Abstract
Background:
HIV-infected patients are reported to have impaired oxidation of fatty acids despite increased availability, suggesting a mitochondrial defect. We investigated whether diminished levels of a key mitochondrial antioxidant, glutathione (GSH), was contributing to defective fatty acid oxidation in older HIV-infected patients, and if so, the metabolic mechanisms contributing to GSH deficiency in these patients.
Methods:
In an open-label design, 8 older GSH-deficient HIV-infected males were studied before and after 14 days of oral supplementation with the GSH precursors cysteine and glycine. A combination of stable-isotope tracers, calorimetry, hyperinsulinemic-euglycemic clamp, and dynamometry were used to measure GSH synthesis, fasted and insulin-stimulated (fed) mitochondrial fuel oxidation, insulin sensitivity, body composition, anthropometry, forearm-muscle strength, and lipid profiles.
Results:
Impaired synthesis contributed to GSH deficiency in the patients and was restored with cysteine plus glycine supplementation. GSH improvement was accompanied by marked improvements in fasted and fed mitochondrial fuel oxidation. Associated benefits included improvements in insulin sensitivity, body composition, anthropometry, muscle strength, and dyslipidemia.
Conclusions:
This work identifies 2 novel findings in older HIV-infected patients: 1) diminished synthesis due to decreased availability of cysteine and glycine contributes to GSH deficiency and can be rapidly corrected by dietary supplementation of these precursors and 2) correction of GSH deficiency is associated with improvement of mitochondrial fat and carbohydrate oxidation in both fasted and fed states and with improvements in insulin sensitivity, body composition, and muscle strength. The role of GSH on ameliorating metabolic complications in older HIV-infected patients warrants further investigation.
Despite increased life expectancy with the advent of effective antiretroviral therapy (1, 2), HIV-infected patients manifest a phenotype of accelerated biological aging (3–5) for which there is no identifiable cause. Recognizing this, the Centers for Disease Control and Prevention has defined the cutoff for aging in an HIV patient to begin at 50 years, and projects that by 2015, over 50% of all patients infected with HIV in the United States will be above the age of 50 years (6). Thus, the landscape of HIV infection may be rapidly changing toward an increased population of older HIV patients with fat accumulation, muscle loss, functional limitations, and frailty (7, 8), for which underlying mechanisms are unknown and therapeutic interventions unavailable.
One component of accelerated aging in HIV is mitochondrial aging (9). HIV-infected patients are reported to have impaired fasted oxidation of nonesterified fatty acids (NEFAs) (10, 11), suggesting a mitochondrial defect. Under physiological conditions, the mitochondrial fuel preference in the fasted state is NEFAs, and in the fed state, it is glucose. Thus, when transitioning from the fasted to fed state, efficiently functioning mitochondria have to suppress NEFA oxidation and increase carbohydrate oxidation (12, 13). Changes in mitochondrial fuel oxidation on transitioning from the fasted to fed state have hitherto not been reported in HIV patients. Mitochondria are also the primary generators of harmful reactive oxygen species (ROS) and depend on antioxidants for protection. Glutathione (GSH; γ-glutamyl-cysteinylglycine), the most abundant endogenous intracellular antioxidant, is also a key mitochondrial antioxidant. Acute depletion of GSH results in mitochondrial injury or irreversible cellular damage (14, 15), and we recently reported that it also decreases fasted mitochondrial NEFA oxidation in mice (16). GSH is synthesized from its precursor amino acids glycine, cysteine, and glutamic acid, and HIV is linked to GSH deficiency (17–19). In an unpublished study, we found that older (≥50 years) HIV patients had significant GSH deficiency, but underlying mechanisms remain unknown.
To understand mechanisms underlying GSH deficiency in older HIV patients and whether GSH deficiency contributes to impaired mitochondrial fuel oxidation in these patients, we proposed and tested the following hypotheses: 1) GSH deficiency results from impaired synthesis due to a deficiency of its precursor amino acids and can be improved by supplementing with GSH precursors and 2) correcting GSH deficiency would improve fasted and fed mitochondrial fuel oxidation, insulin sensitivity, body composition, and muscle strength.
Subjects and Methods
Subjects
The study was approved by the Institutional Review Board at Baylor College of Medicine. Eight HIV-infected older men (age >50 years, consistent with the Centers for Disease Control and Prevention definition of aging in HIV-infected patients) with red blood cell (RBC) GSH deficiency (defined as <2 SD below the mean of age-matched subjects from previous studies published by us) (20, 21) were recruited. Subjects were on stable antiretroviral regimens with suppressed viral loads and without any AIDS-defining infections or hospitalizations for the preceding year; without diabetes, thyroid disorders, hypercortisolemia, hypogonadism, renal impairment, or transaminitis (>2-fold upper limit of normal range); and without a history of treatment with testosterone, anabolic agents, or corticosteroids. Any lipid-lowering medications and nonvitamin supplements (including ω-3 fatty acids) were discontinued from 6 weeks before the first study until the end of the second study, and subjects were instructed to avoid alcohol and acetaminophen-containing medications.
Metabolic study protocol
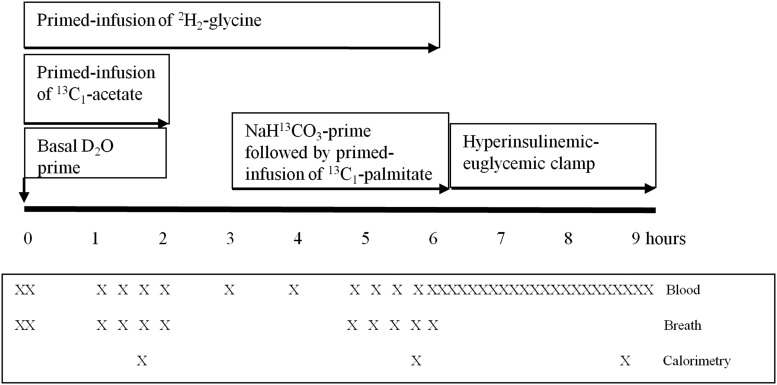
Subjects underwent stable-isotope infusion study at baseline and 2 weeks after receiving oral supplementation with 0.81 mmol/kg/d of cysteine (as N-acetylcysteine) and 1.33 mmol/kg/d of glycine, as capsules prepared by a compounding pharmacist. Compliance was assessed by weekly phone calls and counting of cysteine and glycine capsules at the end of 2 weeks. For 2 days preceding each infusion, subjects consumed a standard diet providing approximately 2500 kcal/d with protein content of 1 g/kg/d to prevent negative energy balance (Figure 1).
Figure 1.
Metabolic infusion protocol.
Subjects were admitted to the adult General Clinical Research Center at 7:00 am after a 10-hour fast and had iv catheters inserted into the veins of both hands for tracer infusions and blood sampling. After collecting basal blood and breath samples, an oral bolus of deuterium oxide (50 mg/kg) was given with primed infusions of [2H2]glycine (prime = 20 μmol/kg; infusion = 15 μmol/kg/h for 6 hours), and [13C1]sodium acetate (prime = 5 μmol/kg; infusion = 5 μmol/kg/h for 2 hours). At hour 3, an iv prime dose of NaH13CO3 (5 μmol/ kg) was administered, followed immediately by a primed infusion of [13C1]potassium palmitate in albumin (prime = 2.4 μmol/kg; infusion = 4.8 μmol/kg/h) from hours 3 to 6. From hours 6 to 9, subjects underwent a hyperinsulinemic-euglycemic clamp initiated with a prime dose of 160 mU/m2/min insulin for 5 minutes and then 80 mU/m2/min for 5 minutes, followed by a constant infusion of 40 mU/m2/min from the hours 6 to 9, with glycemia maintained at the fasted level using a variable infusion of 20% dextrose. Whole-blood glucose concentrations were measured by the glucose oxidase method (Glucose Analyzer; Yellow Springs Instruments) from blood samples collected every 10 minutes throughout the clamp. Subjects also had measures of indirect calorimetry, anthropometry, and grip strength as described below. Blood samples were taken hourly from 0 to 6 hours for measurement of RBC-free and RBC GSH-derived glycine isotopic enrichments, every 15 minutes during the hour 6 for plasma palmitate enrichment and every 10 minutes during the clamp. Breath samples were collected every 15 minutes from hours 1 and 2 and hours 5 and 6 for 13CO2 enrichment. Urine was collected during the protocol. After completing the study protocol, subjects received 14 days of oral dietary supplementation with cysteine and glycine, and same protocol was repeated again.
Indirect calorimetry (Metabolic Cart, CareFusion Inc) was performed during the final 30 minutes of the acetate and palmitate infusions and the final 30 minutes (steady state) of the clamp.
Muscle grip strength was measured with a calibrated Jamar dynamometer in both forearms, using an average of 3 readings on each side.
Anthropometry
Height was measured using a stadiometer and weight by a standardized, calibrated weighing scale. Waist and hip circumferences were measured using a standard tape measure using an averaged value of 3 readings (the waist circumference was measured at the superior border of the iliac crest, per the National Institutes of Health protocol, and the hip circumference at the level of the maximal protrusion of the gluteal muscles with the tape parallel to the floor). The same sites were used for the repeat measurements 2 weeks later.
Blood chemistries
Hemoglobin (Hb), hematocrit, and plasma chemistries were measured in a standard clinical laboratory. Blood was immediately centrifuged and plasma stored at −80°C.
Oxidative stress and oxidant markers
ROS was measured as concentrations of reactive oxygen metabolites as described previously (20, 21). Biomarkers of damage due to oxidative stress were measured by spectrophotometry as plasma concentrations of F2-isoprostanes (Cayman Chemicals).
Glycemic and lipid analyses
Plasma glucose concentrations were measured with a glucose analyzer (Yellow Springs Instruments). Plasma insulin concentrations were measured by highly specific RIA for human insulin (Linco Research). Plasma NEFA concentrations were measured by spectrophotometry (Wako Chemicals). Lipid profiles were measured in a standard clinical laboratory.
RBC enrichment and synthesis rates of GSH and RBC concentrations of GSH, oxidized GSH (GSSG), cysteine, glycine, and glutamic acid were done as previously described by us (20, 21).
The fractional synthesis rate of RBC-GSH
The GSH fractional synthesis rate (FSR) (percent per day) = (IRt2 − IRt1)/(IRRBC × 1200/t2 − t1), where IRt2 − IRt1 is the increase in the isotope ratio of RBC GSH-bound glycine between time points t1 and t2 when the isotope ratio of RBC-free glycine (IRRBC) is at a steady state.
The absolute synthesis rate of RBC-GSH
The absolute synthesis rate (ASR) of GSH (millimolar GSH per gram Hb per day) = RBC-GSH concentration × GSH-FSR.
Lipid kinetics were measured as described by us (11, 22). Briefly, the tracer to tracee ratios of plasma palmitate were determined by negative chemical-ionization gas chromatography mass spectrometer (Agilent Technologies) monitoring ions from mass-to-charge ratios of 255 to 256.
Ra palmitate (flux) = (Tr/TrInf/Tr/Trp − 1) × i, where Tr/TrInf is the tracer to tracee ratio (mole percent) in the infusate, Tr/Trp is the ratio in plasma at the tracer to tracee steady state, and i is the tracer infusion rate.
Whole-body NEFA and carbohydrate oxidation was calculated from calorimetric gas exchange (23).
Acetate oxidation
Recovery of total volume of 13CO2 from [13C1]acetate infused over 2 hours was used as an index of acetate oxidation.
Acetate oxidation (as percent dose of [13C1]acetate oxidized) = V̇CO2 × IECO2/d, where V̇CO2 is the volume of CO2 excreted in breath over 2 hours, IECO2 is the isotopic enrichment of CO2 (atom percent excess), and d is the total dose of [13C1]acetate infused over 2 hours.
Body composition was measured by the deuterated-water technique as previously described (11).
Whole-body insulin sensitivity was measured by the hyperinsulinemic-euglycemic clamp (24). Glucose disposal (M) was calculated as whole-body glucose uptake per kilogram fat-free mass during the final 30 minutes (steady state) of the clamp. The insulin sensitivity index (M/I) was calculated by dividing the M-value by the mean steady-state insulin concentration.
Statistics
Data are expressed as means ± SEM. Data analysis was performed using paired t tests with the Statmate statistical software (GraphPad software version 4). Results were considered to be statistically significant at P < .05.
Results
Subjects were aged 56.1 ± 1.0 years. HIV parameters are provided in Table 1.
Table 1.
Baseline HIV Parameters of GSH-Deficient HIV-Infected Subjects
| Subject | Duration of HIV Infection, y | HIV Viral Load, copies/mL | CD4 Count | Antiretroviral Regimen |
|---|---|---|---|---|
| 1 | 25 | <48 | 635 | Atr, Nor, Rey |
| 2 | 16 | <48 | 1010 | Tzv |
| 3 | 20 | <48 | 655 | Inv, Nor, Vrm |
| 4 | 14 | <48 | 1546 | Atz, Nor, Trv |
| 5 | 13 | <48 | 922 | Atr, Did |
| 6 | 19 | <48 | 364 | Vrm, Tzv |
| 7 | 13 | <48 | 815 | Com, Kal |
| 8 | 13 | <48 | 460 | Epz, Kal |
Abbreviations: Atr, Atripla; Atz, Atazanaivir; Com, Combivir; Did, Didanosine; Epz, Epzicom; Inv, Invirase; Kal, Kaletra; Nor, Norvir; Rey, Reyetaz; Trv, Truvada; Tzv, Trizivir; Vrm, Viramune.
Plasma biochemistry (Table 2)
Table 2.
Serum Biochemistry Before and After Supplementation With Cysteine and Glycinea
| Parameters | Before Supplementation | After Supplementation | P |
|---|---|---|---|
| Hb, g/L | 141 ± 2 | 139 ± 2 | .3 |
| Total bilirubin, mmol/L | 10.3 ± 1.9 | 11.9 ± 1.7 | .5 |
| Alanine transaminase, U/L | 34.6 ± 7.5 | 27.8 ± 4.7 | .3 |
| Aspartate transaminase, U/L | 35.8 ± 6.9 | 26.0 ± 3.1 | .08 |
| Alkaline phosphatase, U/L | 100 ± 12.3 | 96 ± 10.4 | .5 |
| BUN, mmol/L | 5.0 ± 0.4 | 5.6 ± 0.4 | .3 |
| Creatinine, μmol/L | 73.4 ± 5.0 | 71.6 ± 4.2 | .6 |
| HbA1c, % | 5.3 ± 0.1 | 5.3 ± 0.1 | .2 |
| Total cholesterol, mmol/L | 5.8 ± 0.3 | 5.4 ± 0.4 | .3 |
| Triglycerides, mmol/L | 2.2 ± 0.3 | 2.0 ± 0.4 | .3 |
| HDL-cholesterol, mmol/L | 1.2 ± 0.1 | 1.1 ± 0.1 | .2 |
| LDL-cholesterol, mmol/L | 3.4 ± 0.2 | 3.0 ± 0.1 | .02 |
| NEFA, mmol/L | 0.68 ± 0.07 | 0.53 ± 0.09 | .01 |
| Testosterone, nmol/L | 21.5 ± 1.4 | ||
| TSH (mIU/L) | 2.3 ± 0.3 | 2.4 ± 0.3 | .6 |
| Free T4, ng/L | 1.1 ± 0.1 | 1.0 ± 0.1 | .5 |
Abbreviations: BUN, blood urea nitrogen; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Values are mean ± SEM; Means are significantly different at P < .05.
All subjects were euthyroid and eugonadal, with normal renal and liver functions. Supplementation was well-tolerated without changes in liver or renal functions, and there was a trend toward improvement in plasma aspartate transaminase concentrations (P = .08). Fasted plasma low-density lipoprotein cholesterol (P = .02) and NEFA concentrations (P = .01) decreased significantly, without any significant alterations in total or high-density lipoprotein cholesterol, triglyceride, or glucose concentrations or HbA1c.
GSH kinetics and oxidative stress
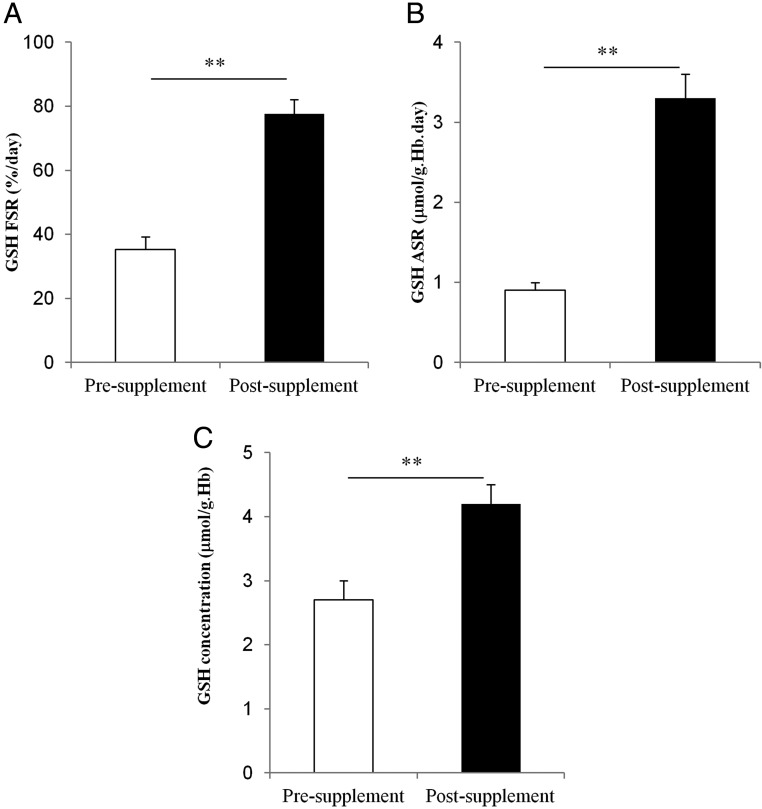
Supplementation with glycine plus cysteine resulted in 32% higher RBC glycine (P = .002), 46% higher RBC cysteine (P = .02), and a 36% increase in RBC glutamic acid (P = .04) concentrations. These changes were associated with a 120% increase in the FSR (P = .0001), a 230% increase in the ASR (P = .00008) of GSH, and a 53% increase in RBC GSH concentration (P = .0003) (Figure 2, A–C). With supplementation, RBC concentrations of GSSG decreased (P = .04) and the GSH to GSSG ratio increased significantly (P = .02), suggesting an improvement in GSH cycling between its oxidized and reduced (active) state. Plasma concentrations of ROS (P = .0003) and F2-isoprotanes (P = .0005) decreased significantly with the rise in GSH (Table 3).
Figure 2.
GSH kinetics in HIV-infected patients before and after supplementation with oral dietary cysteine and glycine. A, RBC GSH FSR, B, RBC GSH ASR. C, RBC GSH concentrations. Values are means ± SEM. Means are considered significant at P < .05 (**, P < .001).
Table 3.
Changes in GSH Kinetics, Biomarkers of Oxidative Stress Mitochondrial Energetics, Insulin Sensitivity, Body Composition, and Muscle Strength Before and After Supplementation With Cysteine and Glycinea
| Parameters | Before Supplementation | After Supplementation | P |
|---|---|---|---|
| GSH kinetics and biomarkers of oxidative stress | |||
| RBC GSH FSR, %/d | 35.3 ± 3.9 | 77.6 ± 4.5 | .0001 |
| RBC GSH ASR, μmol/g Hb/d | 0.9 ± 0.1 | 3.3 ± 0.3 | .00008 |
| RBC GSH, μmol/g Hb | 2.7 ± 0.3 | 4.2 ± 0.3 | .0003 |
| RBC GSSG, μmol/g Hb | 1.3 ± 0.2 | 0.7 ± 0.1 | .04 |
| RBC GSH/GSSG ratio | 2.7 ± 0.6 | 6.1 ± 0.6 | .02 |
| RBC glycine, μmol/g Hb | 0.89 ± 0.04 | 1.19 ± 0.06 | .002 |
| RBC cysteine, μmol/g Hb | 0.26 ± 0.02 | 0.38 ± 0.03 | .02 |
| RBC glutamic acid, μmol/g Hb | 0.56 ± 0.05 | 0.77 ± 0.07 | .04 |
| Plasma reactive oxygen metabolites, U.Carr | 1128 ± 93 | 545 ± 141 | .0003 |
| Plasma F2-isoprostane, pg/ml | 32.4 ± 1.9 | 21.4 ± 2.2 | .0005 |
| Mitochondrial energetics and insulin sensitivity | |||
| Ra palmitate, μmol/kg/h | 91.4 ± 8.2 | 95.9 ± 4.8 | .3 |
| % dose of [13C]acetate oxidized | 18.9 ± 0.5 | 19.1 ± 0.4 | .6 |
| Fasted NEFA oxidation, mg/kg FFM/min | 0.8 ± 0.1 | 1.2 ± 0.10 | .0003 |
| Fasted carbohydrate oxidation, mg/kg FFM/min | 1.7 ± 0.2 | 0.8 ± 0.1 | .002 |
| Fed (clamp) NEFA oxidation, mg/kg FFM/min | 0.6 ± 0.1 | 0.4 ± 0.1 | .01 |
| Fed (clamp) carbohydrate oxidation, mg/kg FFM/min | 2.4 ± 0.2 | 2.9 ± 0.2 | .005 |
| Suppression of NEFA oxidation when transitioning from fasted to fed state, % | 28.2 ± 6.6 | 66.6 ± 4.1 | .0002 |
| Stimulation of carbohydrate oxidation when transitioning from fasted to fed state, % | 44.3 ± 3.9 | 350.9 ± 83.5 | .01 |
| RQfasted | 0.83 ± 0.01 | 0.77 ± 0.01 | .0004 |
| RQfed | 0.87 ± 0.01 | 0.89 ± 0.01 | .01 |
| Fasting glucose, mmol/L | 5.8 ± 0.1 | 5.7 ± 0.1 | .8 |
| Fasting insulin, pmol/L | 70.9 ± 15.3 | 70.0 ± 11.9 | .9 |
| Steady-state clamp glucose, mmol/L | 5.7 ± 0.1 | 5.7 ± 0.1 | .9 |
| Steady-state clamp insulin, pmol/L | 323.6 ± 41.3 | 314.6 ± 41.5 | .7 |
| Glucose disposal rate (M), μmol/kg FFM/min | 28.8 ± 3.1 | 34.3 ± 3.6 | .01 |
| Insulin sensitivity index (M/I), (μmol glucose/kg FFM/min)/(pmol insulin/L) | 0.09 ± 0.02 | 0.12 ± 0.01 | .04 |
| Body composition and muscle strength | |||
| Weight, kg | 81.7 ± 2.4 | 81.0 ± 2.0 | .02 |
| BMI | 27.7 ± 0.9 | 27.5 ± 0.8 | .02 |
| Fat-mass, kg | 21.6 ± 2.3 | 20.0 ± 1.8 | .002 |
| FFM, kg | 60.1 ± 2.7 | 61.0 ± 2.3 | .003 |
| Waist circumference, m | 0.98 ± 0.03 | 0.97 ± 0.02 | .02 |
| Hip circumference, m | 0.95 ± 0.02 | 0.95 ± 0.02 | .4 |
| Waist to hip ratio | 1.03 ± 0.02 | 1.02 ± 0.01 | .01 |
| Muscle strength, kg | |||
| Dominant forearm | 35 ± 1 | 37 ± 1 | .0007 |
| Nondominant forearm | 31 ± 1 | 34 ± 1 | .004 |
Abbreviations: BMI, body mass index; FFM, fat-free mass.
Values are mean ± SEM; Means are significantly different at P < .05.
Fasted fuel oxidation
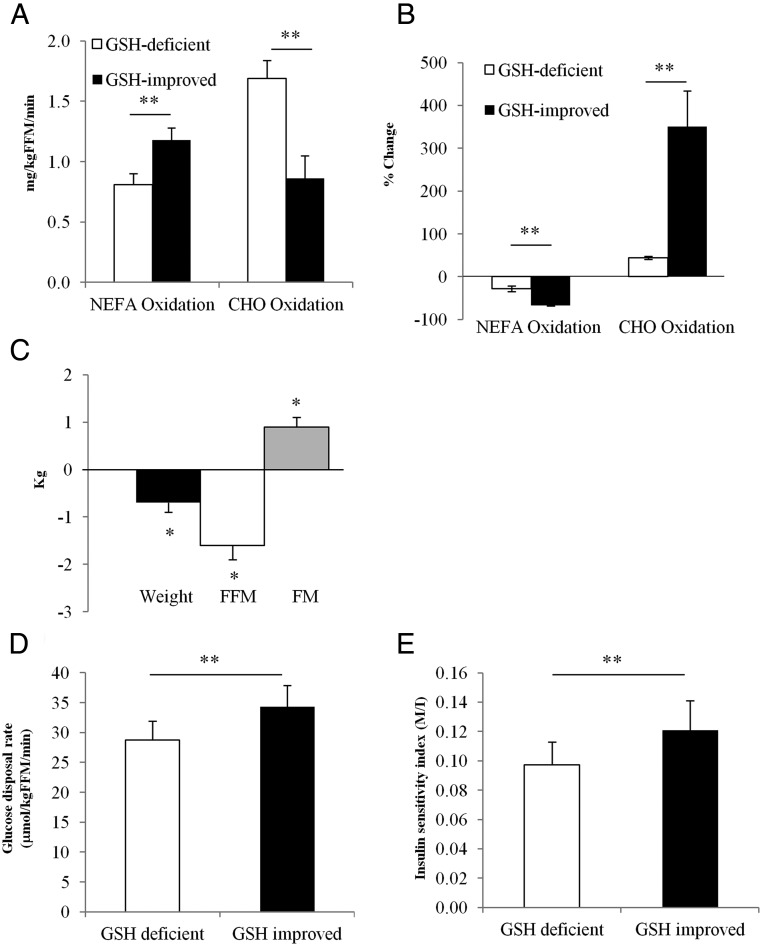
In the GSH-deficient state, fasted NEFA oxidation was lower than carbohydrate oxidation. GSH improvement was associated with a 46% increase (P = .0003) in NEFA oxidation and a 49% decrease (P = .002) in carbohydrate oxidation (Figure 3A), suggesting recovery of impaired fasted mitochondrial fuel oxidation. Fasted plasma acetate oxidation did not change with GSH improvement, suggesting that there were no defects in the Krebs cycle (Table 3).
Figure 3.
Correcting GSH deficiency improves fasted and fed mitochondrial fuel oxidation, glucose disposal, and body composition. A, Fasted NEFA and carbohydrate oxidation. B, Percent change in NEFA and carbohydrate oxidation when transitioning from the fasted to a fed (insulin-stimulated) state. C, Body composition. D, Glucose disposal rate. E, Insulin sensitivity index. Values are means ± SEM. Means are considered significant at P < .05 (*, P < .05; **, P < .001). Abbreviations: CHO, carbohydrate; FFM, fat-free mass; FM, fat-mass; M/I, glucose disposal rate/mean insulin concentration during the final 30 minutes of the clamp ([micromoles glucose per kilogram fat-free mass per minute]/[picomoles insulin per liter]).
Fed fuel oxidation
With GSH improvement, fasted respiratory quotient (RQ) decreased significantly (P = .0004) and fed (insulin-stimulated) RQ increased significantly (P = .01) (Table 3). This was associated with significantly greater suppression of NEFA oxidation (28.2% vs 66.6%; P = .0002) and markedly increased stimulation of carbohydrate oxidation (44.3% vs 350.9%; P = .009) when transitioning from the fasted to fed state (Figure 3B) and suggests that GSH improvement was associated with significant improvements in fasted and fed mitochondrial fuel oxidation.
Body composition, anthropometry, insulin sensitivity, and forearm muscle strength
GSH improvement was associated with 1) significantly lower body mass index (P = .02), waist circumference (P = .02), body weight (P = .02), and fat mass (P = .002) and higher fat-free mass (P = .003) (Figure 3C), without any changes in hip circumference; 2) 16% higher glucose disposal rate (P = .03) (Figure 3D) and 31% increase in the insulin sensitivity index (P = .02) (Figure 3E); and 3) significantly higher muscle strength in the dominant (P = .0007) and nondominant (P = .004) forearm (Table 3).
Discussion
There were two key findings in this study of older HIV-infected patients: 1) deficient intracellular GSH synthesis and decreased GSH regeneration contribute GSH deficiency and can be corrected with short-term cysteine plus glycine supplementation and 2) correcting GSH deficiency was associated with marked improvements in mitochondrial fuel oxidation in the fasted and fed states, insulin sensitivity, muscle strength, and body composition.
GSH deficiency in HIV-infected patients
GSH deficiency is reported in patients with HIV infection (17–19). Results from our study suggest the presence of 2 factors contributing to intracellular GSH deficiency in older HIV-infected patients. The first and major contributor is impaired synthesis due to diminished availability of the amino acid precursors cysteine and glycine (compared with values reported in healthy controls) (20, 21), and the second is increased entrapment of GSH in its oxidized form. Both defects are rapidly overcome with short-term oral dietary supplementation with cysteine and glycine, which increases their intracellular concentrations and that of GSH and also significantly lowers oxidative stress and oxidant damage. Underlying causes for these defects are unclear, and additional studies are needed to investigate mechanisms, but deficiency of cysteine and glycine could be linked to alterations in protein metabolism reported in HIV patients (25).
Link between GSH and mitochondrial fuel oxidation in older HIV subjects
The cardinal finding in this study was that in older HIV-infected patients, mitochondrial fuel oxidation in the fasted and the fed states improved significantly with GSH improvement. Under physiological conditions, fuel preference for energy generation in the fasted state is NEFAs (ie, NEFA oxidation more than carbohydrate oxidation), and fuel preference in the fed state is carbohydrate (carbohydrate oxidation more than NEFA oxidation). The ability of mitochondria to switch efficiently between these fuels in the 2 prandial states represents efficient mitochondrial function (12, 13, 26, 27). However, older HIV-infected patients in our study had a reversal of physiological fuel oxidation in the fasted state with lower NEFA oxidation (despite NEFA availability) and higher fasted carbohydrate oxidation. An increase in GSH levels in these subjects was associated with a return to a physiological pattern of fasted fuel oxidation (NEFA more than carbohydrate). We also found that increasing GSH concentrations was associated with a significant improvement in mitochondrial fuel oxidation on transition from the fasted to the fed state, with a 2.4-fold greater suppression of NEFA oxidation and a 7.9-fold higher increase in glucose oxidation. These data suggest that GSH could play a novel and important role in maintaining optimal mitochondrial fuel oxidation in both prandial states in older HIV-infected patients.
Site of impaired mitochondrial fuel oxidation
Where does the defect in fuel oxidation lie? A clue comes from the elevated fasted carbohydrate oxidation, which argues against a defect in the Krebs cycle. Because the [13C1]acetate tracer enters the acetyl-coenzyme A pool and is oxidized to 13CO2 in the Krebs cycle, we measured recovery of 13CO2 after an infusion of [13C1]acetate (as an index of Krebs cycle function) and found no change with GSH improvement, suggesting that Krebs cycle function was not altered and that the defect must lie before it. Data from a recent study in older HIV-infected patients presented at the 19th AIDS Conference (28) did not find any changes in expression or activity of carnitine palmitoyl transferase 1 but decreased activity of hydoxyacyl coenzyme A dehydrogenase, which suggests that mitochondrial entry of NEFAs is not impaired in these patients and that the defects could lie in impaired mitochondrial β-oxidation.
How does improvement of GSH improve mitochondrial fuel oxidation?
Aging is associated with increased production of ROS, which can damage cellular proteins, lipids, and DNA (29–31). We have previously shown that correcting GSH deficiency in aging decreases ROS levels and ROS-mediated biomarkers of cellular damage (21). In aging, mitochondrial DNA is susceptible to oxidative damage due to increased levels of ROS (32),and is associated with a 17-fold higher rate of mutations compared with nuclear DNA (33). Therefore, it is conceivable that impaired mitochondrial fuel oxidation could occur due to damaged mitochondrial DNA leading to defects in transcription, translation, or activity of enzymes regulating NEFA oxidation and that improving GSH concentrations could protect against such ROS-induced mitochondrial damage. Although the precise mechanisms by which GSH regulates mitochondrial fuel oxidation are not clear, our study supports the need for additional work to investigate underlying mechanisms.
Changes in body composition with GSH improvement
Although impaired NEFA oxidation is a risk factor for weight gain (34), it is unclear whether improving NEFA oxidation could induce weight loss. Our study shows improved mitochondrial fuel oxidation on GSH improvement was associated with a modest but significant reduction in body weight. However, there was a striking decrease in total fat mass, waist circumference, and waist to hip ratios without any change in the hip circumference, which suggests that the fat loss may have preferentially occurred in the central abdominal adipose tissue compartment. Interestingly, there was a simultaneous and significant increase in fat-free mass, which remains unexplained and could be linked to changes in protein metabolism reported to occur in HIV infection (25).
Changes in glucose disposal and insulin sensitivity with GSH improvement
GSH improvement significantly improved glucose disposal rate and insulin sensitivity. Although the underlying mechanisms are not clear, 2 factors could have played a role: elevated levels of plasma NEFA concentrations (36) and ROS (by impairment of insulin signaling [37] and decreased Glut4 transcription [38, 39]) have both been linked to insulin resistance. Decreases in elevated plasma concentrations of both NEFA and ROS concentrations could have contributed to improved insulin sensitivity.
Accelerated aging in HIV
Mitochondrial dysfunction has been linked to the aging process. Results from our study suggest that impaired mitochondrial fuel oxidation improves on correcting GSH deficiency. One parameter indicative of accelerated aging is decreased muscle strength. Using gender-specific normative values for grip strength (40), we found that GSH-deficient older HIV-infected subjects in our study (average age 56 years) had a muscle strength equivalent to an 80-year-old, which improved to that of a 70-year-old with an increase in GSH. This suggests an improvement toward their chronological age by 10 years within a 2-week timeframe and raises the question of whether GSH deficiency acting through impaired mitochondrial fuel oxidation could contribute to accelerated aging in HIV.
Study limitations
The limitations of our study are a small sample size, short-term duration of supplementation, and lack of a placebo group. Despite these limitations, results show a significant impact of GSH improvement on mitochondrial fuel metabolism, insulin resistance, body composition, and muscle strength and supports the need for additional long-term studies to investigate the role GSH on metabolic disease in older HIV-infected patients.
Conclusions
These data suggest that GSH adequacy promotes metabolic health in older HIV-infected patients. Diminished GSH synthesis caused by deficiency of its precursors cysteine and glycine is an important contributor to GSH deficiency in these patients and can be rapidly corrected by supplementing these amino acids in the diet. Improvement of GSH is associated with restoration of a physiological pattern of mitochondrial fuel oxidation, lower total body fat, waist circumference, and insulin resistance and higher fat-free mass and muscle strength, suggesting that GSH deficiency could contribute to abnormalities in these parameters. These findings also raise the intriguing question of whether GSH deficiency could underlie or contribute to accelerated aging in HIV-infected patients and warrants further investigation.
Acknowledgments
We thank the nursing, pharmacy, and dietary staff of the adult general clinical research center (GCRC) at Baylor College of Medicine for meticulous care with the conduction of studies.
This work was supported by the Baylor-UT Council for AIDS Research, a National Institutes of Health (NIH)-funded program (AI036211); the Baylor Alkek Bridge fund; Baylor GCRC (NIH RR-0188); and the Baylor NIH-Diabetes Research Center (NIH-P30DK079638).
D.N. contributed to protocol execution, sample analyses, and manuscript review. J.W.H. contributed to sample analyses. F.J. contributed to manuscript review. R.V.S. conceived the project; designed, coordinated, and supervised studies and sample analyses; analyzed and interpreted data; and drafted the manuscript.
Disclosure Summary: None of the authors have any conflicts of interest to disclose. R.V.S. is an inventor on U.S. patent US20110077303 with rights held by Baylor College of Medicine.
Footnotes
- ASR
- absolute synthesis rate
- FSR
- fractional synthesis rate
- GSH
- glutathione
- GSSG
- oxidized GSH
- Hb
- hemoglobin
- NEFA
- nonesterified fatty acid
- RBC
- red blood cell
- ROS
- reactive oxygen species
- RQ
- respiratory quotient.
References
- 1. Palella FJ, J., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860 [DOI] [PubMed] [Google Scholar]
- 2. Mack KA, Ory MG. AIDS and older Americans at the end of the twentieth century. J Acquir Immune Defic Syndr. 2003;33(Suppl 2):S68–S75 [DOI] [PubMed] [Google Scholar]
- 3. Pathai S, Lawn SD, Shiels PG, et al. Corneal endothelial cells provide evidence of accelerated cellular senescence associated with HIV infection: a case-control study. PLoS One. 2013;8(2):e57422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, Cheng WJ, Paukovics G, Palmer CS, Novak RM, Jaworowski A, Landay AL, Crowe SM. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One. 2013;8(1):e55279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatia R, Ryscavage P, Taiwo B. Accelerated aging and human immunodeficiency virus infection: emerging challenges of growing older in the era of successful antiretroviral therapy. J Neurovirol. 2012;18(4):247–255 [DOI] [PubMed] [Google Scholar]
- 6. CDC Fact Sheet: HIV/AIDS Among Persons Aged 50 and Older Centers for Disease Control and Prevention website. http://www.cdc.gov/hiv/pdf/library_factsheet_HIV_among_PersonsAged50andOlder.pdf
- 7. Önen NF, Overton ET. A review of premature frailty in HIV-infected persons; another manifestation of HIV-related accelerated aging. Curr Aging Sci. 2011;4(1):33–41 [PubMed] [Google Scholar]
- 8. Erlandson KM, Allshouse AA, Jankowski CM, Mawhinney S, Kohrt WM, Campbell TB. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J Acquir Immune Defic Syndr. 2013;1;63(2):209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Payne BA, Wilson IJ, Hateley CA, et al. Mitochondrial aging is accelerated by anti-retroviral therapy through the clonal expansion of mtDNA mutations. Nat Genet. 2011;43(8):806–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vassimon HS, Albuquerque de Paula FJ, Machado AA, Monteiro JP, Jordão AA., Jr Hypermetabolism and altered substrate oxidation in HIV-infected patients with lipodystrophy. Nutrition. 2012;28(9):912–916 [DOI] [PubMed] [Google Scholar]
- 11. Sekhar RV, Jahoor F, White AC, et al. Metabolic basis of HIV-lipodystrophy syndrome. Am J Physiol Endocrinol Metab. 2002;283:E332–E337 [DOI] [PubMed] [Google Scholar]
- 12. Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a re-examination. Diabetes. 2000;49(5):667–683 [DOI] [PubMed] [Google Scholar]
- 14. Ghosh S, Pulinilkunnil T, Yuen G, et al. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol. 2005;289:H768–H76 [DOI] [PubMed] [Google Scholar]
- 15. Mastrocola R, Restivo F, Vercellinatto I, et al. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol. 2005;187:37–44 [DOI] [PubMed] [Google Scholar]
- 16. Nguyen D, Samson SL, Reddy VT, Gonzalez EV, Sekhar RV. Impaired mitochondrial fatty acid oxidation and insulin resistance in aging: novel role of glutathione. Aging Cell. 2013;12(3):415–425 [DOI] [PubMed] [Google Scholar]
- 17. Vassimon HS, Deminice R, Machado AA, Monteiro JP, Jordao AA. The association of lipodystrophy and oxidative stress biomarkers in HIV-infected men. Curr HIV Res. 2010;8(5):364–369 [DOI] [PubMed] [Google Scholar]
- 18. Sbrana E, Paladini A, Bramanti E, Spinetti MC, Raspi G. Quantitation of reduced glutathione and cysteine in human immunodeficiency virus-infected patients. Electrophoresis. 2004;25(10–11):1522–1529 [DOI] [PubMed] [Google Scholar]
- 19. Nakamura H, Masutani H, Yodoi J. Redox imbalance and its control in HIV infection. Antioxid Redox Signal. 2002;4(3):455–464 [DOI] [PubMed] [Google Scholar]
- 20. Sekhar RV, McKay SV, Guthikonda AP, Patel SG, Reddy VT, Balasubramanyam A, Jahoor F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34(1):162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sekhar RV, Patel SG, Guthikonda AP, et al. Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation. Am J Clin Nutr. 2011;94(3):847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sekhar RV, Patel SG, D'Amico S, et al. Effects of rosiglitazone on abnormal lipid kinetics in HIV-associated dyslipidemic lipodystrophy: a stable isotope study. Metabolism. 2011;60(6):754–760 [DOI] [PubMed] [Google Scholar]
- 23. Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634 [DOI] [PubMed] [Google Scholar]
- 24. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 25. Reeds DN, Cade WT, Patterson BW, Powderly WG, Klein S, Yarasheski KE. Whole-body proteolysis rate is elevated in HIV-associated insulin resistance. Diabetes. 2006;55(10):2849–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. Am J Physiol Endocrinol Metab. 2008;295(5):E1009–E1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63(2):363–368 [DOI] [PubMed] [Google Scholar]
- 28. Ortmeyer H, Ryan A, Hafer-Macko C, Oursler KA. Reduced skeletal muscle mitochondrial function in older HIV-infected men is associated with low aerobic exercise capacity. In: Proceedings from the 19th International AIDS Conference; July 22–27, 2012; Washington, DC Abstract WEPE091 [Google Scholar]
- 29. Chakravarti B, Chakravarti DN. Oxidative modification of proteins: age-related changes. Gerontology. 2007;53(3):128–139 [DOI] [PubMed] [Google Scholar]
- 30. Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17(10):1195–1214 [DOI] [PubMed] [Google Scholar]
- 31. Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutation Res. 2004;567(1):1–61 [DOI] [PubMed] [Google Scholar]
- 32. Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995;27:647–653 [DOI] [PubMed] [Google Scholar]
- 33. Wallace DC, Ye JH, Neckelmann SN, Singh G, Webster KA, Greenberg BD. Sequence analysis of cDNAs for the human and bovine ATP synthase beta subunit: mitochondrial DNA genes sustain seventeen times more mutations. Curr Genet. 1987;12:81–90 [DOI] [PubMed] [Google Scholar]
- 34. Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259(5):E650–657 [DOI] [PubMed] [Google Scholar]
- 35. Corpeleijn E, Saris WH, Blaak EE. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev. 2009;10(2):178–193 [DOI] [PubMed] [Google Scholar]
- 36. Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46(1):3–10 [PubMed] [Google Scholar]
- 37. Mouzannar R, McCafferty J, Benedetto G, Richardson C. Transcriptional and phospho-proteomic screens reveal stem cell activation of insulin resistance and transformation pathways following a single minimally toxic episode of ROS. Int J Genomics Proteomics. 2011;2(1):34–49 [PMC free article] [PubMed] [Google Scholar]
- 38. Bloch-Damti A, Bashan N. Proposed mechanisms for the induction of insulin resistance by oxidative stress. Antioxid Redox Signal. 2005;7(11–12):1553–1567 [DOI] [PubMed] [Google Scholar]
- 39. Karnieli E, Armoni M. Transcriptional regulation of the insulin-responsive glucose transporter GLUT4 gene: from physiology to pathology. Am J Physiol Endocrinol Metab. 2008;295(1):E38–E45 [DOI] [PubMed] [Google Scholar]
- 40. Peters MJ, van Nes SI, Vanhoutte EK, et al. ; PeriNomS Study Group Revised normative values for grip strength with the Jamar dynamometer. J Peripher Nerv Syst. 2011;16(1):47–50 [DOI] [PubMed] [Google Scholar]