCl2 to a final concentration of 10 mm. The sample was heated at 70 °C for 15 min, and the reaction was stopped by adding EDTA and incubating on ice for 10 min. The lysate was then incubated with protein A/G conjugated with mouse anti-human hnRNP K antibody (Abcam, ab39975, Cambridge, MA) or a goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA) at 4 °C for 3 h, and eluted with 0.1 m glycine (pH 3.0). Total RNA was released by digestion with proteinase K, and extracted by Trizol reagent (Invitrogen).
Before cDNA synthesis, total RNA was polyadenylated with the E. coli Poly(A) polymerase (NEB). The cDNA was synthesized using M-MuLV reverse transcriptase (NEB, Cambridge, MA) and an anchored oligo dT primer (5′-TTT TTT TTT TTT TTT TTT TTT TTT TTV-3′; V = A, C, or G), followed by RNase H (NEB) digestion to remove RNA from cDNA/RNA complex. HCV 5′-UTR sense (5′-AGC CAT GGC GTT AGT ATG AGT GTC-3′) and 5′-UTR antisense (5′- ACA AGG CCT TTC GCG ACC CAA C-3′) primers were used to amplify HCV 5′ UTR-specific sequences. Quantification of the number of copies of the HCV RNA has been described previously (22).
RESULTS
Identification of Proteins that Recognize HCV SL1 and SL3d RNA Structures
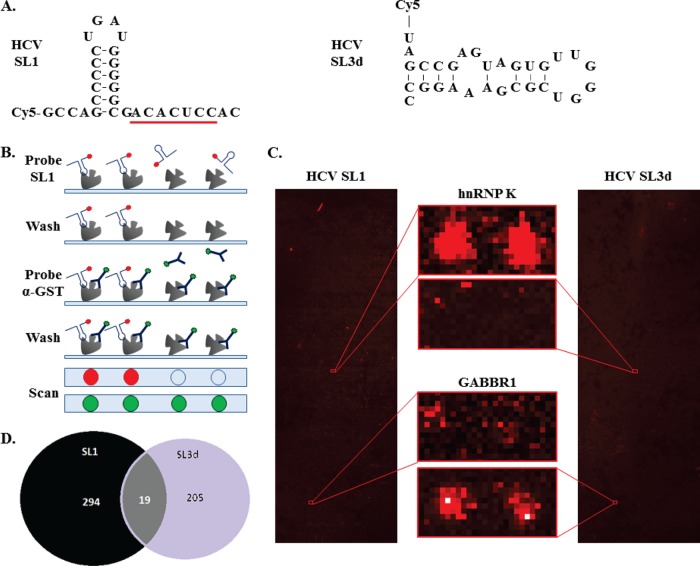
SL1 plays an important role in the early stage of HCV infection by initiating binding to miR-122 (23–24). Identification of proteins that interact with SL1 could provide novel targets for antiviral therapies. To determine whether the bound proteins are specific to SL1, SL3d that is also present in the 5′ UTR of HCV RNA was used as a control (Fig. 1A and supplemental Fig. S1). Both RNAs are 30-nt in length and both were labeled with Cy5 fluorophore at their 5′-ends. After probing the human proteome microarrays fabricated in the same batch, the relative amount of proteins in each spot of the array was determined by probing with DyLight™549-conjugated anti-GST antibody to allow signal normalization (Fig. 1B). Proteins that yielded Cy5/DyLight™549 signals that were greater than three standard deviations above the means were categorized as hits. Examples of SL1-specific hits as compared with the SL3d probing are shown in Fig. 1C. Under such criteria, 313 and 224 protein hits were found to bind HCV SL1 and SL3d, respectively (Fig. 1D and Supplemental Table S1). Among those proteins, only 19 proteins were shared by both RNA probes.
Fig. 1.
Multiple host proteins bind to SL1 in the HCV genome. A, Sequences and putative structures of SL1 and SL3d used to identify interacting proteins in human protein microarrays. SL1 (nt 1–30 of the HCV genome; left panel) and SL3d (nt 251–280 of the HCV genome; right panel) were both synthesized to contain a Cy5 dye at their 5′ termini. The seed sequence for miR-122 binding in SL1 is underlined. B, Schematic of the screening process for the protein microarrays. The RNAs are shown as hairpins with a red circle to denote Cy5. The DyLight™549-conjugated anti-GST antibody used to assess the amount of each protein spot is shown as a Y with a green circle. C, Two examples of hits from the human protein arrays probed respectively with the HCV SL1 (left panel) and HCV SL3d (right panel). The central panels show the spots corresponding to hnRNP K and GABBR1 and how they specifically bind to HCV SL1 and SL3d, respectively. D, Summary of the hits from the screens. In total 313 and 224 proteins were found to bind HCV SL1 and SL3d, respectively. Note that 19 proteins bind to both RNAs.
Bioinformatics Analysis of Identified HCV SL1-binding Proteins
To eliminate obvious false positive hits from our screens, we integrated information of tissue specificity and annotation of the SL1-binding proteins that were identified. Among the 313 SL1-binding proteins, 152 were retrieved from the UniProt Knowledgebase; 83 (54.6%) of them were annotated as liver-expressing proteins. Among the 83 proteins, 14 (16.9%) of them were highly expressed in liver (Supplemental Table S1). Of the proteins highly expressed in the liver, 12 were annotated to bind HCV proteins or to be transactivated during HCV replication (Table I). Therefore, we decided to focus on these 12 host proteins for further study.
Table I. Identified HCV stem loop 1 binding proteins reported to be hepatitis-related.
| Name | UniProt accession number | Relevant feature |
|---|---|---|
| SEPSECS | Q9HD40 | Potential diagnostic marker for autoimmune hepatitis |
| FKBP8 | Q14318 | Interacts with HCV nonstructural protein NS5A and inhibits apoptosis in Huh7 hepatoma cells |
| HFE | Q30201 | Positively correlated with chronic HCV infection in North American patients with porphyria cutanea tarda |
| KIAA0020 | Q15397 | Transactivated by hepatitis B virus X antigen |
| PPARA | Q07869 | Interacts with HCV core protein to modulate its transcriptional activity and facilitates HCV infection |
| SLC35F5 | Q8WV83 | Transactivated by HCV NS5A protein |
| HDDC2 | Q7Z4H3 | Transactivated by HCV NS5A protein |
| hnRNP K | P61978 | Interacts with HCV core protein |
| MEMO1 | Q9Y316 | Transactivated by HCV NS5A protein |
| NOP16 | Q9Y3C1 | Transregulated by hepatitis B virus pre-S2 protein |
| NPM1 | P06748 | Interacts with hepatitis delta antigens and modulates the hepatitis delta virus RNA replication |
| PSMA3 | P25788 | Interacts with HCV F protein |
| TBC1D23 | Q9NUY8 | Transactivated by nonstructural protein 4A of hepatitis C virus (NS4ATP1) mRNA |
| YWHAE | P62258 | Interacts with HCV core protein and activates the kinase Raf-1 |
Validation of HCV SL1-binding Proteins Using UV-crosslinking Assays
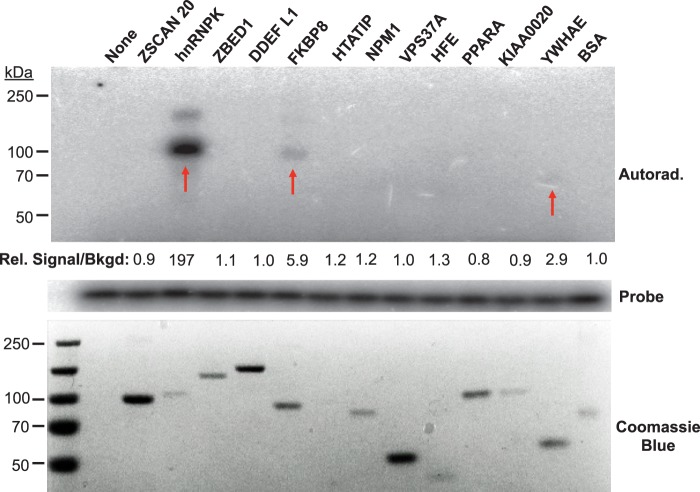
We employed denaturing electrophoresis mobility shift assay (EMSA) to confirm the interaction between the 12 candidate proteins and HCV SL1 RNA. Hence, each candidate protein was expressed and purified from yeast as a GST fusion and tested individually for binding to radiolabeled SL1 RNA after UV irradiation (Fig. 2). EMSA was previously used to identify RNA-binding proteins (25–26). All proteins had purity in excess of 90%, but variable concentration (Fig. 2, bottom panel). Of the 12 proteins, hnRNP K, FKBP8, and YWHAE formed stable complexes with the HCV SL1 RNA with signal that are at least 2.9-fold above the background (Fig. 2, upper panel). Interestingly, despite hnRNP K being present at lower concentration relative to many other proteins tested, it exhibited the strongest binding signal to SL1 (Fig. 2, left arrow). Notably, hnRNP K was reported to interact with the HCV core protein, although it was not previously known to bind HCV RNA (27). As a comparison, hnRNP K did not bind to SL3d, indicating its specificity to SL1 RNA (Fig. 1C and supplemental Table S1). There was also a lower amount of a second band that migrated close to mass expected of a hnRNP K dimer, suggesting that more than one hnRNP K molecule binds to SL1 or a fraction of hnRNP K undergoes a conformational change following interaction with SL1 (Fig. 2).
Fig. 2.
Biochemical validation of the HCV SL1-host protein interactions identified in the human proteome chips. The 12 protein hits from the SL1 screen were purified as GST fusions from yeast (16), and were subjected to UV-crosslinking assays with HCV SL1. The proteins were separated on SDS-PAGE and HCV SL1-bound proteins were detected by autoradiography (upper panel). The lower panel indicates the input proteins in the EMSA. The amount of probe presented in each reaction is shown in the slice of the autoradiogram in the middle of the figure. Among the proteins of interest, hnRNP K, FKBP8, and YWHAE showed the binding affinity to HCV SL1 in the crosslinking analysis as indicated by red arrows. The signal intensities of the radiolabeled EMSA bands and the Coomassie blue stained protein bands were quantified using the Image Quant 5.2 software and the ratio of the signal was normalized to that of BSA.
Knockdown of SL1-binding Proteins Affects HCV Replication
To determine the physiological relevance of identified SL1-binding proteins in HCV replication, siRNAs against the genes for the 12 SL1 hits (Supplemental Table S2) were individually transfected into Huh7.5 cells harboring HCV replicon, with the reporter Renilla luciferase (RLuc), to assess HCV replication efficiency. The RLuc activity was measured and normalized to the mock-treated cells at 48 h post transfection. All of the experiments were conducted in triplicate, and a scrambled siRNA duplex was employed as a negative control. Quantitative analysis revealed that knockdown of eight of the 12 candidate genes resulted in >25% reduction in HCV replication efficiency. Notably, knockdown of the three proteins (hnRNP K, FKBP8, and YWHAE), which could form complexes with SL1, led to a marked reduction in HCV replication (Fig. 3). Among them, FKBP8 knockdown showed the greatest reduction in HCV RNA level by 60% (Fig. 3). None of the siRNAs affected the levels of the β-actin mRNA, suggesting that the siRNAs had no general detrimental effect on cellular gene expression (Fig. 3B). Altogether, these results suggest that the majority of the 12 SL1-binding proteins play a positive role in HCV infection.
Fig. 3.
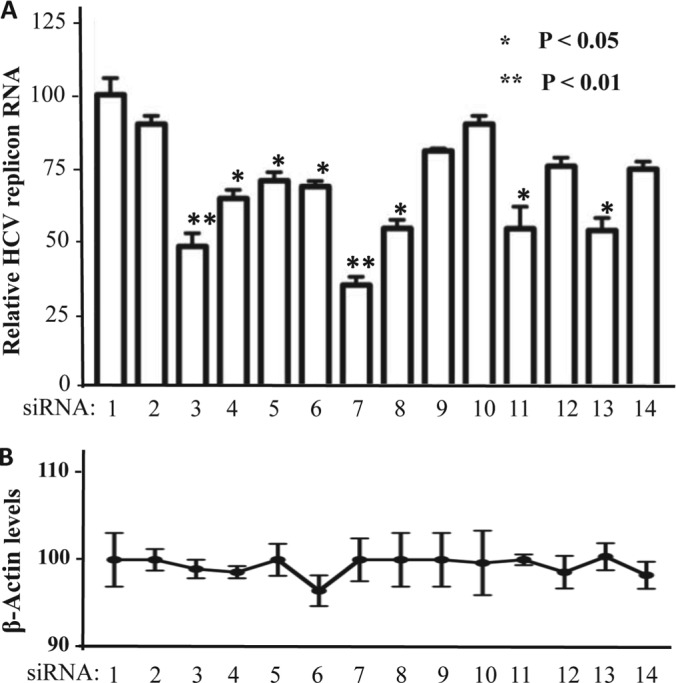
Knockdown of SL1-binding proteins reduced HCV replication efficiency. A, bar graph shows the luciferase level from Huh7.5 cells electroporated with an HCV replicon that expresses RLuc (18). The cells were then transfected with 20 nm of the siRNA indicated below each bar. Each transfection was done in triplicate. The mean and standard error of the results are shown. B, Amount of β-actin expressed in the cells analyzed for HCV RLuc expression. The siRNAs used in the 14 samples were: 1: mock; 2: nonspecific siRNA control (a scrambled siRNA duplex); 3: ZSCAN20; 4: hnRNP K; 5: ZBED1; 6: DDEFL1; 7: FKBP8; 8: HTATIP; 9: VPS37A; 10: HFE; 11: PPARA; 12: KIAA0020; 13: YWHAE; 14: NPM1.
Because hnRNP K exhibited the strongest interactions to the HCV RNA, we further focused on hnRNP K study. Because luciferase reporter activity cannot accurately measure HCV replication efficiency, we used quantitative RT-PCR in hnRNP K-depleted cells to further assess HCV replication. As shown in Fig. 4A, transfection of two different concentrations of hnRNP K siRNA into HCV replicon cells resulted in a dose-dependent reduction in the levels of hnRNP K protein (Fig. 4A). Additionally, the reduction in hnRNP K protein expression led from 25% to 75% reduction in the HCV RNA levels (Fig. 4B). Altogether, these data indicate that hnRNP K plays a role in HCV genome replication.
Fig. 4.
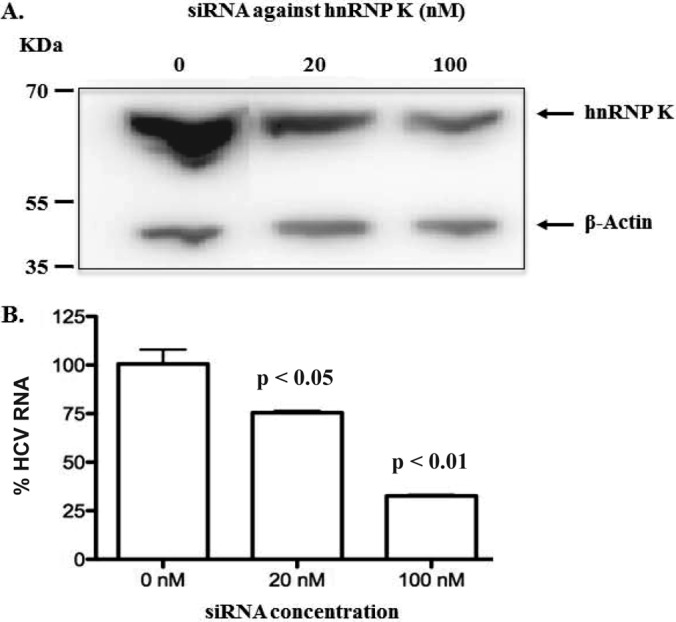
Knockdown of hnRNP K inhibited HCV RNA accumulation in a dose-response manner. A, hnRNP K siRNA was transfected into HCV replicon cells at 0, 20, and 100 nm. At 48 h post-transfection, hnRNP K expression was determined by Western blot. B, Quantitation of HCV RNA levels in cell treated with hnRNP K siRNA. The HCV RNA was quantified by qRT-PCR, and the values were divided by a mock-treated control (0 nm) to generate the percent changes (n = 3).
A Proportion of hnRNP K is Relocated to the Cytoplasm in HCV Replicon Cells
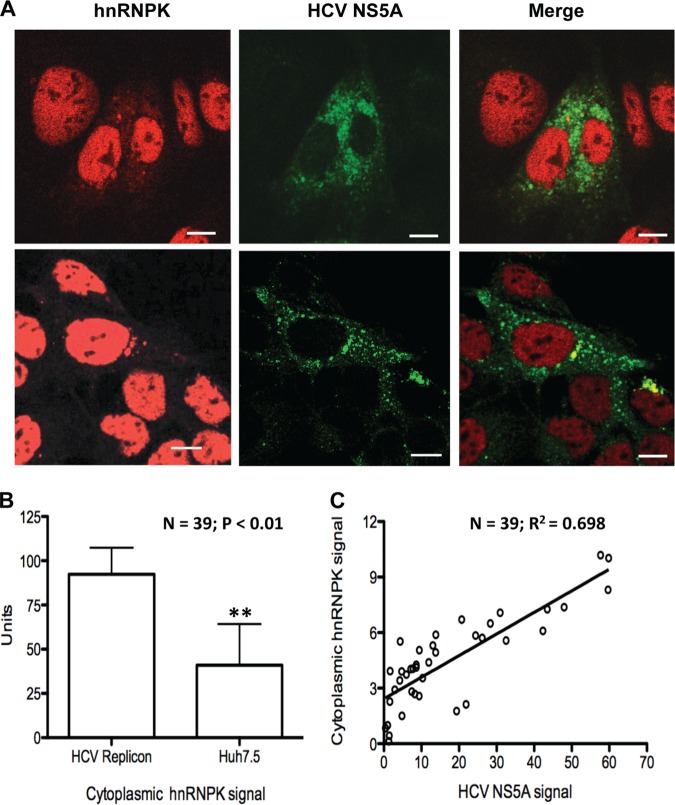
hnRNP K protein is normally localized to the nucleus, where it participates in pre-mRNA splicing through incorporation into the spliceosome complex (28). Because HCV RNA replication occurs in the cytoplasm, an interaction between hnRNP K and HCV RNA will require hnRNP K relocalization to the cytoplasm. Thus, confocal microscopy was used to determine the subcellular distribution of hnRNP K in Huh7.5 cells with or without HCV replicon. As expected, the vast majority of the hnRNP K was localized to the nucleus (Fig. 5A). However, in cells expressing the HCV replicon with GFP-fused nonstructural protein 5A (NS5A-GFP replicon) (20), ca. 2–5% of the hnRNP K was translocated to the cytoplasm (Fig. 5A). Although it is a small fraction of hnRNP K translocation, it could have a significant impact on HCV RNA replication because of the high abundance of hnRNP K in the cell. In fact, it has been reported that even a small amount of protein changes can impose a conspicuous effect on HCV life cycle (29). In a previous study of HCV core protein and hnRNP K, Hsieh et al. (30) also found that only partial hnRNP K was colocalized with HCV core protein, and might contribute to the pathogenesis of HCV. In this study, we also found that the amount of cytoplasmic hnRNP K was significantly lower in Huh7.5 cells that lacked the HCV replicon (Fig. 5B). Finally, Huh7.5 cells harboring the replicon usually express a range of HCV products and can be detected by the differing levels of NS5A-GFP expressed. Interestingly, cells with higher levels of NS5A-GFP had higher amounts of hnRNP K translocated to the cytoplasm (Fig. 5C). These results demonstrate that hnRNP K could re-localize from the nucleus to the cytoplasm to affect HCV RNA replication.
Fig. 5.
HCV replication increases hnRNP K abundance in the cytoplasm. A, Immunofluorescence analysis of representative Huh7.5 cells with or without HCV NS5A-GFP replicon (20). Four representative cells that contained the HCV replicon (two in each of the upper and lower panels) are shown. The endogenous hnRNP K was visualized with anti-hnRNP K antibody and Alexa Fluor 594-conjugated secondary antibody. The NS5A protein, in the NS5A-GFP replicon, was detected via GFP fluorescence. hnRNP K was observed in all nuclei; it was also found as the hazy red color in the cytoplasm of the four representative cells. Note that NS5A subcellular distribution (middle and right-most panels) correlates with the hnRNP K fluorescence. B, Quantification of the amount of cytoplasmic signal (red) for hnRNP K in 39 independently selected Huh7.5 cells with or without HCV NS5A-GFP replicon. Quantification of the cytoplasmic fluorescence was performed with the Image J program. Statistical analysis was performed with the Student t test. C, A correlation between the level of NS5A-GFP expression and the abundance of hnRNP K presented in the cytoplasm.
HCV RNA is Associated with hnRNP K
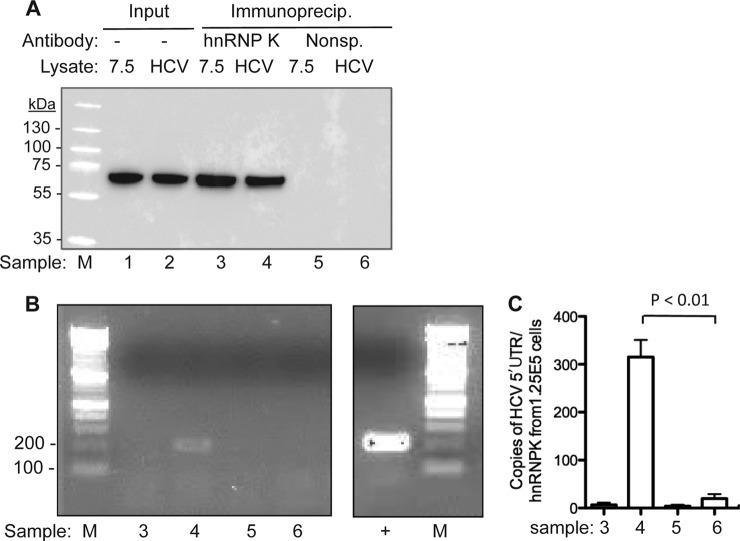
To determine whether hnRNP K could contact the HCV RNA in Huh7.5 cells, we performed a crosslinking-immunoprecipitation (CLIP) assay, as described previously (31). Briefly, Huh7.5 cells or Huh7.5 cells harboring the HCV replicon were crosslinked by UV irradiation, followed by RNA fragmentation in the presence of ZnCl2 in the lysate. RNAs in complex with hnRNP K was immunoprecipitated with antibody against hnRNP K and a nonspecific mAb was used as a negative control. RNAs CLIP'ed with hnRNP K were released by proteolysis and converted to cDNA for quantification and DNA sequence analysis. In this assay, hnRNP K was specifically immunoprecipitated by the specific antibody and not by the control antibody (Fig. 6A). The sample processed from the replicon-containing Huh7.5 cells (Sample 4, panel A), but not Huh7.5 cells (Sample 3, panel A), also yielded the ca. 200 bp DNA fragment expected from the HCV replicon (Fig. 6B). In quantitative PCR, the sequence corresponding to the 5′ UTR of the HCV RNA was ∼2 orders of magnitude more abundant than in the controls (Fig. 6C). Finally, sequencing of 4 independent clones of the amplified cDNA indicates that all contained the sequence from the HCV 5′ UTR in Huh7.5 cells. These results support the binding of hnRNP K to the 5′ UTR of the HCV RNA, likely SL1.
Fig. 6.
HCV RNA can co-immunoprecipitate with hnRNP K protein. Analysis of hnRNP K binding to HCV replicon RNA was assessed using the CLIP assay (31). A, Western blot analysis of the levels of hnRNP K in Huh7.5 cells with or without HCV replicon. The amount of input lysate loaded in the Western blot analysis was equivalent to 10% of the amounts processed for immunoprecipitation. The sample number at the bottom of the Western blot image is used consistently in all panels of this figure. B, CLIP products from Huh7.5 cells harboring the HCV replicon (lane 4) contained the 5′ UTR of the HCV genome. The image is of a 1.5% agarose gel containing PCR products amplified from the CLIP reaction. The sample identified with a “+” is the amplification of the total lysate from Huh7.5 cells harboring the HCV replicon. C, Quantitative RT-PCR to determine the number of HCV 5′ UTR precipitated from either Huh7.5 cells with or without the HCV replicon. Each sample was from the products of three independent CLIP reactions.
DISCUSSION
The emerging high-throughput proteome microarray technology has shown its use and superiority in identifying protein-RNA interactions (25–26). In this study, we used the human proteome microarrays that consisted of ∼17,000 full-length nonredundant human proteins to identify HCV SL1-binding proteins. The proteome-wide screening led to the identification of a total of 313 HCV SL1-binding proteins. The statistical analysis and data mining processes were also valuable parts of the screen process. It showed that 54.6% of the identified SL1-binding proteins were annotated for expression in liver. In addition, 12 SL1-binding proteins (Table I and supplemental Table 1) have been linked to hepatitis C virus (www.uniprot.org). In the UV-crosslinking assay, hnRNP K showed the strongest binding signal to the HCV SL1 RNA (Fig. 2). Notably, hnRNP K did not bind SL3d that also resides within the 5′ UTR of the HCV genome, indicating that there is specificity in hnRNP K recognition of SL1 (Fig. 1). To demonstrate the biological relevance of SL1-binding proteins in HCV replication, siRNAs against the 12 candidate genes were individually transfected into HCV replicon cells and 8 siRNAs, including hnRNP K siRNA, resulted in decreased HCV RNA replication efficiency (Fig. 3). Importantly, some hnRNP K was retargeted to the cytoplasm at sites where the HCV replication complex-associated NS5A protein was also localized (Fig. 5). Finally, immunoprecipitation of hnRNP K co-precipitated the HCV RNA (Fig. 6). Altogether, these findings indicate that hnRNP K regulates HCV replication via interaction with SL1 in the 5′ UTR of the virus genome.
hnRNP K is a multifunctional protein that plays important roles in transcription, mRNA transport, RNA splicing, and signal transduction (32). In its various roles, hnRNP K can associate with numerous binding partners, acting as a docking platform to integrate signaling cascades by facilitating cross-talk between kinases and factors that mediate nucleic-acid-directed processes (32). Several viruses have evolved to use hnRNP K as positive regulator to enhance viral infection by directly binding to viral genome or proteins, including Sindbis Virus (SINV) nonstructural proteins and subgenomic RNA, Herpes Simplex Virus immediate early protein IE63 (20), Dengue Virus core protein, the Epstein-Barr Virus-encoded EBNA2 protein and Chikungunya Virus nsP2 (33–38). Therefore, to prevent productive viral infection, cells could sequester hnRNP K. For example, cytidine deaminase APOBEC3 may bind hnRNP K to prevent its interaction with the hepatitis B virus enhancer II protein, thus suppressing HBV replication (39). Granzyme M may cleave hnRNP K and result in a reduction of the immediate-early 2 protein of Human cytomegalovirus (HCMV), hence inhibiting HCMV infection (40).
A yeast two-hybrid screen had previously identified hnRNP K to interact with HCV core protein (27). The protein microarray results indicate that hnRNP K is capable of directly interacting with the 5′ UTR of the HCV genome. Notably, a similar role for hnRNP K was also observed in the Enterovirus 71 (EV71) infection, in which hnRNP K binds to EV71 5′ UTR and this interaction is required for efficient EV71 replication (38). The consequence of hnRNP K interaction with HCV SL1 remains to be elucidated. However, because SL1 also contains the seed sequence to initiate binding to miR-122, one intriguing possibility is that hnRNP K could play a role in mediating miR-122's interaction with the HCV 5′ UTR.
Supplementary Material
Footnotes
* This research is supported in part by grant NHRI-EX102-10233SC from National Health Research Institute, Taiwan, NSC99-2627-M-008-003, NSC100-2627-M-008-003, and NSC100-2320-B-008-001 from the National Science Council, Taiwan to C-S Chen; grants RAI075015A and AI073335 from the National Institute of Allergy and Infectious Diseases to C. Kao; and grants R01 GM076102, U54 RR020839, U24 CA160036, and U54 HG006434 to H. Zhu.
 This article contains supplemental Tables S1 and S2 and Fig. S1.
This article contains supplemental Tables S1 and S2 and Fig. S1.
1 The abbreviations used are:
- HCV
- hepatitis C virus
- 5′UTR
- 5′ untranslated region
- SL
- stem loop
- EMSA
- electrophoresis mobility shift assay.
REFERENCES
- 1. Georgel P., Schuster C., Zeisel M. B., Stoll-Keller F., Berg T., Bahram S., Baumert T. F. (2010) Virus-host interactions in hepatitis C virus infection: implications for molecular pathogenesis and antiviral strategies. Trends Mol. Med. 16, 277–286 [DOI] [PubMed] [Google Scholar]
- 2. Nagy P. D., Pogany J. (2012) The dependence of viral RNA replication on co-opted host factors. Nature Rev. 10, 137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tellinghuisen T. L., Rice C. M. (2002) Interaction between hepatitis C virus proteins and host cell factors. Current Opinion Microbiol. 5, 419–427 [DOI] [PubMed] [Google Scholar]
- 4. Shepard C. W., Finelli L., Alter M. J. (2005) Global epidemiology of hepatitis C virus infection. Lancet 5, 558–567 [DOI] [PubMed] [Google Scholar]
- 5. Jopling C. L., Yi M., Lancaster A. M., Lemon S. M., Sarnow P. (2005) Modulation of Hepatitis C Virus RNA Abundance by a Liver-Specific MicroRNA. Science 309, 1577–1581 [DOI] [PubMed] [Google Scholar]
- 6. Pileri P., Uematsu Y., Campagnoli S., Galli G., Falugi F., Petracca R., Weiner A. J., Houghton M., Rosa D., Grandi G., Abrignani S. (1998) Binding of hepatitis C virus to CD81. Science 282, 938–941 [DOI] [PubMed] [Google Scholar]
- 7. Evans M. J., von Hahn T., Tscherne D. M., Syder A. J., Panis M., Wolk B., Hatziioannou T., McKeating J. A., Bieniasz P. D., Rice C. M. (2007) Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446, 801–805 [DOI] [PubMed] [Google Scholar]
- 8. Hopkins S., Dimassimo B., Rusnak P., Heuman D., Lalezari J., Sluder A., Scorneaux B., Mosier S., Kowalczyk P., Ribeill Y., Baugh J., Gallay P. (2012) The cyclophilin inhibitor SCY-635 suppresses viral replication and induces endogenous interferons in patients with chronic HCV genotype 1 infection. J. Hepatol. [DOI] [PubMed] [Google Scholar]
- 9. Albecka A., Belouzard S., Op de Beeck A., Descamps V., Goueslain L., Bertrand-Michel J., Terce F., Duverlie G., Rouille Y., Dubuisson J. (2012) Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology 55, 998–1007 [DOI] [PubMed] [Google Scholar]
- 10. Otto G. A., Puglisi J. D. (2004) The pathway of HCV IRES-mediated translation initiation. Cell 119, 369–380 [DOI] [PubMed] [Google Scholar]
- 11. Spahn C. M., Kieft J. S., Grassucci R.A., Penczek P. A., Zhou K., Doudna J. A., Frank J. (2001) Hepatitis C virus IRES RNA-induced changes in the conformation of the 40s ribosomal subunit. Science 291, 1959–1962 [DOI] [PubMed] [Google Scholar]
- 12. Moradpour D., Penin F., Rice C. M. (2007) Replication of hepatitis C virus. Nature Rev. 5, 453–463 [DOI] [PubMed] [Google Scholar]
- 13. Shimakami T., Yamane D., Jangra R. K., Kempf B. J., Spaniel C., Barton D. J., Lemon S. M. (2012) Stabilization of hepatitis C virus RNA by an Ago2-miR-122 complex. Proc. Natl. Acad. Sci. U. S. A. 109, 941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu J., Gopinath K., Murali A., Yi G., Hayward S. D., Zhu H., Kao C. (2007) RNA-binding proteins that inhibit RNA virus infection. Proc. Natl. Acad. Sci. U. S. A. Proc. Natl. Acad. Sci. U. S. A. 104, 3129–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeong J. S., Jiang L., Albino E., Marrero J., Rho H. S., Hu J., Hu S., Vera C., Bayron-Poueymiroy D., Rivera-Pacheco Z. A., Ramos L., Torres-Castro C., Qian J., Bonaventura J., Boeke J. D., Yap W. Y., Pino I., Eichinger D. J., Zhu H., Blackshaw S. (2012) Rapid identification of monospecific monoclonal antibodies using a human proteome microarray. Mol. Cell. Proteomics 11, O111.016253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deleted in proof.
- 17. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular cloning: A laboratory manual, 2 ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 18. Targett-Adams P., McLauchlan J. (2005) Development and characterization of a transient-replication assay for the genotype 2a hepatitis C virus subgenomic replicon. J. Gen. Virol. 86, 3075–3080 [DOI] [PubMed] [Google Scholar]
- 19. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 20. Moradpour D., Evans M. J., Gosert R., Yuan Z., Blum H. E., Goff S. P., Lindenbach B. D., Rice C. M. (2004) Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. J. Virol. 78, 7400–7409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bolte S., CordeliÈRes F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microscopy 224, 213–232 [DOI] [PubMed] [Google Scholar]
- 22. Vaughan R., Fan B., You J. S., Kao C. C. (2012) Identification and functional characterization of the nascent RNA contacting residues of the hepatitis C virus RNA-dependent RNA polymerase. RNA 18, 1541–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yi M., Lemon S. M. (2003) Structure–function analysis of the 3′ stem-loop of hepatitis C virus genomic RNA and its role in viral RNA replication. RNA 9, 331–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y. P., Gottwein J. M., Scheel T. K., Jensen T. B., Bukh J. (2011) MicroRNA-122 antagonism against hepatitis C virus genotypes 1–6 and reduced efficacy by host RNA insertion or mutations in the HCV 5′ UTR. Proc. Natl. Acad. Sci. U. S. A. 108, 4991–4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu J., Gopinath K., Murali A., Yi G., Hayward S. D., Zhu H., Kao C. (2007) RNA-binding proteins that inhibit RNA virus infection. Proc. Natl. Acad. Sci. U. S. A. 104, 3129–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu S., Xie Z., Qian J., Blackshaw S., Zhu H. (2011) Functional protein microarray technology. Systems Biol. Med. 3, 255–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsieh T. Y., Matsumoto M., Chou H. C., Schneider R., Hwang S. B., Lee A. S., Lai M. M. (1998) Hepatitis C virus core protein interacts with heterogeneous nuclear ribonucleoprotein K. J. Biol. Chem. 273, 17651–17659 [DOI] [PubMed] [Google Scholar]
- 28. Matunis M. J., Michael W. M., Dreyfuss G. (1992) Characterization and primary structure of the poly(C)-binding heterogeneous nuclear ribonucleoprotein complex K protein. Mol. Cell. Biol. 12, 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Z., Choi J., Lu W., Ou J. H. (2003) Hepatitis C virus F protein is a short-lived protein associated with the endoplasmic reticulum. J. Virol. 77, 1578–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsieh T. Y., Matsumoto M., Chou H. C., Schneider R., Hwang S. B., Lee A. S., Lai M. M. C. (1998) Hepatitis C virus core protein interacts with heterogeneous nuclear ribonucleoprotein K. J. Biol. Chem. 273, 17651–17659 [DOI] [PubMed] [Google Scholar]
- 31. Konig J., Zarnack K., Rot G., Curk T., Kayikci M., Zupan B., Turner D. J., Luscombe N. M., Ule J. (2011) iCLIP - Transcriptome-wide mapping of protein-RNA interactions with individual nucleotide resolution. J. Vis. Exp. e2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bomsztyk K., Denisenko O., Ostrowski J. (2004) hnRNP K: One protein multiple processes. BioEssays 26, 629–638 [DOI] [PubMed] [Google Scholar]
- 33. Burnham A. J., Gong L., Hardy R. W. (2007) Heterogeneous nuclear ribonuclear protein K interacts with Sindbis virus nonstructural proteins and viral subgenomic mRNA. Virology 367, 212–221 [DOI] [PubMed] [Google Scholar]
- 34. Wadd S., Bryant H., Filhol O., Scott J. E., Hsieh T. Y., Everett R. D., Clements J. B. (1999) The multifunctional herpes simplex virus IE63 protein interacts with heterogeneous ribonucleoprotein K and with casein kinase 2. J. Biol. Chem. 274, 28991–28998 [DOI] [PubMed] [Google Scholar]
- 35. Chang C. J., Luh H. W., Wang S. H., Lin H. J., Lee S. C., Hu S. T. (2001) The heterogeneous nuclear ribonucleoprotein K (hnRNP K) interacts with dengue virus core protein. DNA Cell Biol. 20, 569–577 [DOI] [PubMed] [Google Scholar]
- 36. Gross H., Hennard C., Masouris I., Cassel C., Barth S., Stober-Grässer U., Mamiani A., Moritz B., Ostareck D., Ostareck-Lederer A., Neuenkirchen N., Fischer U., Deng W., Leonhardt H., Noessner E., Kremmer E., Grässer F. A. (2012) Binding of the heterogeneous ribonucleoprotein K (hnRNP K) to the epstein-barr virus nuclear antigen 2 (EBNA2) enhances viral LMP2A expression. PLoS ONE 7, e42106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bouraï M., Lucas-Hourani M., Gad H.H., Drosten C., Jacob Y., Tafforeau L., Cassonnet P., Jones L. M., Judith D., Couderc T., Lecuit M., André P., Kümmerer B. M., Lotteau V., Desprès P., Tangy F., Vidalain P. O. (2012) Mapping of Chikungunya virus interactions with host proteins identified nsP2 as a highly connected viral component. J. Virol. 86, 3121–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin J. Y., Li M. L., Huang P. N., Chien K. Y., Horng J. T., Shih S. R. (2008) Heterogeneous nuclear ribonuclear protein K interacts with the enterovirus 71 5′ untranslated region and participates in virus replication. J. Gen. Virol. 89, 2540–2549 [DOI] [PubMed] [Google Scholar]
- 39. Zhang W., Zhang X., Tian C., Wang T., Sarkis P. T. N., Fang Y., Zheng S., Yu X. F., Xu R. (2008) Cytidine deaminase APOBEC3B interacts with heterogeneous nuclear ribonucleoprotein K and suppresses hepatitis B virus expression. Cell. Microbiol. 10, 112–121 [DOI] [PubMed] [Google Scholar]
- 40. van Domselaar R., de Poot S. A. H., Remmerswaal E. B. M., Lai K. W., ten Berge I. J. M., Bovenschen N. (2013) Granzyme M targets host cell hnRNP K that is essential for human cytomegalovirus replication. Cell Death Differentiation 20, 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.