Abstract
Context:
Maternal hypothyroidism during pregnancy is associated with adverse neuropsychological development in the offspring.
Objective:
The objective of the study was to evaluate the effect of maternal thyroid dysfunction during pregnancy on a child's attention-deficit/hyperactivity disorder (ADHD) symptoms.
Design, Settings, and Participants:
The prospective, population-based Northern Finland Birth Cohort 1986 (9362 pregnancies; 9479 infants) included analysis of maternal TSH, free T4, and thyroid-peroxidase antibodies (TPO-Abs) from early pregnancy samples (5791 women). Teachers evaluated the children's ADHD symptoms at 8 years using the Rutter B2 scale (5131 mother-child pairs), in which a high score indicated probable psychiatric disorders and three questions focused directly on ADHD.
Main outcome measures:
The odds ratios (ORs) and 95% confidence intervals (95% CIs) of child having ADHD symptoms and/or a high Rutter B2 score after exposure to increases in maternal TSH levels (after logarithmic transformation), low free T4 levels, and TPO-Ab positivity was tested with logistic regression, adjusting for maternal/family covariates. Data were stratified by the child's gender due to interaction.
Results:
Among girls the odds of inattention (OR 1.18, 95% CI 1.02–1.37), high Rutter B2 total score (OR 1.23, 95% CI 1.03–1.48), and combined ADHD symptoms (OR 1.39, 95% CI 1.07–1.80) significantly increased with every natural log increase in maternal TSH concentrations. Such findings were not evident in boys. No associations were seen between ADHD symptoms and low maternal free T4 levels or TPO-Ab positivity.
Conclusions:
Increases in maternal TSH in early pregnancy showed weak but significant association with girls' ADHD symptoms.
Early pregnancy is a crucial time in fetal neurodevelopment, during which the fetus totally depends on maternal thyroid hormone supply (1, 2). Thyroid hormones are required for normal neuronal migration and maturation (3), and lack of thyroid hormones during pregnancy due to severe maternal iodine deficiency leads to mental retardation in the child (4).
Women with limited thyroidal capacity, due to autoimmunity, disease, or iodine deficiency, are at high risk of hypothyroidism or hypothyroxinemia (low serum free T4 with normal TSH concentrations) during pregnancy (5). Insufficiently treated maternal hypothyroidism and hypothyroxinemia have been associated with reductions in the intelligence quotient (IQ) of the offspring (6–8), reduced performance in motor skills (1, 7–10), and poorer reaction time (11). Hypothyroxinemia has also been associated with expressive language and cognitive delay (10, 12) and autism (13). However, results from a randomized trial showed no improvement in the child's IQ after adequately treated maternal hypothyroidism or hypothyroxinemia (14).
It is also suggested that untreated maternal hypothyroidism associates with delays in attention at 7–9 years (7), and increases in maternal serum TSH and thyroid-peroxidase antibody (TPO-Ab) levels might elevate the risk of externalizing symptoms in early childhood (15, 16). The prevalence of attention-deficit/hyperactivity disorder (ADHD) might be higher in areas with iodine deficiency (17). The effects of maternal thyroid function during pregnancy on ADHD symptoms of children still remain unclear.
We studied the associations between maternal thyroid function during pregnancy and ADHD symptoms of the offspring at the age of 8 years in the prospective, population-based Northern Finland Birth Cohort 1986. We hypothesized that untreated maternal thyroid dysfunction increases the odds of ADHD symptoms in children.
Materials and Methods
The Northern Finland Birth Cohort 1986 (NFBC 1986)
The prospective NFBC 1986 comprises of 99% of all births between July 1, 1985, and June 30, 1986, drawn from the two northernmost provinces of Finland (9362 mothers and 9479 children). Maternal and family demographics, maternal health data, and data on pregnancy, delivery, and neonatal outcomes were collected during routine visits at communal free-of-charge maternity welfare clinics (overall participation rate 99.8% in Finland) and via questionnaires during the index pregnancies. The cohort has been followed up since the first maternity welfare clinic visit at 8–12 weeks of gestation, and all mothers were recruited to the study by 24 weeks (18, 19).
Since birth, data on the health of the cohort children and familial demographic data have been obtained via visits to free-of-charge community child welfare clinics and via questionnaires, supplemented with data from various national registers. Informed consent was obtained from all subjects. The Ethics Committees of the Northern Ostrobothnia Hospital District and the National Institute for Health and Welfare approved this study.
Study population
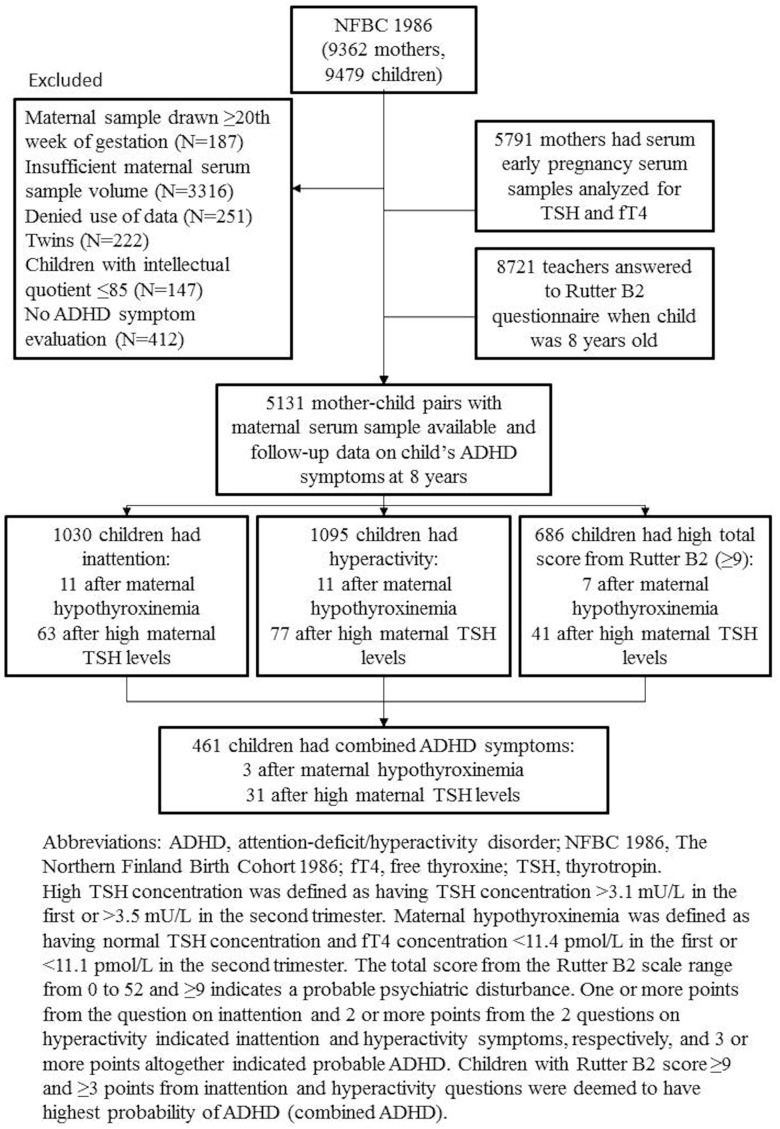
Of the total 9479 children, we excluded twins (n = 222), those refusing use of data (n = 251), those with diagnosed intellectual disabilities (full scale IQ ≤ 85; n = 147), and those missing data on maternal thyroid function analyses (n = 3316) or on ADHD symptoms (n = 412) (Figure 1). The final study population consisted of 5131 mothers with thyroid function analyses carried out during early pregnancy and their 5131 children with follow-up data on ADHD symptoms at 8 years.
Figure 1.
Study progression and results of the 8-year questionnaires of NFBC 1986 children.
Maternal thyroid function assessment
Mothers of NFBC 1986 underwent infectious disease screening in early pregnancy (mean gestational age at sampling 10.7 wk, SD 2.8), with leftover serum samples stored at the premises of the Finnish Maternity Cohort, frozen at −25°C. These samples (5791, 61.1% of the whole NFBC 1986 cohort) were analyzed for TSH, free T4, and TPO-Abs using the Abbott Architect i2000 method (Abbott Diagnostics) in 2006. Laboratory data collection, analysis (20), and the effect of long-term storage on these laboratory parameters have been reported previously (21). The mothers with and without laboratory analyses did not have significant differences in maternal demographic characteristics and birth outcomes (20).
ADHD symptoms
Children's ADHD symptoms at 8 years of age were evaluated by their main teachers (participation rate 92%). The teachers used the official Finnish translations of the Rutter scale B2 with 29 questions. The scale has one question on inattention (“child is not able to concentrate on anything for a longish period”) and two on hyperactivity (“child is restless, does not have patience to sit down for a long period of time” and “child wriggles and is restless”). The teachers answered the questions in one of three ways: does not apply, applies to some degree, or applies well, and these were rated 0, 1, and 2 points accordingly. The Rutter B2 scale also includes questions on general behavior during classes and on friends, mood swings, headaches, and school absences. The total score from the Rutter B2 scale ranges from 0 to 52, in which 9 or greater indicates a probable psychiatric disturbance (22). Children were categorized as inattentive if they scored 1 or 2 points on inattention and as hyperactive if they scored 2 or more points combined on the questions on hyperactivity. Children with total Rutter B2 scores of 9 or greater and 3 or more points from ADHD questions were deemed to have the highest probability of ADHD and will henceforth be referred to as having combined ADHD symptoms (22). Among the families with data on thyroid function during pregnancy, 5089 teacher questionnaires had all questions answered and a total of 5131 questionnaires had at least one answered item on ADHD.
Statistical analyses
Maternal and family characteristics of mothers with high serum TSH concentrations or hypothyroxinemia during pregnancy vs the total cohort were compared by using Student's t tests for continuous variables with normal distributions and by using the Mann-Whitney U test for those with non-Gaussian distributions. Categorical variables were compared by using χ2 tests.
Maternal serum TSH and free T4 concentrations were categorized on the basis of trimester and population-specific reference limits (23). High TSH was defined as a concentration above the upper reference limit of 3.1 mU/L in the first trimester or 3.5 mU/L in the second trimester. Maternal hypothyroxinemia was defined as having a normal serum TSH concentration and a low free T4 concentration, less than 11.4 pmol/L in the first trimester, or less than 11.1 pmol/L in the second trimester. Analyses of maternal TSH concentrations were also conducted on continuous measures, after logarithmically transforming the data to achieve normality.
χ2 tests were used to evaluate the prevalence of ADHD symptoms among children of mothers with and without high TSH levels or hypothyroxinemia. Logistic regression was used to calculate odds ratios (ORs) with 95% confidence intervals (CIs) to estimate the risk of ADHD symptoms associated with high TSH levels, hypothyroxinemia, and maternal TSH increases in the natural log scale.
Our first-line analyses were conducted among all children, adjusting for child's gender, number of children in the family at the time of ADHD symptom evaluation (two or more vs one) maternal smoking (yes vs no), maternal education (<11 y vs ≥ 11y), and maternal age (<20 or > 35 y vs 20–35 y). Because adjusting for child's gender modified our effect estimates, all further analyses were performed for boys and girls separately to deal with potential interaction in the analyses. Other covariates were selected based on literature (24–26) and retained if they affected one or more of the ADHD symptoms at a statistically significant level when included in the model. Because the unadjusted and adjusted analyses were similar, we present only the adjusted analyses.
As a sensitivity analysis, we analyzed all data by including and excluding TPO-Ab-positive mothers (TPO-Ab concentration > 167.7 IU/mL) and mothers with thyroid medication use currently or in the past (n = 98 of whom 96% used levothyroxine) were retained in the analysis. Data were also stratified to term and preterm children to study if preterm birth modified the association.
All statistical analyses were performed by using SPSS version 18.0 software (IBM Statistics).
Results
Characteristics of the NFBC 1986 population are shown in Table 1. Mothers with high serum TSH concentrations had a higher prepregnancy body mass index (BMI) (calculated as weight in kilograms per height squared in square meters), and they smoked less than the total cohort. Hypothyroxinemic mothers also had a higher BMI and they were older than the total cohort. The number of children in the family when the child was 8 years old was higher among hypothyroxinemic mothers than in the total cohort. Otherwise, the two maternal thyroid function groups did not differ from the cohort with respect to maternal education, time of maternal serum sampling, number of preterm (<37 wk) births, and the proportion of male children.
Table 1.
Maternal and Family Characteristics Grouped by Maternal Thyroid Function
| Characteristics | High Maternal TSH (n = 348)a | Hypothyroxinemia (n = 66)b | NFBC 1986 With Sufficient Maternal Serum Samples (n = 5131) |
|---|---|---|---|
| Median (IQR) maternal TSH concentration, mU/L | 4.1 (3.6–5.5)c | 1.3 (0.9–2.0) | 1.2 (0.7–1.9) |
| Median (IQR) maternal free T4, pmol/L | 13.9 (12.5–15.3)c | 11.0 (10.7–11.2)c | 15.1 (13.8–16.7) |
| Median (IQR) maternal TPO-Ab, IU/mL | 23.2 (4.8–296.5)c | 3.4 (2.3–5.7)d | 4.3 (3.1–6.7) |
| Mean (SD) maternal age at birth, y | 28.5 (5.4) | 29.9 (6.2)d | 28.2 (5.3) |
| >35 y, n, % | 43 (12.4) | 16 (24.2)d | 614 (12.0) |
| <20 y, n, % | 13 (3.7) | 4 (6.1) | 201 (3.9) |
| Mean (SD) BMI, kg/m2 | 22.6 (3.6)d | 23.7 (4.8)d | 22.2 (3.4) |
| Overweight/obese (BMI ≥ 25 kg/m2), n, % | 71 (20.6)d | 16 (26.7)d | 812 (16.2) |
| Smoking during pregnancy, n, % | 48 (13.9)d | 17 (26.2) | 1066 (20.9) |
| Maternal education | |||
| ≥ 11 y, n, % | 180 (58.8) | 32 (55.2) | 2764 (61.2) |
| < 11 y, n, % | 126 (41.2) | 26 (44.8) | 1753 (38.8) |
| Mean (SD) gestational age at maternal serum sampling, wk | 10.7 (2.8) | 10.4 (2.7) | 10.7 (2.8) |
| Preterm births (<37 wk), n, % | 11 (3.2) | 4 (6.1) | 186 (3.6) |
| Male children, n, % | 181 (52.0) | 39 (59.1) | 2596 (50.6) |
| Children in the family when child was 8 y old (minimum-maximum), n | 3.4 (1–17) | 4.2 (1–17)d | 3.3 (1–19) |
Abbreviations: IQR, interquartile range.
High TSH concentration was defined as having a serum TSH concentration greater than 3.1 mU/L in the first trimester or greater than 3.5 mU/L in the second trimester.
Maternal hypothyroxinemia was defined as having a normal TSH concentration and free T4 concentrations less than 11.4 pmol/L in the first trimester or less than 11.1 pmol/L in the second trimester.
P < .001 when maternal thyroid function group was compared with all women with laboratory data with t tests or Mann-Whitney U test (continuous variables) or χ2 test (categorical variables).
P < .05 when maternal thyroid function group was compared with all women with laboratory data with t tests or Mann-Whitney U test (continuous variables) or χ2 test (categorical variables).
There were altogether 1030 children (20.1%) with inattention symptoms, 1095 children (21.3%) with hyperactivity symptoms, 686 children with total Rutter B2 scores of 9 or greater (13.4%), and 461 children (9.0%) with combined ADHD symptoms (inattention and hyperactivity symptoms with total Rutter B2 scores ≥ 9) based on the teachers' evaluation. Overall, boys had higher prevalence of ADHD symptoms, Rutter B2 scores of 9 or greater, and combined ADHD symptoms than girls (Table 2). There were no statistically significant differences in the prevalence or odds of ADHD symptoms in children born to mothers with high and normal serum TSH concentrations or hypothyroxinemia and normal free T4 concentrations (Table 2).
Table 2.
Prevalence and Estimated Odds of ADHD Symptoms in Children of Mothers With High and Normal Serum TSH Concentrations and With and Without Hypothyroxinemia in the NFBC 1986
| Child's ADHD Symptom | Maternal Thyroid Function During Early Pregnancy |
|||||
|---|---|---|---|---|---|---|
| Normal TSH (n = 4763)a | High TSH (n = 348)a | aOR (95% CI)b | Normal Free T4 (n = 4370)a | Hypothyroxinemia (n = 66)a | aOR (95% CI)c | |
| Inattention, n, % | ||||||
| Boys | 700 (14.7) | 44 (12.6) | 0.76 (0.51–1.12) | 641 (14.7) | 9 (9.1) | 0.75 (0.33–1.69) |
| Girls | 260 (5.5) | 19 (5.5) | 1.14 (0.66–1.97) | 242 (5.5) | 2 (3.0) | 0.66 (0.15–2.83) |
| Hyperactivity, n, % | ||||||
| Boys | 745 (15.6) | 56 (16.1) | 1.07 (0.75–1.52) | 680 (15.6) | 8 (12.1) | 0.69 (0.31–1.56) |
| Girls | 267 (5.6) | 21 (6.0) | 1.20 (0.70–2.03) | 247 (5.7) | 3 (4.5) | 1.07 (0.31–3.65) |
| Rutter B2 scores ≥ 9, n, % | ||||||
| Boys | 462 (9.7) | 27 (7.8) | 0.77 (0.49–1.24) | 423 (9.7) | 7 (10.6) | 0.75 (0.28–1.96) |
| Girls | 177 (3.7) | 14 (4.0) | 1.30 (0.70–2.42) | 162 (3.7) | 0 | NA |
| Combined ADHD, n, % | ||||||
| Boys | 330 (6.9) | 21 (6.0) | 0.89 (0.52–1.50) | 299 (6.8) | 3 (4.5) | 0.61 (0.18–2.03) |
| Girls | 95 (2.0) | 10 (2.9) | 1.69 (0.79–3.56) | 86 (2.0) | 0 | NA |
Abbreviations: aOR, adjusted odds ratio; NA, not applicable.
Numbers may vary due to missing maternal TSH and/or free T4 measurements or availability of teacher evaluation of child's symptoms. Forty-two teacher questionnaires were not totally filled out.
The ORs with 95% CIs for child's ADHD symptoms after exposure to high maternal TSH concentrations during early pregnancy are calculated by logistic regression after adjusting for having more than two children in family, maternal smoking, maternal education, and maternal age. High TSH concentration was defined as having TSH concentration greater than 3.1 mU/L in the first or greater than 3.5 mU/L in the second trimester.
The ORs with 95% CIs for child's ADHD symptoms after exposure to maternal hypothyroxinemia during early pregnancy are calculated by logistic regression after adjusting for having more than two children in family, maternal smoking, maternal education, and maternal age. Maternal hypothyroxinemia was defined as having normal TSH concentration and free T4 concentration less than 11.4 pmol/L in the first or less than 11.1 pmol/L in the second trimester.
Table 3 shows the children's estimated odds of ADHD symptoms by natural log increases in maternal serum TSH concentrations. In boys, we observed no association between ADHD symptoms and log increases in maternal TSH. Girls had a 1.2-fold odds of inattention and Rutter scores of 9 or greater and 1.4-fold odds of combined ADHD symptoms with every natural log increase in maternal TSH.
Table 3.
Prevalence and Odds of ADHD symptoms in Children After Exposure to Increasing Maternal Serum Thyroid Stimulating Hormone Concentration in Early Pregnancy
| All Mother-Children Pairs With Sufficient Maternal Serum Samples and Children's ADHD Symptom Evaluation Available (n = 5131) | ||
|---|---|---|
| Child's ADHD symptom | n (%) | aOR (95% CI)a |
| Inattention | ||
| Boys | 749 (14.6) | 1.00 (0.90–1.12) |
| Girls | 281 (5.5) | 1.18 (1.02–1.37) |
| Hyperactivity | ||
| Boys | 806 (15.7) | 1.00 (0.90–1.10) |
| Girls | 289 (5.6) | 1.10 (0.95–1.25) |
| Rutter B2 scores of 9 or greater | ||
| Boys | 494 (9.6) | 1.02 (0.90–1.15) |
| Girls | 192 (3.7) | 1.23 (1.03–1.48) |
| Combined ADHD | ||
| Boys | 355 (6.9) | 1.17 (1.00–1.36) |
| Girls | 106 (2.1) | 1.39 (1.07–1.80) |
Abbreviations: aOR, adjusted odds ratio.
The ORs with 95% CIs for ADHD symptoms in the child per 1 natural logarithmic unit increase in maternal early-pregnancy TSH concentrations are calculated with logistic regression after adjusting for having more than two children in family, maternal smoking, maternal education, and maternal age.
In our sensitivity analyses, the exclusion of mothers taking thyroid medication and of those with TPO-Ab positivity affected our results only minimally. Risk increases among full-term children were no different to those among all children, but we were underpowered to study the associations in preterm children.
Discussion
In our large, population-based cohort study, we observed a modest association between increases in maternal serum TSH concentrations in early pregnancy and ADHD symptoms in girls. To our knowledge, our study is the first using maternal thyroid hormone measurements from early pregnancy coupled with objectively evaluated ADHD symptom scoring by the child's teachers.
Few previous studies concerning maternal thyroid dysfunction and children's ADHD symptoms exist, and our results partly support them (7, 15). In a study by Haddow et al (7), high maternal TSH concentrations were found to be associated with lower full-scale IQ and attention scores when comparing children of women with hypothyroidism with controls. Ghassabian et al (15) found that high maternal TSH concentrations increased the risk of high externalizing scores (eg, aggressive behavior and attention problems). An independent association between maternal TPO-Ab concentrations and ADHD symptoms has also been shown (16). In our study, the exclusion of TPO-Ab-positive mothers did not change children's risk of ADHD symptoms.
The overall prevalence of ADHD symptoms in NFBC 1986 was consistent with that reported in previous studies in Northern Finland and abroad (27, 28). The prevalence of both inattention and hyperactivity were slightly higher in boys than girls, being in line with previous data (27, 28). In our study, ADHD symptoms of the children were evaluated by the teacher with the Rutter B2 scale. The scale has been reported to be a more reliable method to find children's probable psychiatric disturbances at the time of intervention than parental reports (Rutter A2) and child interviews (Children's Depression Inventory) (29). Teachers are more objective when assessing a child's ADHD symptoms because they are able to observe children in a more socially challenging environment in which calm behavior is required (29). Children's behavior problems may also be different at home and at school (30). In addition, in Finland all children attend public elementary schools and all elementary school teachers are highly educated. The accuracy of parents as estimators may vary, depending on educational level and on the amount of stress associated with the child's behavior (31, 32). We believe that using teacher-estimated symptom scoring based on a validated questionnaire has given us a more accurate estimation of children's ADHD symptoms than in studies in which only parental data have been used.
We cannot explain for certain why boys and girls showed somewhat different results in our study. According to our results, girls may be more sensitive to altered maternal thyroid hormone concentrations during pregnancy. Such an interesting gender difference has not been reported in previous literature. It is known, however, that ADHD is globally approximately 3 times as prevalent among boys, depending on subjects and population (27, 28). It could therefore be speculated that boys and girls have somewhat different etiologies with regard to ADHD symptoms.
Although prematurity is known to affect the prevalence of ADHD symptoms (33), our results did not change after excluding preterm children. The number of preterm children was not sufficient to estimate their risk of ADHD, but prematurity seems not to be a major confounding factor in our study. The possible effects of TPO-Ab positivity and maternal thyroid medications were also acknowledged, but these factors did not affect the results.
The strengths of our study are the mainly first-trimester maternal thyroid function analyses, homogeneity of the Finnish school system, and the use of the population-based cohort NFBC 1986, which has been followed up since early pregnancy. The excellent participation rate with regard to the 8-year-old children's questionnaire (92%) led to more than 5000 mother-child pairs with teacher evaluation of children's ADHD symptoms and maternal thyroid function test results available. The effect of the country's iodine status must also be acknowledged because the prevalence of ADHD might be higher in areas of iodine deficiency (17). Recent results indicate that iodine deficiency may also have a significant effect on the overall neurodevelopment (34). Iodine insufficiency during pregnancy should not confound our results because in Finland iodine supplementation has been in use since the 1940s (35, 36). In 1986, Finland had the highest iodine intake of the European countries, about 300 μg/d (37), and at that time Finland was considered iodine sufficient.
Unfortunately, in our study setting, it was not possible to assign clinical ADHD diagnoses to the children because the evaluation was carried out via questionnaires. However, the Rutter B2 scale is a valid screening instrument for ADHD symptoms (29), and our possibility to use total Rutter scale scores increased the reliability of the evaluation. Another limitation was the fact that we did not have data on parental ADHD symptoms, and ADHD is a highly heritable disorder in up to 60%–90% (24). We did not presume that parental ADHD would have an effect on maternal thyroid function during pregnancy, suggesting that our observed association is not confounded by the presence of parental ADHD symptoms. Some environmental factors such as maternal alcohol use and smoking during pregnancy also affect a child's risk of ADHD, at least in those who already have a genetic susceptibility (25, 26). We were able to account for maternal smoking by adjusting for it in the analyses. It is likely that maternal thyroid dysfunction during pregnancy can also act as an environmental trigger among those who already have a genetic predisposition in addition to the direct influence that maternal thyroid dysfunction has on the developing fetal brain (2, 3, 17). We were also lacking a portion of maternal serum samples, but it has been ensured that mothers without laboratory data did not differ from the rest with regard to background and family characteristics (20). We acknowledge that that serum free T4 measurements with immunoassays may not be totally reliable during pregnancy, although they are clinically used in addition to TSH measurements (38). However, we were able to use population- and trimester-specific reference intervals to define hypothyroxinemia, as currently recommended when the gold standard methods are not available (5). Because there has been shown to be small intraindividual variability in thyroid function tests over the course of pregnancy (5), we expect that most women would remain euthyroid or hypothyroid throughout pregnancy (if untreated).
According to our results, girls had slightly increased odds of ADHD symptoms with increasing maternal early pregnancy serum TSH concentrations. The mechanisms are unclear and merit further study. However, the association was weak in our study, and no data exist as to whether treating women with high TSH levels would prevent ADHD in their children. In addition, maternal thyroid function screening would not help in discovering those at risk of ADHD. However, because a possible association exists, abnormal maternal serum TSH concentrations should be treated as early in pregnancy as possible, especially if there is a history of ADHD or other neuropsychological disorders in the family (24).
Acknowledgments
We thank Ms Sarianna Vaara, Ms Aljona Amelina, and Ms Jenna Aavavirta (National Institute for Health and Welfare), and Ms Tuula Ylitalo (Institute of Health Sciences, Oulu University) for their valuable work regarding the Northern Finland Birth Cohort 1986 and the Finnish Maternity Cohort serum bank.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and in part by grants from the Alma and K. A. Snellman Foundation (Oulu, Finland), the Jalmari and Rauha Ahokas Foundation (Finland), the Northern Ostrobothnia Hospital District (Finland), the Finnish Medical Association of Clinical Chemistry, and the Academy of Finland.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADHD
- attention-deficit/hyperactivity disorder
- BMI
- body mass index
- CI
- confidence interval
- IQ
- intelligence quotient
- NFBC 1986
- Northern Finland Birth Cohort 1986
- OR
- odds ratio
- TPO-Ab
- thyroid-peroxidase antibody.
References
- 1. de Escobar GM, Obregon MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–248 [DOI] [PubMed] [Google Scholar]
- 2. Cao XY, Jiang XM, Dou ZH, et al. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994;331(26):1739–1744 [DOI] [PubMed] [Google Scholar]
- 3. Auso E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145(9):4037–4047 [DOI] [PubMed] [Google Scholar]
- 4. Gardner L. Historical notes on cretinism. In: Gardner LI, ed. Endocrine and Genetic Diseases of Childhood and Adolescence. 2nd ed Philadelphia: W. B. Saunders; 1975 [Google Scholar]
- 5. Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21(10):1081–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Man EB, Jones WS, Holden RH, Mellits ED. Thyroid function in human pregnancy. 8. retardation of progeny aged 7 years: relationships to maternal age and maternal thyroid function. Am J Obstet Gynecol. 1971;109:9–12 [PubMed] [Google Scholar]
- 7. Haddow JE, Palomaki GE, Allan WC, ET AL. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–555 [DOI] [PubMed] [Google Scholar]
- 8. Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin Endocrinol (Oxf). 2010;72(6):825–829 [DOI] [PubMed] [Google Scholar]
- 9. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149–155 [DOI] [PubMed] [Google Scholar]
- 10. Craig WY, Allan WC, Kloza EM, et al. Mid-gestational maternal free thyroxine concentration and offspring neurocognitive development at age two years. J Clin Endocrinol Metab. 2012;97(1):E22–E28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Finken M, van Eijsden M, Loomans E, Vrijkotte T, Rotteveel J. Maternal hypothyroxinemia in early pregnancy predicts reduced performance in reaction time tests in 5- to 6-year-old offspring. J Clin Endocrinol Metab. 2013;98(4):1417–1426 [DOI] [PubMed] [Google Scholar]
- 12. Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the Generation R study. J Clin Endocrinol Metab. 2010;95(9):4227–4234 [DOI] [PubMed] [Google Scholar]
- 13. Román GC, Ghassabian A, Bongers-Schokking JJ, et al. Association of gestational maternal hypothyroxinemia and increased autism risk [published online August 13, 2013]. Ann Neurol. doi:10.1002/ana.23976 [DOI] [PubMed] [Google Scholar]
- 14. Lazarus JH, Bestwick JP, Channon S, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med. 2012;366(6):493–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghassabian A, Bongers-Schokking JJ, Henrichs J, et al. Maternal thyroid function during pregnancy and behavioral problems in the offspring: The Generation R study. Pediatr Res. 2011;69(5 Pt 1):454–459 [DOI] [PubMed] [Google Scholar]
- 16. Ghassabian A, Bongers-Schokking JJ, de Rijke YB, et al. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R study. Thyroid. 2012;22(2):178–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: a possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89(12):6054–6060 [DOI] [PubMed] [Google Scholar]
- 18. Järvelin MR, Hartikainen-Sorri AL, Rantakallio P. Labour induction policy in hospitals of different levels of specialisation. Br J Obstet Gynaecol. 1993;100:310–315 [DOI] [PubMed] [Google Scholar]
- 19. Järvelin MR, Elliott P, Kleinschmidt I, et al. Ecological and individual predictors of birthweight in a Northern Finland Birth Cohort 1986. Paediatr Perinat Epidemiol. 1997;11(3):298–312 [DOI] [PubMed] [Google Scholar]
- 20. Männistö T, Vääräsmäki M, Pouta A, et al. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: a prospective population-based cohort study. J Clin Endocrinol Metab. 2009;94(3):772–779 [DOI] [PubMed] [Google Scholar]
- 21. Männistö T, Surcel HM, Bloigu A, et al. The effect of freezing, thawing, and short- and long-term storage on serum thyrotropin, thyroid hormones, and thyroid autoantibodies: Implications for analyzing samples stored in serum banks. Clin Chem. 2007;53(11):1986–1987 [DOI] [PubMed] [Google Scholar]
- 22. Almqvist F, Tuompo-Johansson E, Panelius E, Aronen E, Kairemo A. Screening for psychiatric symptoms on the basis of the Rutter teacher questionnaire. Psychiatric symptoms in Finnish children. Rep Psychiatr Fennica. 1991;91:35–46 [Google Scholar]
- 23. Männistö T, Surcel HM, Ruokonen A, et al. Early pregnancy reference intervals of thyroid hormone concentrations in a thyroid antibody-negative pregnant population. Thyroid. 2011;21(3):291–298 [DOI] [PubMed] [Google Scholar]
- 24. Biederman J. Attention-deficit/hyperactivity disorder: A selective overview. Biol Psychiatry. 2005;57(11):1215–1220 [DOI] [PubMed] [Google Scholar]
- 25. Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatr. 2005;57(6):359–371 [PubMed] [Google Scholar]
- 26. Huizink A, Mulder E. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41 [DOI] [PubMed] [Google Scholar]
- 27. Smalley SL, McGough JJ, Moilanen IK, et al. Prevalence and psychiatric comorbidity of attention-deficit/hyperactivity disorder in an adolescent Finnish population. J Am Acad Child Adolesc Psychiatry. 2007;46:1575–1583 [DOI] [PubMed] [Google Scholar]
- 28. Akinbami LJ, Liu X, Pastor PN, Reuben CA. Attention deficit hyperactivity disorder among children aged 5–17 years in the United States, 1998–2009. NCHS Data Brief. 2011;(70):1–8 [PubMed] [Google Scholar]
- 29. Kresanov K, Tuominen J, Piha J, Almqvist F. Validity of child psychiatric screening methods. Eur Child Adolesc Psychiatry. 1998;7(2):85–95 [DOI] [PubMed] [Google Scholar]
- 30. Kolko DJ, Kazdin AE. 1993 Emotional/behavioral problems in clinic and nonclinic children: correspondence among child, parent and teacher reports. J Child Psychol Psychiatry. 1993;34:991–1006 [DOI] [PubMed] [Google Scholar]
- 31. De Los Reyes A, Kazdin AE. Informant discrepancies in the assessment of childhood psychopathology: a critical review, theoretical framework, and recommendations for further study. Psychol Bull. 2005;131(4):483–509 [DOI] [PubMed] [Google Scholar]
- 32. Collishaw S, Goodman R, Ford T, Rabe-Hesketh S, Pickles A. How far are associations between child, family and community factors and child psychopathology informant-specific and informant-general? J Child Psychol Psychiatry. 2009;50:571–580 [DOI] [PubMed] [Google Scholar]
- 33. Lindström K, Lindblad F, Hjern A. Preterm birth and attention-deficit/hyperactivity disorder in schoolchildren. Pediatrics. 2011;127(5):858–865 [DOI] [PubMed] [Google Scholar]
- 34. Bath S, Steer C, Golding J, Emmett P, Rayman M. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013;382(9889):331–337 [DOI] [PubMed] [Google Scholar]
- 35. Erkkola M, Karppinen M, Järvinen A, Knip M, Virtanen SM. Folate, vitamin D, and iron intakes are low among pregnant Finnish women. Eur J Clin Nutr. 1998;52(10):742–748 [DOI] [PubMed] [Google Scholar]
- 36. Lamberg BA, Haikonen M, Makela M, Jukkara A, Axelson E, Welin MG. Further decrease in thyroidal uptake and disappearance of endemic goitre in children after 30 years of iodine prophylaxis in the east of Finland. Acta Endocrinol. 1981;98(2):205–209 [DOI] [PubMed] [Google Scholar]
- 37. Lamberg BA. Endemic goitre in finland and changes during 30 years of iodine prophylaxis. Endocrinol Exp Mar. 1986;20(1):35–47 [PubMed] [Google Scholar]
- 38. Feldt-Rasmussen U, Bliddal Mortensen AS, Rasmussen AK, Boas M, Hilsted L, Main K. Challenges in interpretation of thyroid function tests in pregnant women with autoimmune thyroid disease. J Thyroid Res. 2011;2011:598712. [DOI] [PMC free article] [PubMed] [Google Scholar]