Abstract
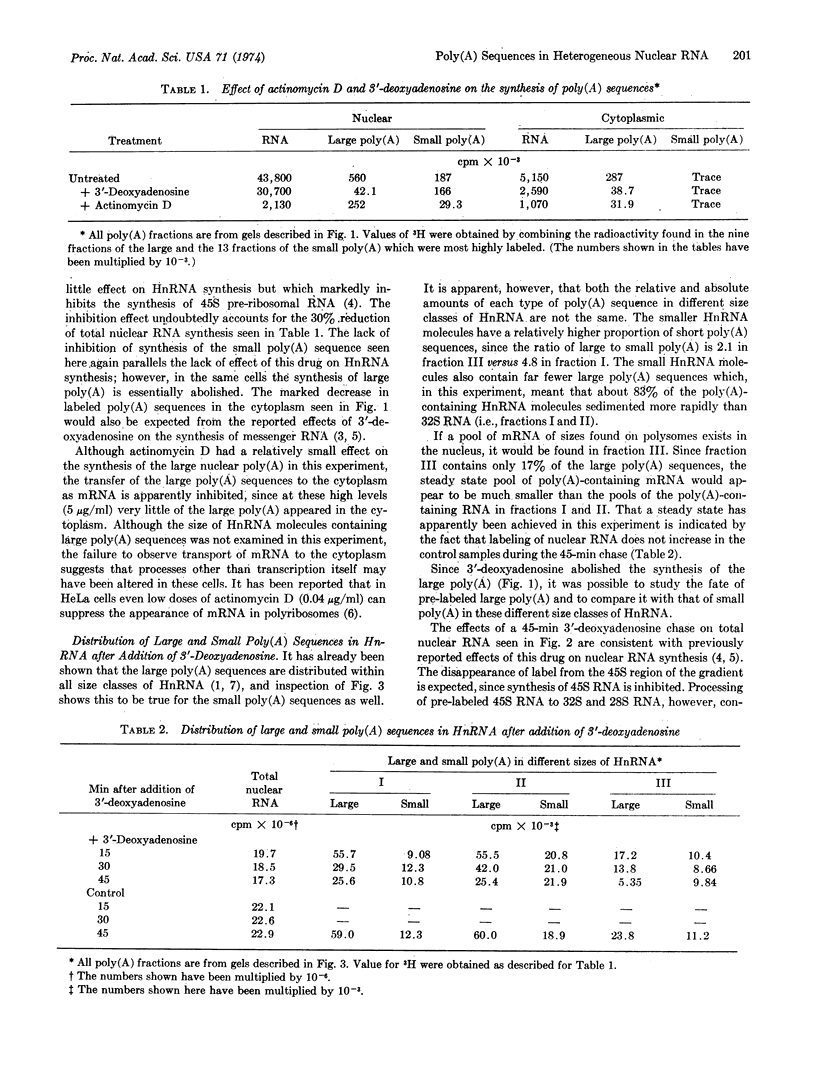
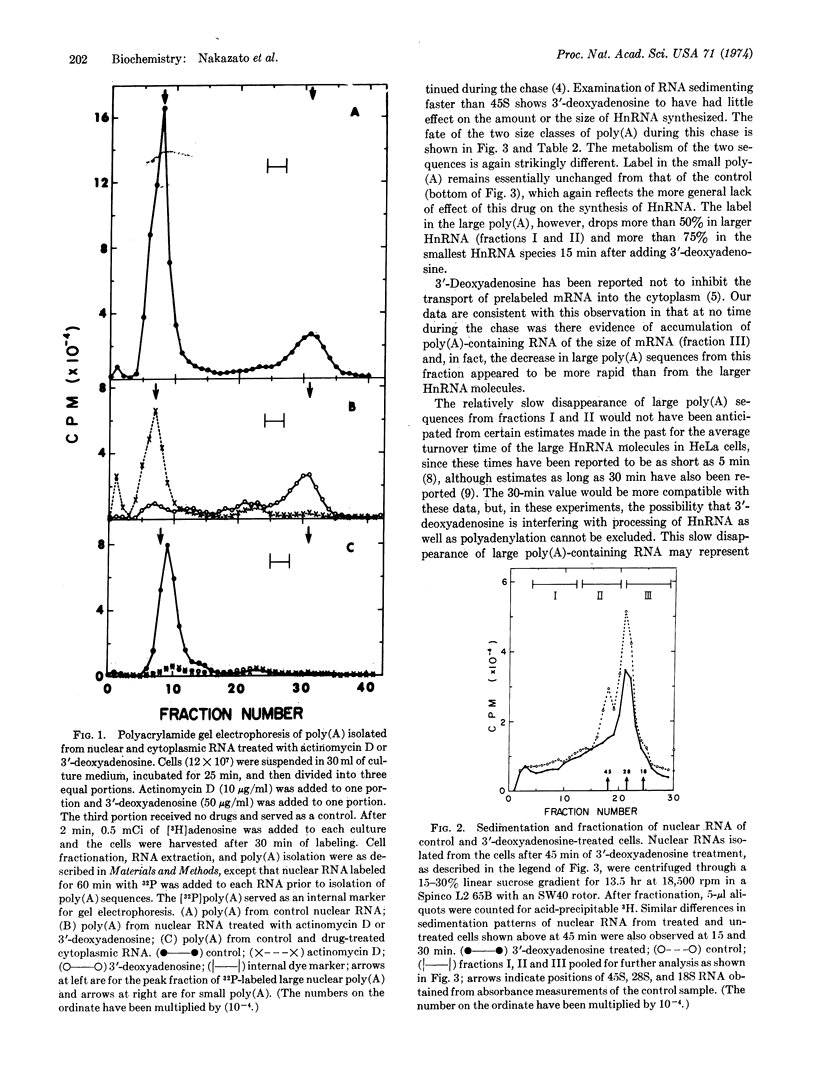
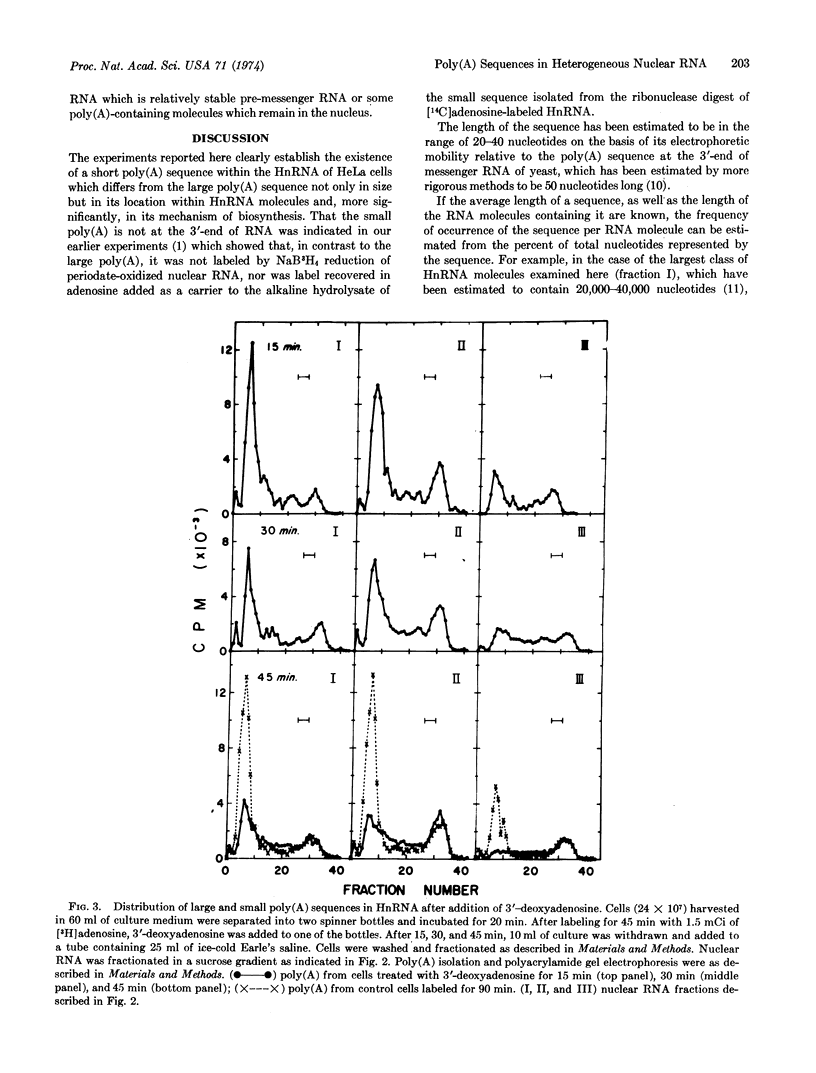
The heterogeneous RNA of the HeLa cell nucleus contains short internal poly(A) sequences which, in contrast to the longer poly(A) sequences at the 3′ ends, are not found in messenger RNA of the cytoplasm. A distinct origin for each of these homologous sequences is evident when the effects of actinomycin D and 3′-deoxyadenosine on their biosynthesis are compared. The shorter poly(A) appears to be transcribed, while the longer one does not. A kinetic analysis of the metabolism of each type of poly(A) sequence within different size classes of HnRNA after treatment of the cells with 3′-deoxyadenosine reveals distinctive distribution and metabolism of these sequences and also provides insight into mechanisms proposed for the generation of messenger RNA from these large RNA molecules in the nucleus.
Keywords: transcriptional synthesis, messenger RNA, 3′-deoxyadenosine, actinomycin D
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Mitchel R. E., Straus N. A. Analysis of long pyrimidine polynucleotides in HeLa cell nuclear DNA: absence of polydeoxythymidylate. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2189–2192. doi: 10.1073/pnas.70.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jelinek W. R., Molloy G. R. Biogenesis of mRNA: genetic regulation in mammalian cells. Science. 1973 Sep 28;181(4106):1215–1221. doi: 10.1126/science.181.4106.1215. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG I. H., RABINOWITZ M., REICH E. Basis of actinomycin action. I. DNA binding and inhibition of RNA-polymerase synthetic reactions by actinomycin. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2094–2101. doi: 10.1073/pnas.48.12.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Persson T. Isolation of mRNA from KB-cells by affinity chromatography on polyuridylic acid covalently linked to Sepharose. Eur J Biochem. 1972 Dec 4;31(2):246–254. doi: 10.1111/j.1432-1033.1972.tb02527.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Molloy G. R., Thomas W. L., Darnell J. E. Occurrence of uridylate-rich oligonucleotide regions in heterogeneous nuclear RNA of HeLa cells. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3684–3688. doi: 10.1073/pnas.69.12.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato H., Kopp D. W., Edmonds M. Localization of the polyadenylate sequences in messenger ribonucleic acid and in the heterogeneous nuclear ribonucleic acid of HeLa cells. J Biol Chem. 1973 Feb 25;248(4):1472–1476. [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Philipson L., Wall R., Glickman G., Darnell J. E. Addition of polyadenylate sequences to virus-specific RNA during adenovirus replication. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2806–2809. doi: 10.1073/pnas.68.11.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siev M., Weinberg R., Penman S. The selective interruption of nucleolar RNA synthesis in HeLa cells by cordycepin. J Cell Biol. 1969 May;41(2):510–520. doi: 10.1083/jcb.41.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Vaughan M. H., Warner J. R., Darnell J. E., Jr The turnover of nuclear DNA-like RNA in HeLa cells. J Cell Biol. 1968 Oct;39(1):112–118. doi: 10.1083/jcb.39.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]


