Abstract
Context:
Bone mineral density (BMD) and calcified atherosclerotic plaque (CP) demonstrate inverse relationships. Sclerostin, an endogenous regulator of the Wnt pathway and bone formation, has been associated with impaired osteoblast activation and may play a role in vascular calcification.
Objective:
Our objective was to assess the relationships between sclerostin, BMD, and CP.
Design:
Generalized linear models were fitted to test for associations between sclerostin, volumetric BMD (vBMD), and CP.
Participants:
A targeted population of 450 unrelated African Americans (AAs) with type 2 diabetes (T2D) was 56% female with mean/SD/median age of 55.4/9.5/55.0 years and a diabetes duration of 10.3/8.2/8.0 years.
Main Outcome Measures:
Plasma sclerostin, computed tomography-derived thoracic and lumbar vertebrae trabecular vBMD, coronary artery, carotid artery, and aortoiliac CP were measured.
Results:
Plasma sclerostin was 1119/401/1040 pg/mL, thoracic vBMD was 206.3/52.4/204.8 mg/cm3, lumbar vBMD was 180.7/47.0/179.0 mg/cm3, coronary artery CP score was 284/648/13, carotid artery CP score was 46/132/0, and aortoiliac CP score was 1613/2910/282. Sclerostin levels were higher in men than women (P < .0001). Before and after adjusting for age, sex, body mass index, blood pressure, smoking, hemoglobin A1c, and low-density lipoprotein-cholesterol, plasma sclerostin levels were positively associated with thoracic and lumbar vertebrae vBMD (P < .0001). Sex-stratified analyses verified significant relationships in both men and women (both P < .001). Sclerostin was not associated with CP except for an inverse relationship with carotid CP in men (fully adjusted model, P = .03).
Conclusions:
In this cross-sectional study of AA men and women with T2D, circulating sclerostin was positively associated with vBMD in the spine in both sexes and inversely associated with carotid artery CP in men. Sclerostin may play a role in skeletal mineral metabolism in AA but fails to explain inverse relationships between BMD and CP.
Considerable evidence supports a relationship between low bone mineral density (BMD) and the presence of calcified atherosclerotic plaque (CP) (1, 2), a phenomenon observed in cross-sectional and longitudinal studies of individuals with European and African ancestry (3–11). Mouse knockout models of genes involved in bone and soft tissue mineralization, including osteoprotegerin (OPG), matrix gla protein (MGP), and klotho (KL), lead to accelerated vascular calcification (12–14), supporting linkage of molecular pathways between bone disease and formation of atherosclerotic CP.
Population ancestry is associated with differential susceptibility to osteoporosis and atherosclerosis. Despite exposure to higher levels of conventional cardiovascular disease (CVD) risk factors, diabetic and nondiabetic African Americans (AAs) have lower amounts of coronary artery, carotid bifurcation, and abdominal aortoiliac CP relative to European Americans (EAs) (15–19). AAs with equivalent healthcare access as EAs in Kaiser Permanente, Veterans Administration, and Medicare-supported dialysis programs have 50% lower rates of myocardial infarction (20–22), suggesting a biologic role for this phenomenon. Relative to EAs, AAs have reduced risk for osteoporosis despite lower dietary calcium ingestion and serum 25-hydroxyvitamin D concentrations (23–25).
The Wnt/β-catenin signaling pathway is involved in maintaining bone health through regulation of osteoblast differentiation, proliferation, and survival. Sclerostin, the product of the SOST gene, and Dickkopf-1 (DKK1), the product of the DKK1 gene, are secreted protein regulators of the Wnt canonical signaling pathway. Both proteins are involved in bone developmental processes, leading to their pursuit as potential therapeutic targets for bone disease (26). DKK1 acts by binding to single-pass transmembrane receptor proteins (Kremen 1 and Kremen 2) as well as to plasma membrane-localized Wnt coreceptors low-density lipoprotein receptor-related protein family (LRP5 and LRP6) (27). High DKK1 levels have been associated with impaired osteoblast activation and bone loss. We reported that circulating DKK1 levels were inversely associated with coronary and aortoiliac CP but not spinal BMD measures derived from computed tomography (CT) in the AA-Diabetes Heart Study (AA-DHS) cohort. Sclerostin is associated with skeletal phenotypes in genome-wide association studies (28) and competes with DKK1 for binding to LRP5/6. In addition, some studies suggest that sclerostin is present in atherosclerotic plaques and may be involved in atherosclerosis and vascular calcification (29–32). The purpose of this study was to evaluate relationships between circulating sclerostin and BMD and CP in the AA-DHS.
Subjects and Methods
Study population
Self-reported, unrelated AAs with type 2 diabetes (T2D) were recruited from internal medicine clinics and community advertising in the AA-DHS (25). Participant examinations were carried out in the Clinical Research Unit of the Wake Forest School of Medicine and included interviews for medical history and health behaviors, anthropometric measures, resting blood pressure (BP), 12-lead electrocardiography (ECG), fasting blood sampling, and spot urine collection for the albumin to creatinine ratio (ACR). T2D was defined as a diagnosis of diabetes after 30 years of age in the absence of historical evidence of diabetic ketoacidosis. Medical histories were obtained by trained and certified interviewers. History of CVD was provided by participant report and medical record review. Individuals with a history of myocardial infarction or stroke were included. Participants with a surgical history of coronary artery bypass grafting or carotid artery endartectomy had that vascular segment excluded for measurement of CP. Hypertension was based on physician diagnosis or, if coded in medical records, BP >140/90 mm Hg or use of antihypertensive medications. Plasma samples were stored at −80°C until assayed for sclerostin. The study was approved by the Institutional Review Board at the Wake Forest School of Medicine, and all participants provided written informed consent.
Sclerostin assay
Plasma sclerostin was measured in duplicate using EDTA-plasma and human Quantikine ELISA kits (DSST00; R&D Systems) according to the manufacturer's instructions. All assays were performed using a single lot of reagents and calibrators.
CT imaging of arterial CP
CP was determined in the coronary arteries, carotid arteries, and abdominal aortoiliac bed using 4 or 16 channel multidetector CT (LightSpeed Qxi and 16 Pro, GE Healthcare, Waukesha, WI, USA) (2, 25). Participants were placed in the supine position on the CT couch over a calibration phantom containing verified concentrations of calcium hydroxyl apatite (Image Analysis, Inc) for thoracic and abdominal scans. Coronary imaging was performed without iv contrast and with ECG gating in late diastole (75% of the RR interval). Series through the neck for the carotid bifurcation and abdomen for the aortoiliac arteries were performed without ECG gating using helical scan mode. CT scans were analyzed on a GE Advantage Windows Workstation with the SmartScores software package (GE Healthcare) using a modified Agatston scoring method adjusting for slice thickness and using the conventional threshold of 130 Hounsfield units as well as a calcium mass score using a 90–Hounsfield-unit threshold. The calcium mass score was employed to have a more stable measure of CP across the 3 vascular beds and to be more consistent with CT measures of BMD. Coronary CP mass score was the sum of CP in epicardial coronary arteries (left main, anterior descending, circumflex, and right and posterior descending). Carotid CP mass score was the sum of plaque in the common and internal carotid arteries. Aortoiliac CP mass was the sum of CP present in the abdominal aorta below the renal arteries and in the right and left common iliac arteries.
Bone imaging
Trabecular volumetric BMD (vBMD) in milligrams per cubic centimeter in the thoracic spine (T8–T11) and lumbar spine (T12–L3) were measured using QCT-5000 volumetric software and the previously described calcium calibration phantom included in each participant's CT examination (Image Analysis) (2, 25). Coefficients of variation for these measures were <1% for thoracic vBMD (TvBMD) and lumbar vBMD (LvBMD), and in sequential studies performed in the same individual, the precision error was 2.3%.
Statistical methods
Generalized linear models were fitted to test for associations between plasma sclerostin concentration and renal parameters (estimated glomerular filtration rate [eGFR] and urine ACR), vBMD, coronary CP, carotid CP, and aortoiliac CP. The Box-Cox method (33) was applied to identify the appropriate transformation best approximating the distributional assumptions of conditional normality and homogeneity of variance of the residuals. This method suggested taking the natural logarithm of (coronary CP + 1), (carotid CP + 1), (abdominal aortoiliac CP + 1), and (urine ACR + 1) and the square root of LvBMD and TvBMD. After an unadjusted analysis, age, sex, and body mass index (BMI) adjustments were incorporated. The fully adjusted analysis also included mean arterial pressure, low-density lipoprotein-cholesterol, HbA1c, and smoking status.
Results
The study population consisted of 450 unrelated AAs with T2D. The mean/SD/median age was 55.4/9.5/55.0 years, diabetes duration was 10.3/8.2/8.0 years, 56.2% were female, and 50.2% were hypertensive (Table 1). Across all subjects, the mean/SD/median levels of plasma sclerostin were 1119/401/1040 pg/mL. Coronary CP was present in 62.9% of participants, 47.1% had detectable carotid CP, and 76.8% had detectable aortoiliac CP (Table 2). Because the distributions of CP were skewed, mean values should be interpreted with caution, and values were log-transformed for analysis. Median CP scores were 13.0 for coronary, 0 for carotid, and 282 for aortoiliac.
Table 1.
Demographic Characteristics of Study Participantsa
| Variable | Men (n = 197) | Women (n = 253) | All (n = 450) | P Value |
|---|---|---|---|---|
| Age, y | 55.8 ± 9.9 (55.0) | 55.1 ± 9.1 (54.0) | 55.4 ± 9.5 (55.0) | .45 |
| Age at diabetes onset, y | 45.9 ± 10.1 (46.0) | 45.0 ± 9.6 (45.0) | 45.4 ± 9.8 (46.0) | .31 |
| Diabetes duration, y | 10.5 ± 9.0 (8.0) | 10.1 ± 7.5 (8.0) | 10.3 ± 8.2 (8.0) | .71 |
| BMI, kg/m2 | 33.0 ± 7.6 (31.5) | 37.7 ± 8.9 (36.3) | 35.6 ± 8.7 (34.0) | <.0001 |
| Systolic BP, mm Hg | 132.4 ± 18.2 (131.0) | 133.4 ± 19.6 (131.0) | 133.0 ± 19.0 (131.0) | .69 |
| Diastolic BP, mm Hg | 78.8 ± 11.0 (79.0) | 76.5 ± 11.3 (77.0) | 77.5 ± 11.2 (77.0) | .03 |
| Hypertension, % | 49.7 | 50.6 | 50.2 | .86 |
| Lipid meds, % | 51.3 | 53.2 | 52.4 | .69 |
| History of cardiovascular disease, % | 28.4 | 26.2 | 27.2 | .60 |
| Smokers, % | .002 | |||
| Current | 41.1 | 30.4 | 35.1 | |
| Past | 25.9 | 19.8 | 22.4 | |
| Insulin use, % | 43.1 | 39.5 | 43.1 | .44 |
| Hormone replacement therapy, % | 28.3 | 28.3 |
Unless indicated otherwise, results are shown as mean ± SD (median). P values are derived from a test comparing the distributions between men and women.
Table 2.
Laboratory and Imaging Results of Study Samplea
| Variable | Men (n = 197) | Women (n = 253) | All (n = 450) | P Value |
|---|---|---|---|---|
| Sclerostin, pg/mL | 1217 ± 447 (1119) | 1043 ± 343 (988) | 1119 ± 401 (1040) | <.0001 |
| Aortoiliac CP mass, mg Ca2+ | 1834 ± 3370 (328) | 1442 ± 2494 (224) | 1613 ± 2910 (282) | .0717 |
| Aortoiliac CP mass > 0, % | 81.3 | 73.3 | 76.8 | .0466 |
| Carotid CP mass, mg Ca2+ | 61.6 ± 165.5 (1.0) | 34.2 ± 98.0 (0.0) | 46.2 ± 132.4 (0.0) | .0211 |
| Carotid CP mass > 0, % | 51.8 | 43.4 | 47.1 | .079 |
| Coronary CP mass, mg Ca2+ | 373.4 ± 678.8 (45.0) | 215.4 ± 616.0 (4.0) | 284.3 ± 648.2 (13.0) | .0005 |
| Coronary CP mass > 0, % | 68.2 | 58.7 | 62.9 | .0398 |
| Serum creatinine, mg/dL | 1.1 ± 0.3 (1.0) | 0.9 ± 0.3 (0.8) | 1.0 ± 0.3 (0.9) | <.0001 |
| C-reactive protein, mg/dL | 0.7 ± 1.1 (0.3) | 1.4 ± 2.3 (0.7) | 1.1 ± 1.9 (0.5) | <.0001 |
| MCP-1, pg/mL | 248.6 ± 107.4 (227.6) | 236.6 ± 101.0 (212.7) | 243.3 ± 104.7 (218.7) | .1867 |
| Urine ACR, mg/g | 144.7 ± 490.1 (24.1) | 148.3 ± 598.9 (11.1) | 146.7 ± 552.9 (15.0) | .0166 |
| GFR,(ml/min/1.73 m2 | 97.0 ± 26.6 (92.6) | 93.8 ± 27.7 (93.7) | 95.2 ± 27.3 (93.3) | .217 |
| Fasting glucose, mg/dL | 158.1 ± 68.6 (140.0) | 144.4 ± 61.5 (130.0) | 150.4 ± 65.0 (134.5) | .0299 |
| HbA1c, % | 8.2 ± 1.9 (7.9) | 8.0 ± 2.0 (7.6) | 8.1 ± 2.0 (7.7) | .2643 |
| Total cholesterol, mg/dL | 173.9 ± 47.5 (169.0) | 184.1 ± 44.6 (176.0) | 179.7 ± 46.1 (173.0) | .007 |
| HDL-cholesterol, mg/dL | 44.5 ± 12.1 (43.0) | 50.5 ± 14.3 (48.0) | 47.8 ± 13.7 (46.0) | <.0001 |
| LDL-cholesterol, mg/dL | 102.4 ± 35.7 (98) | 109.3 ± 37.8 (100) | 106.3 ± 37 (100) | .576 |
| Triglycerides, mg/dL | 139.4 ± 178.6 (105.0) | 123.4 ± 104.2 (97.0) | 130.4 ± 141.7 (101.0) | .336 |
| TvBMD, mg/cm3 | 202.2 ± 48.7 (199.7) | 209.5 ± 55.0 (205.3) | 206.3 ± 52.4 (204.8) | .203 |
| LvBMD, mg/cm3 | 179.4 ± 44.5 (179.5) | 181.7 ± 48.9 (177.8) | 180.7 ± 47.0 (179.0) | .6464 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MCP-1, monocyte chemotactic protein-1.
Unless indicated otherwise, results are shown as mean ± SD (median). P values are derived from the test comparing the distributions between men and women.
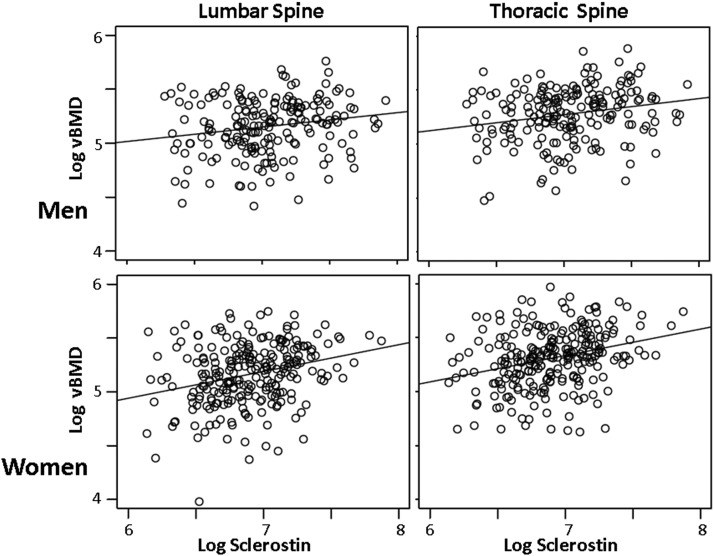
Results of the association analyses between sclerostin and CP and BMD before and after stratification for sex are shown in Table 3. In unadjusted analyses and after adjustment for covariates, plasma sclerostin levels were positively associated with thoracic (log TvBMD) and lumbar (log LvBMD) spine bone density. These associations remained strong in stratified analyses in both men and women. Figure 1 illustrates the unadjusted data depicting positive relationships between sclerostin and TvBMD and LvBMD in both men and women.
Table 3.
Unadjusted and Fully Adjusted Sclerostin Association Analysesa
| Outcome | All Subjects |
Men |
Women |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P Value | Estimate | SE | P Value | Estimate | SE | P Value | |
| Log (TvBMD) | |||||||||
| Model 1 | 0.180 | 0.035 | <.0001 | 0.149 | 0.050 | .003 | 0.245 | 0.051 | <.0001 |
| Model 2 | 0.263 | 0.032 | <.0001 | 0.236 | 0.048 | <.0001 | 0.272 | 0.043 | <.0001 |
| Model 3 | 0.294 | 0.048 | <.0001 | 0.274 | 0.070 | .000 | 0.310 | 0.070 | <.0001 |
| Log (LvBMD) | |||||||||
| Model 1 | 0.181 | 0.037 | <.0001 | 0.133 | 0.052 | .011 | 0.249 | 0.055 | <.0001 |
| Model 2 | 0.265 | 0.032 | <.0001 | 0.227 | 0.048 | <.0001 | 0.284 | 0.043 | <.0001 |
| Model 3 | 0.322 | 0.048 | <.0001 | 0.258 | 0.075 | .001 | 0.369 | 0.067 | <.0001 |
| Log (Cor CP + 1) | |||||||||
| Model 1 | 0.746 | 0.378 | .049 | 0.907 | 0.572 | .115 | 0.020 | 0.514 | .969 |
| Model 2 | −0.110 | 0.345 | .749 | −0.234 | 0.511 | .648 | −0.123 | 0.475 | .796 |
| Model 3 | −0.362 | 0.502 | .472 | −0.963 | 0.807 | .236 | 0.090 | 0.667 | .893 |
| Log (Car CP + 1) | |||||||||
| Model 1 | 0.257 | 0.285 | .368 | 0.241 | 0.437 | .582 | 0.026 | 0.389 | .947 |
| Model 2 | −0.318 | 0.269 | .238 | −0.624 | 0.406 | .126 | −0.113 | 0.364 | .757 |
| Model 3 | −0.653 | 0.398 | .102 | −1.452 | 0.651 | .028 | 0.136 | 0.501 | .786 |
| Log (Ao CP + 1) | |||||||||
| Model 1 | 0.522 | 0.447 | .244 | 0.407 | 0.632 | .520 | 0.316 | 0.657 | .631 |
| Model 2 | −0.502 | 0.393 | .202 | −0.927 | 0.564 | .102 | −0.038 | 0.554 | .946 |
| Model 3 | −0.301 | 0.519 | .563 | −1.018 | 0.824 | .220 | 0.612 | 0.682 | .372 |
| Log (ACR) | |||||||||
| Model 1 | 0.538 | 0.241 | .026 | 0.376 | 0.341 | .272 | 0.497 | 0.354 | .162 |
| Model 2 | 0.469 | 0.250 | .061 | 0.522 | 0.357 | .146 | 0.489 | 0.356 | .171 |
| Model 3 | 0.640 | 0.339 | .060 | 0.870 | 0.493 | .081 | 0.620 | 0.485 | .204 |
| GFR | |||||||||
| Model 1 | −20.700 | 3.636 | <.0001 | −25.248 | 5.016 | <.0001 | −20.094 | 5.389 | .000 |
| Model 2 | −19.010 | 3.589 | <.0001 | −19.911 | 5.109 | .000 | −19.269 | 5.111 | .000 |
| Model 3 | −14.849 | 5.216 | .005 | −21.393 | 8.616 | .015 | −9.010 | 6.643 | .178 |
Abbreviations: Ao, aortoiliac; Car, carotid artery; Cor, coronary artery.
Model 1 is unadjusted. Model 2 is adjusted for age, gender, and BMI. Model 3 is adjusted for age, gender, BMI, low-density lipoprotein-cholesterol, mean arterial BP, smoking status, and HbA1c.
Figure 1.
Association between sclerostin and BMD (unadjusted).
Sclerostin was positively associated with log (coronary CP + 1) before, but not after, adjustment for covariates. In sex-stratified analyses, the only significant association with sclerostin was an inverse relationship observed with the fully adjusted model in men for carotid CP [log (carotid CP + 1)]. Sclerostin was also inversely associated with eGFR in unadjusted and adjusted models across all subjects and after stratification by sex, with the sole exception of the fully adjusted model in females.
Discussion
This study evaluated relationships between plasma sclerostin and subclinical CVD (CP) and spinal trabecular vBMD in AAs with T2D. Before and after adjustment for potential confounders, sclerostin levels were strongly and positively associated with trabecular vBMD in the thoracic and lumbar spine in men and women. The positive association between sclerostin and BMD was not affected by sex, BMI, use of lipid-lowering or antihypertensive medications, or glycemic control (HbA1c). In unadjusted models, sclerostin was inversely associated with coronary artery CP in all subjects, but not in fully adjusted models. In fully adjusted models, sclerostin was associated with carotid artery CP in men. These data suggest that circulating sclerostin may not be a critical link between BMD and vascular calcification in AAs.
Strong correlations between sclerostin and vBMD across vertebral sites in both sexes, before and after adjustment, suggest a significant mechanistic link between these phenotypes. The mechanisms underlying these relationships are unclear, but could relate to local regulation of sclerostin concentrations in bone, or other interactions between circulating sclerostin and the skeleton. Higher plasma sclerostin levels could indicate decreased skeletal/osteoblast binding (with decreased inhibition) of the Wnt pathway, potentially leading to increases in bone mass and density. Our findings regarding circulating sclerostin and bone density support observations in other populations, (32, 34–38), indicating the finding is not unique to AAs with T2D. Sclerostin has been associated with lower fracture risk, reduced rate of bone turnover, and better trabecular bone microarchitectural features in older men (34, 35); low sclerostin levels have been associated with idiopathic osteoporosis in men (39), and genome-wide association studies also implicate the SOST gene in BMD phenotypes (27).
Previous reports have described relationships between sclerostin and vascular disease. Sclerostin levels were higher in subjects with T2D classified as having carotid or aortic atherosclerotic disease based on carotid ultrasound or lateral radiography and were positively associated with carotid intimal-medial thickness, although adjustment for age abolished the relationship in men (30). Hampson et al (32) observed positive relationships between sclerostin and aortic calcium as well as pulse-wave velocity, a measure of vascular stiffness. Elevations in sclerostin expression have been associated with calcification of cultured mouse vascular smooth muscle cells (29). Relationships between sclerostin and CP were positive in the present study, although not statistically significant, in the unadjusted data. Once age was considered, the relationship became negative but was significant only in the fully adjusted data for men. It is also possible that increased soluble circulating sclerostin could modestly inhibit mineralization pathways in arterial tissue, although such a relationship does not appear to be strong and could be an epiphenomenon dependent upon other pathways. Whether these different outcomes relate to subject selection, differences in population ancestry, or other factors remains unknown. AAs have a lower prevalence of vascular calcification and exhibit opposite relationships between arterial calcification and serum vitamin D concentrations compared with EAs (24, 25). Additional studies are required to identify the mechanistic relationships between circulating sclerostin and bone density. The lack of a strong association between sclerostin and vascular calcification in the present study suggests that sclerostin is not a key factor involved in the observed age-independent associations between BMD and vascular calcification. Alternatively, the dissociation of these relationships in the AA-DHS could reflect the presence of diabetes or other competing factors.
Previous work in the AA-DHS population demonstrated significant inverse relationships between plasma DKK1 and coronary and aortoiliac CP but no relationship with BMD (40). Here we found sclerostin was strongly associated with BMD but only minimally associated with CP. DKK1 and sclerostin both bind to LRP5 and LRP6 as well as LRP4 and other proteins to inhibit their association with Wnts (26). DKK1 can interfere with sclerostin binding to LRP5 (27). Although the known molecular mechanisms of action appear similar, there appear to be unique features in the biology of DKK1 and sclerostin that require further exploration.
The inverse association between sclerostin and eGFR is consistent with 2 reports in populations with chronic kidney disease (41, 42). Sclerostin is expressed in the kidney and could have direct effects on kidney function. It is important to note that the AA-DHS population was screened to exclude patients with end-stage kidney disease; as such, kidney function was relatively well preserved.
The AA-DHS cohort has numerous strengths and some limitations. It is a large and well-characterized population facilitating evaluation of molecular biomarkers with potential impact on bone health and subclinical atherosclerosis. The AA-DHS allowed us to provide sensitive molecular characterization in AAs, a population known to display different patterns of bone disease and subclinical CVD relative to EAs. Limitations include the cross-sectional nature of measurements and that study results may not generalize to other population groups. Relative to the data presented here, it will be critical to define the relationships between DKK1 and sclerostin. Longitudinal studies characterizing the relationship between sclerostin and change in vBMD remain necessary to perform.
We conclude that plasma sclerostin concentrations manifest strong positive associations with BMD and negative associations with eGFR in AAs with T2D without significant evidence of association with CP except in the carotid artery in men.
Acknowledgments
We acknowledge the cooperation of our participants, study recruiter Cassandra Bethea, and research technician Christian O'Rourke.
This work was supported by General Clinical Research Center of the Wake Forest School of Medicine Grant M01 RR07122; National Institutes of Health Grants RO1 DK071891 (to B.I.F.), AR48797 (to J.J.C.), and HL67348 (to D.W.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- African American
- AA-DHS
- AA-Diabetes Heart Study
- ACR
- albumin to creatinine ratio
- BMD
- bone mineral density
- BMI
- body mass index
- BP
- blood pressure
- CP
- calcified atherosclerotic plaque
- CT
- computed tomography
- CVD
- cardiovascular disease
- EA
- European American
- ECG
- electrocardiography
- eGFR
- estimated glomerular filtration rate
- HbA1c
- hemoglobin A1c
- LRP
- low-density lipoprotein receptor-related protein
- LvBMD
- lumbar vBMD
- T2D
- type 2 diabetes
- TvBMD
- thoracic vBMD
- vBMD
- volumetric BMD.
References
- 1. Carr JJ, Register TC, Hsu FC, et al. Calcified atherosclerotic plaque and bone mineral density in type 2 diabetes: the diabetes heart study. Bone. 2008;42:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Divers J, Register TC, Langefeld CD, et al. Relationships between calcified atherosclerotic plaque and bone mineral density in African Americans with type 2 diabetes. J Bone Miner Res. 2011;26:1554–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson J, Barnett E, Nordin BE. The relation between osteoporosis and aortic calcification. Br J Radiol. 1964;37:910–912 [DOI] [PubMed] [Google Scholar]
- 4. Elkeles A. A comparative radiological study of calcified atheroma in males and females over 50 years of age. Lancet. 1957;273:714–715 [DOI] [PubMed] [Google Scholar]
- 5. Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR. Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke. 1993;24:940–946 [DOI] [PubMed] [Google Scholar]
- 6. Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O'Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276 [DOI] [PubMed] [Google Scholar]
- 7. Marcovitz PA, Tran HH, Franklin BA, O'Neill WW, Yerkey M, Boura J, Kleerekoper M, Dickinson CZ. Usefulness of bone mineral density to predict significant coronary artery disease. Am J Cardiol. 2005;96:1059–1063 [DOI] [PubMed] [Google Scholar]
- 8. Ness J, Aronow WS. Comparison of prevalence of atherosclerotic vascular disease in postmenopausal women with osteoporosis or osteopenia versus without osteoporosis or osteopenia. Am J Cardiol. 2006;97:1427–1428 [DOI] [PubMed] [Google Scholar]
- 9. Silverman SL, Delmas PD, Kulkarni PM, Stock JL, Wong M, Plouffe L., Jr Comparison of fracture, cardiovascular event, and breast cancer rates at 3 years in postmenopausal women with osteoporosis. J Am Geriatr Soc. 2004;52:1543–1548 [DOI] [PubMed] [Google Scholar]
- 10. Tankó LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–1920 [DOI] [PubMed] [Google Scholar]
- 11. Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab. 2004;89:4246–4253 [DOI] [PubMed] [Google Scholar]
- 12. Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Maadawy S, Kaartinen MT, Schinke T, Murshed M, Karsenty G, McKee MD. Cartilage formation and calcification in arteries of mice lacking matrix Gla protein. Connect Tissue Res. 2003;44 Suppl 1:272–278 [PubMed] [Google Scholar]
- 14. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51 [DOI] [PubMed] [Google Scholar]
- 15. Newman AB, Naydeck BL, Whittle J, Sutton-Tyrrell K, Edmundowicz D, Kuller LH. Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol. 2002;22:424–430 [DOI] [PubMed] [Google Scholar]
- 16. Freedman BI, Hsu FC, Langefeld CD, et al. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia. 2005;48:2511–2518 [DOI] [PubMed] [Google Scholar]
- 17. Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–1320 [DOI] [PubMed] [Google Scholar]
- 18. Carnethon MR, Bertoni AG, Shea S, et al. Racial/ethnic differences in subclinical atherosclerosis among adults with diabetes: the Multiethnic Study of Atherosclerosis. Diabetes Care. 2005;28:2768–2770 [DOI] [PubMed] [Google Scholar]
- 19. Budoff MJ, Nasir K, Mao S, et al. Ethnic differences of the presence and severity of coronary atherosclerosis. Atherosclerosis. 2006;187:343–350 [DOI] [PubMed] [Google Scholar]
- 20. Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26:2392–2399 [DOI] [PubMed] [Google Scholar]
- 21. Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–2527 [DOI] [PubMed] [Google Scholar]
- 22. Young BA, Rudser K, Kestenbaum B, Seliger SL, Andress D, Boyko EJ. Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: the USRDS. Kidney Int. 2006;69:1691–1698 [DOI] [PubMed] [Google Scholar]
- 23. Aloia JF. African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr 2008;88:545S–550S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freedman BI, Wagenknecht LE, Hairston KG, et al. Vitamin d, adiposity, and calcified atherosclerotic plaque in African-Americans. J Clin Endocrinol Metab. 2010;95:1076–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Freedman BI, Register TC. Effect of race and genetics on vitamin D metabolism, bone and vascular health. Nat Rev Nephrol. 2012;8:459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ke HZ, Richards WG, Li X, Ominsky MS. Sclerostin and Dickkopf-1 as Therapeutic Targets in Bone Diseases. Endocr Rev. 2012;33:747–783 [DOI] [PubMed] [Google Scholar]
- 27. Balemans W, Piters E, Cleiren E, et al. The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif Tissue Int. 2008;82:445–453 [DOI] [PubMed] [Google Scholar]
- 28. Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet. 2012;44:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M. Proteomics characterization of extracellular space components in the human aorta. Mol Cell Proteomics. 2010;9:2048–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu D, Mackenzie NC, Millán JL, Farquharson C, MacRae VE. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One. 2011;6:e19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morales-Santana S, García-Fontana B, García-Martín A, et al. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care. 2013;36:1667–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hampson G, Edwards S, Conroy S, Blake GM, Fogelman I, Frost ML. The relationship between inhibitors of the Wnt signalling pathway (Dickkopf-1(DKK1) and sclerostin), bone mineral density, vascular calcification and arterial stiffness in post-menopausal women. Bone. 2013;56:42–47 [DOI] [PubMed] [Google Scholar]
- 33. Box GE, Cox DR. An analysis of transformations. J Roy Stat Soc Ser. 1964;26:211–246 [Google Scholar]
- 34. Szulc P, Bertholon C, Borel O, Marchand F, Chapurlat R. Lower fracture risk in older men with higher sclerostin concentration: A prospective analysis from the MINOS study. J Bone Miner Res. 2013;28:855–864 [DOI] [PubMed] [Google Scholar]
- 35. Szulc P, Boutroy S, Vilayphiou N, et al. Correlates of bone microarchitectural parameters and serum sclerostin levels in men: the STRAMBO study. J Bone Miner Res. 2013;28:1760–1770 [DOI] [PubMed] [Google Scholar]
- 36. Garnero P, Sornay-Rendu E, Munoz F, Borel O, Chapurlat RD. Association of serum sclerostin with bone mineral density, bone turnover, steroid and parathyroid hormones, and fracture risk in postmenopausal women: the OFELY study. Osteoporos Int. 2013;24:489–494 [DOI] [PubMed] [Google Scholar]
- 37. Gaudio A, Pennisi P, Bratengeier C, et al. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 2010;95:2248–2253 [DOI] [PubMed] [Google Scholar]
- 38. Amrein K, Amrein S, Drexler C, et al. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab. 2012;97:148–154 [DOI] [PubMed] [Google Scholar]
- 39. Lapauw B, Vandewalle S, Taes Y, et al. Serum sclerostin levels in men with idiopathic osteoporosis. Eur J Endocrinol. 2013;168:615–620 [DOI] [PubMed] [Google Scholar]
- 40. Register TC, Hruska KA, Divers J, et al. Plasma Dickkopf1 (DKK1) concentrations negatively associate with atherosclerotic calcified plaque in African-Americans with type 2 diabetes. J Clin Endocrinol Metab. 2013;98:E60–E65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thambiah S, Roplekar R, Manghat P, et al. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int. 2012;90:473–480 [DOI] [PubMed] [Google Scholar]
- 42. Pelletier S, Dubourg L, Carlier MC, Hadj-Aissa A, Fouque D. The relation between renal function and serum sclerostin in adult patients with CKD. Clin J Am Soc Nephrol. 2013;8:819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]