Abstract
Context:
The effect of the female sex steroids, estradiol and progesterone, on muscle protein turnover is unclear. Therefore, it is unknown whether the changes in the hormonal milieu throughout the life span in women contribute to the changes in muscle protein turnover and muscle mass (eg, age associated muscle loss).
Objective:
The objective of this study was to provide a comprehensive evaluation of the effect of sex hormones on muscle protein synthesis and gene expression of growth-regulatory factors [ie, myogenic differentiation 1 (MYOD1), myostatin (MSTN), follistatin (FST), and forkhead box O3 (FOXO3)].
Subjects and Design:
We measured the basal rate of muscle protein synthesis and the expression of muscle growth-regulatory genes in 12 premenopausal women and four groups of postmenopausal women (n = 24 total) who were studied before and after treatment with T, estradiol, or progesterone or no intervention (control group). All women were healthy, and pre- and postmenopausal women were carefully matched on body mass, body composition, and insulin sensitivity.
Results:
The muscle protein fractional synthesis rate was approximately 20% faster, and MYOD1, FST, and FOXO3 mRNA expressions were approximately 40%–90% greater (all P < .05) in postmenopausal than premenopausal women. In postmenopausal women, both T and progesterone treatment increased the muscle protein fractional synthesis rate by approximately 50% (both P < .01), whereas it was not affected by estradiol treatment and was unchanged in the control group. Progesterone treatment increased MYOD1 mRNA expression (P < .05) but had no effect on MSTN, FST, and FOXO3 mRNA expression. T and estradiol treatment had no effect on skeletal muscle MYOD1, MSTN, FST, and FOXO3 mRNA expression.
Conclusion:
Muscle protein turnover is faster in older, postmenopausal women compared with younger, premenopausal women, but these age-related differences do not appear to be explained by the age- and menopause-related changes in the plasma sex hormone milieu.
Sexual dimorphism in body composition (more fat and less muscle in women than men) (1, 2) is thought to be a secondary sexual characteristic due to differences in the sex hormone milieu between men and women. T is a well-known anabolic steroid; it increases muscle protein synthesis (3–7) and muscle mass (8–11) and is most likely responsible for the greater increase in lean mass after the onset of puberty in boys compared with girls (12). Female sex steroids, on the other hand, have long been considered innocent bystanders with regard to muscle protein turnover and mass. About a decade ago, however, a study on rodents showed that ovariectomy increases and progesterone and estradiol suppress the rate of muscle protein synthesis (13). This suggests that female sex steroids might be important regulators of muscle mass. The results from subsequent studies evaluating the effect of female sex steroids on muscle protein metabolism in human subjects have been conflicting though. We found that the basal rate of muscle protein synthesis is greater in older, postmenopausal compared with younger, premenopausal women (14), and other investigators reported that the basal rate of muscle protein synthesis is less in women who received estrogen replacement therapy after hysterectomy than age-matched postmenopausal women (15) and less in women who use oral, hormonal contraceptives compared with those who do not (16). Others, however, reported no difference in the rate of muscle protein synthesis between hormone users and nonusers (16), between young and old women (17), or between women who were studied during the follicular or the luteal phases of their menstrual cycle (18).
These studies are difficult to interpret, however, because of their cross-sectional design and differences in subject characteristics within and between studies [eg, body composition (14, 17)] as well as potential confounding influences [eg, differences in free T, IGF-I, and insulin concentrations between hormone treated and untreated women (15, 16)], which may have been induced by the oral hormone treatments that were used in these studies. Evidence is emerging that increased adiposity may affect the rate of muscle protein turnover (19), and it is well known that oral administration of synthetic female sex hormone derivatives alters the concentration of anabolic and catabolic hormones (eg, IGF-I, cortisol) in plasma, whereas systemic delivery of unmodified hormones does not (20–22).
The purpose of the study presented here was to evaluate the effect of menopausal status on muscle protein synthesis and to provide a comprehensive evaluation of the effect of systemically delivered sex hormones (to avoid oral hormone treatment induced changes in anabolic/catabolic plasma hormone availability) on muscle protein synthesis. Accordingly, we measured the basal rate of muscle protein synthesis in premenopausal women and four groups of postmenopausal women who were studied before and after treatment with T, estradiol, or progesterone or no intervention (control group). Pre- and postmenopausal women were healthy and carefully matched on body mass, body composition, and insulin sensitivity. We hypothesized that the rate of muscle protein synthesis would be greater in postmenopausal compared with premenopausal women and that T administration would increase and estradiol and progesterone treatment would suppress the basal rate of muscle protein synthesis. We also measured the expression of genes involved in the regulation of muscle mass [ie, myogenic differentiation 1 (MYOD1), a myogenic growth factor (23); myostatin (MSTN), a muscle growth inhibitor (24–26); follistatin (FST), which binds to and thereby inhibits myostatin (27, 28); and forkhead box O3 (FOXO3), which induces the transcription of ubiquitin ligases (29)] to gain information regarding potential sex hormone-mediated transcriptional changes.
Materials and Methods
Subjects and prestudy testing
Twelve premenopausal and 24 postmenopausal, sedentary (<1.5 h of exercise/ per week) and weight-stable (<2 kg change for at least 6 mo) women participated in this study. Written informed consent was obtained from all subjects before participation in the study, which was approved by the Institutional Review Board of Washington University School of Medicine (St Louis, Missouri).
All subjects were considered to be in good health after completing a comprehensive medical examination, including a detailed history and physical examination, a resting electrocardiogram, standard blood tests, and an oral glucose tolerance test (OGTT). None of the subjects had evidence of chronic illness or significant organ dysfunction (eg, diabetes mellitus, cirrhosis) or were taking medications (including hormone replacement therapy or hormonal contraceptives) that could interfere with muscle protein metabolism, and none reported excessive alcohol intake or consumed tobacco products. Body fat mass, fat-free mass, and appendicular muscle mass were determined by using dual-energy X-ray absorptiometry (Hologic QDR 1000/w).
Experimental design
Protein metabolism study
All subjects completed a baseline protein metabolism study after having been instructed to adhere to their usual diet, to avoid alcohol intake, and to refrain from vigorous physical activities for 3 days. They were admitted to the Clinical Research Unit in the late afternoon, the day before the protein metabolism study, consumed a standard dinner between 6:00 and 7:00 pm, and then fasted, except for water, until the next morning. At 6:00 am, a catheter was inserted into an arm vein for the infusion of a stable isotope labeled leucine tracer; a second catheter was inserted into a vein of the contralateral hand, which was warmed to 55°C by using a thermostatically controlled box to obtain arterialized blood samples. The sampling catheter was kept open with a slow, controlled infusion of 0.9% NaCl solution (30 mL/h). At 7:00 am (time = 0), a continuous infusion of [5,5,5-2H3]leucine (0.12 μmol/kg body weight · min; priming dose: 7.2 μmol/kg body weight) dissolved in 0.9% NaCl solution was started and maintained for 5 hours.
Blood samples were obtained immediately before administering the tracer and 60, 120, 180, 240, and 300 minutes after starting the tracer infusion to determine plasma hormone and substrate concentrations and the leucine tracer to tracee ratio (TTR) in plasma. Muscle tissue (∼100 mg) was obtained under local anesthesia (lidocaine 2%) from the quadriceps femoris by using a Tilley-Henkel forceps at 120 minutes and 300 minutes to determine the basal rate of muscle protein synthesis (labeled leucine incorporation into muscle protein; see Calculations) and the mRNA expressions (initial biopsy only) of MYOD1, MSTN, FST, and FOXO3.
Hormone treatment and repeat protein metabolism studies (postmenopausal women only)
After completing the baseline study, postmenopausal women were randomized to one of four groups (n = 6 per group): control (no intervention), and T, estradiol, or progesterone treatment, which was started within 2 weeks after completing the baseline study. Subjects randomized to the T group applied 1.25 g/d of T transdermally (Androgel 1%; Abbvie Inc) for 21 days. Assuming an absorption rate of 10%, this regimen was expected to deliver approximately 1250 μg of T daily and raise plasma T concentration by approximately 7-fold (6). This increase in concentration is comparable with the concentration typically found in women with hyperandrogenemia due to polycystic ovary syndrome (30, 31) but somewhat below the normal range for eugonadal men (32). Subjects randomized to the estradiol group applied estradiol transdermally for 14 days by using continuous delivery patches designed to deliver 0.1 mg estradiol per day (31 cm2 patches applied once a week; Mylan Pharmaceuticals Inc), followed by a 14-day period without treatment. The 14-day treatment period was repeated two more times with a 14-day no-treatment period in between. This regimen was expected to raise (during the active drug delivery phase) plasma estrogens to concentrations comparable with those in young healthy women during the mid- to late-follicular phase of the menstrual cycle (33). Subjects randomized to the progesterone group applied micronized progesterone (100 mg/d) by using a vaginal insert (Endometrin; Ferring Pharmaceuticals Inc) for 14 days followed by a 14-day period without treatment. The 14-day treatment period was repeated two more times with a 14-day no-treatment period in between. This regimen was expected to raise (during the active drug delivery phase) plasma progesterone to concentrations similar to those in premenopausal women during the luteal phase of the menstrual cycle (34).
Subjects in the control group repeated the protein metabolism study after 46 ± 7 days (four subjects in the control group completed their second study 31–38 days after their baseline study, one completed it after 56 days, and another after 78 days); subjects randomized to the treatment groups repeated the protein metabolism study on the day after the last dose of T (d 22 after start of treatment) or progesterone (d 71 after start of the treatment) administration or the day after removal of the last estradiol patch (d 71 after start of the treatment), respectively. All subjects were instructed to continue their normal diet and exercise habits and to maintain their body weight during the intervention. Compliance with the interventions was confirmed by regular phone interviews and pill count at the end of the study.
Sample collection, processing, and analyses
Blood samples were collected in chilled tubes containing either heparin (to determine glucose and insulin concentrations) or EDTA (all other analyses). Samples were placed in ice and plasma was separated by centrifugation within 30 minutes of collection and then stored at −80°C until final analyses. Muscle samples were rinsed in ice-cold saline immediately after collection, cleared of visible fat and connective tissue, frozen in liquid nitrogen, and stored at −80°C until final analysis.
Plasma glucose concentration was determined on an automated glucose analyzer (Yellow Spring Instruments Co). Serum total and bioavailable T concentrations were determined at the Mayo Medical Laboratories. The bioavailable T concentration in serum was determined by adding known amounts of tritium-labeled T to the serum, precipitating the SHBG-bound fraction with ammonium sulfate and measuring the radioactivity of the supernatant as described by Wheeler (35). Total T concentration was determined by using liquid chromatography-tandem mass spectrometry as described by Wang et al (36). Commercially available ELISAs were used to measure insulin (EMD Millipore), FSH, SHBG, estrone (E1), 17β-estradiol (E2), progesterone, cortisol (all from IBL-America Inc), IGF-I and IGF-binding protein 3 (IGF-BP3) (R&D Systems Inc). Plasma concentrations of triacylglycerol, cholesterol (total), high-density lipoprotein (HDL)-cholesterol, and low-density lipoprotein (LDL)-cholesterol were measured in the Washington University Core Laboratory for Clinical Studies by using a Hitachi 917 autoanalyzer.
Plasma leucine concentration and enrichment were determined by using gas chromatography-mass spectrometry (MSD 5973 system; Hewlett-Packard); after adding a known amount of nor-leucine to aliquots of each plasma sample, plasma proteins were precipitated, and the supernatant, containing free amino acids, was collected to prepare the t-butyldimethylsilyl derivatives of leucine and nor-leucine (37). To determine leucine labeling in muscle proteins and in tissue fluid, samples (∼20 mg) were homogenized in 1 mL trichloroacetic acid solution [3% (wt/vol)], proteins were precipitated by centrifugation, and the supernatant, containing free amino acids was collected. The pellet containing muscle proteins was washed and then hydrolyzed in 6 N HCl at 110°C for 24 hours. Amino acids in the protein hydrolysate and supernatant samples were purified on cation-exchange columns (Dowex 50W-X8–200; Bio-Rad Laboratories). The t-butyldimethylsilyl derivative was prepared for the analysis of muscle free leucine and the N-heptafluorobutyryl n-propyl ester for the analysis of leucine in muscle proteins to determine their TTR by electron impact and chemical ionization gas chromatography-mass spectrometry (MSD 5973 system) analysis, respectively (6, 38). The extent of leucine labeling in plasma, muscle tissue fluid, and muscle protein was calculated based on the simultaneously measured TTR of standards of known isotope labeling.
MYOD1, MSTN, FST, and FOXO3 gene expression in muscle was evaluated by using real-time PCR. Total RNA was isolated from frozen muscle biopsy samples in Trizol reagent (Invitrogen), quantified spectrophotometrically (NanoDrop 1000; Thermo Scientific) and reverse transcribed (high capacity cDNA reverse transcription kit; Invitrogen). Gene expression was determined on an ABI 7500 real-time PCR system (Invitrogen) by using the SYBR Green master mix (Invitrogen) and the following primer sequences: MYOD1, forward, CGCCATCCGCTATATCGAGG, reverse, CTGTAGTCCATCATGCCGTCG; MSTN, forward, TCCTCAGTAAACTTCGTCTGGA, reverse, CTGCTGTCATCCCTCTGGA; FST, forward, ACGTGTGAGAACGTGGACTG, reverse, CACATTCATTGCGGTAGGTTTTC; and FOXO3, forward, CGGACAAACGGCTCACTCT, reverse, GGACCCGCATGAATCGACTAT. The expression of each gene of interest was normalized against the threshold cycle value for the GAPDH gene (forward, TTGCCATCAATGACCCCTTCA, reverse, CGCCCCACTTGATTTTGGA) because it has been reported that GAPDH is a valid housekeeping gene across a wide age range in human skeletal muscle (39, 40). To ascertain the validity of GAPDH as a housekeeping gene in our hormone interventions, we also compared the expression of our genes of interest against β-actin (ACTB, forward, CATGTACGTTGCTATCCAGGC, reverse, CTCCTTAATGTCACGCACGAT) and the results were the same (data not shown).
Calculations
The fractional synthesis rate (FSR) of mixed muscle protein was calculated from the rate of incorporation of [2H3]leucine into muscle protein, using a standard precursor-product model as follows: FSR = ΔEp/Eic × 1/t × 100; where ΔEp is the change between two consecutive biopsies in extent of labeling (TTR) of protein-bound leucine. Eic is the mean labeling over time of the precursor for protein synthesis, and t is the time between biopsies. The free leucine labeling in muscle tissue fluid was chosen to represent the immediate precursor for muscle protein synthesis (ie, aminoacyl-t-RNA) (41). The homeostasis model assessment of insulin resistance score was calculated as the product of plasma insulin (microunits per milliliter−1) and glucose (millimoles per liter−1) concentrations divided by 22.5 (42).
Statistical analyses
All data sets were tested for normality by using the Kolmogorov-Smirnov test. Differences between pre- and postmenopausal women were evaluated by using a Student's t test for independent samples (for normally distributed data) or a Mann-Whitney U test (for skewed data sets). A repeated-measures ANOVA and a Tukey's post hoc procedure were used to evaluate the significance of hormone treatment-induced changes in plasma substrate and hormone concentrations and the muscle protein FSR. Skewed data sets were log transformed for ANOVA. Statistical contrasts focused on treatment vs control comparisons. A Kruskal-Wallis one-way ANOVA and a Bonferroni-corrected Mann-Whitney U post hoc analyses were used to evaluate differences in gene expression between the control and individual treatment groups. The potential impact of time between studies on the change in muscle protein FSR in the control group was assessed by using the Pearson product-moment correlation coefficient.
A value of P ≤ .05 was considered statistically significant. Data in the text are presented as means ± SEM; data in tables and figures are expressed as indicated in the legends. All analyses were carried out with SPSS version 20 for Windows (IBM).
Results
Pre- vs postmenopausal women
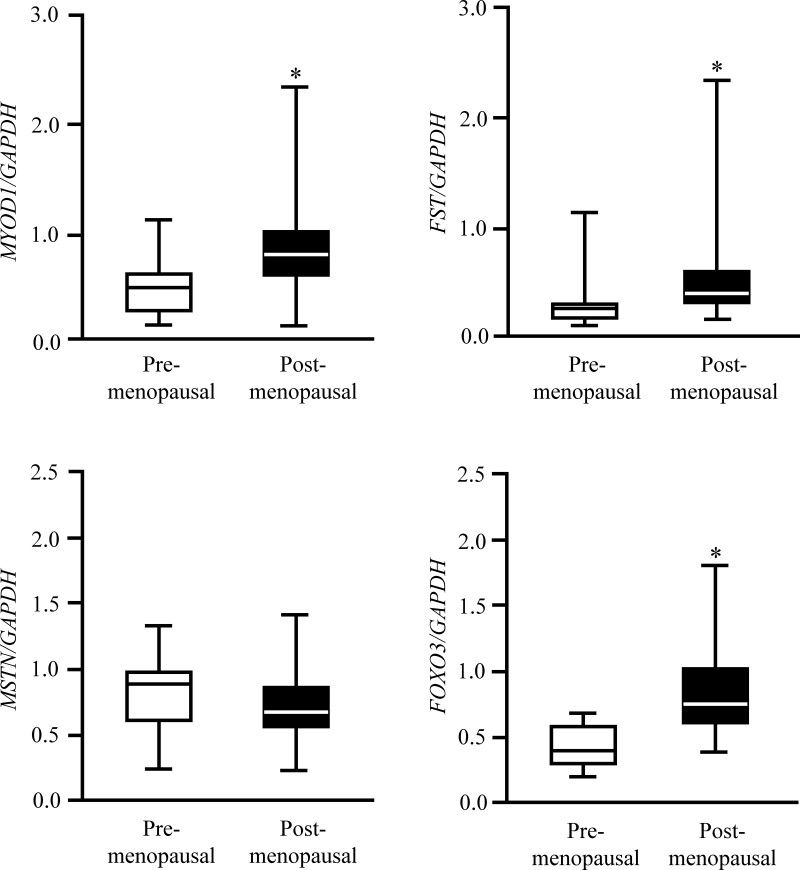
Pre- and postmenopausal women were well matched on total body mass, body mass index, body composition, and insulin sensitivity (Table 1). However, postmenopausal women were older (as expected) and had a somewhat worse plasma lipid profile than premenopausal women (Table 1). Total and bioavailable plasma T, leucine, and IGF-BP3 concentrations were not different in pre- and postmenopausal women, whereas plasma progesterone, estrogen, IGF-I, and SHBG concentrations were significantly lower and FSH and cortisol concentrations were greater in post- compared with premenopausal women (all P < .01; Table 1). The muscle protein FSR was approximately 20% greater (P = .02; Figure 1) and MYOD1, FST, and FOXO3 mRNA expressions were approximately 40%–90% greater (P < .05; Figure 2) in postmenopausal than premenopausal women. MSTN mRNA expression was not different in premenopausal and postmenopausal women (Figure 2).
Table 1.
Subjects' Age, Body Composition, and Baseline Plasma Substrate and Hormone Concentrations
| Premenopausal (n = 12) | Postmenopausal (n = 24) | P Value | |
|---|---|---|---|
| Age, y | 33 ± 2 | 61 ± 2 | <.01 |
| Body mass index, kg/m2 | 29.7 ± 1.7 | 29.8 ± 1.2 | .96 |
| Body mass, kg | 78 ± 5 | 79 ± 3 | .83 |
| Fat-free mass, kg | 48 ± 2 | 46 ± 1 | .45 |
| Appendicular muscle mass, kg | 19.4 ± 1.0 | 17.8 ± 0.5 | .12 |
| Body fat, % | 38 ± 2 | 41 ± 1 | .19 |
| Fasting insulin, mU/L | 3.9 (3.1, 5.0) | 3.5 (2.5, 4.4) | .25 |
| Fasting insulin, pM | 27 (22, 35) | 24 (18, 30) | |
| Fasting glucose, mg/dL | 88 ± 2 | 92 ± 1 | .10 |
| Fasting glucose, mM | 4.88 ± 0.10 | 5.09 ± 0.07 | |
| 2-Hour post-OGTT plasma glucose, mg/dL | 105 ± 5 | 116 ± 4 | .14 |
| 2-Hour post-OGTT plasma glucose, mM | 5.83 ± 0.28 | 6.42 ± 0.24 | |
| HOMA-IR score | 0.8 (0.7, 1.1) | 0.7 (0.5, 1.0) | .38 |
| Leucine, mg/dL | 1.37 ± 0.08 | 1.31 ± 0.05 | .47 |
| Leucine, μM | 105 ± 6 | 100 ± 4 | |
| Triacylglyerol, mg/dL | 62 ± 7 | 94 ± 8 | .01 |
| Triacylglyerol, mM | 0.71 ± 0.08 | 1.06 ± 0.09 | |
| Total cholesterol, mg/dL | 164 ± 8 | 197 ± 6 | <.01 |
| Total cholesterol, mM | 4.26 ± 0.22 | 5.10 ± 0.15 | |
| HDL-cholesterol, mg/dL | 57 ± 4 | 66 ± 3 | .10 |
| HDL-cholesterol, mM | 1.47 ± 0.09 | 1.71 ± 0.09 | |
| LDL-cholesterol, mg/dL | 95 ± 7 | 113 ± 5 | .04 |
| LDL-cholesterol, mM | 2.46 ± 0.18 | 2.93 ± 0.12 | |
| Total T, ng/dL | 26 ± 2 | 21 ± 3 | .23 |
| Total T, nM | 0.92 ± 0.08 | 0.71 ± 0.11 | |
| Bioavailable T, ng/dL | 0.6 ± 0.1 | 0.4 ± 0.1 | .26 |
| Bioavailable T, nM | 0.020 ± 0.004 | 0.015 ± 0.002 | |
| E1 + E2, pg/mL | 144 ± 11 | 84 ± 6 | <.01 |
| E1 + E2, pM | 529 ± 40 | 311 ± 21 | |
| Progesterone, ng/mL | 3.63 ± 1.49 | 0.16 ± 0.04 | <.01 |
| Progesterone, nM | 11.5 ± 4.7 | 0.5 ± 0.1 | |
| FSH, U/L | 5 ± 1 | 71 ± 4 | <.01 |
| SHBG, mg/L | 7.88 ± 1.26 | 4.71 ± 0.44 | <.01 |
| SHBG, nM | 70 ± 11 | 42 ± 4 | |
| Cortisol, ng/dl | 70 (29, 111) | 129 (107, 144) | <.01 |
| Cortisol, nM | 193 (81, 305) | 356 (295, 398) | |
| IGF-I, ng/dL | 90 (88, 139) | 66 (61, 87) | <.01 |
| IGF-I, nM | 11.8 (11.5, 18.2) | 8.7 (8.0, 11.4) | |
| IGF-BP3, ng/dL | 1822 ± 119 | 1773 ± 85 | .74 |
| IGF-BP3, nM | 66 ± 4 | 64 ± 3 |
Abbreviation: HOMA-IR, homeostasis model assessment of insulin resistance. Values are mean ± SEM or median (quartiles) for normally distributed and skewed data sets, respectively.
Figure 1.
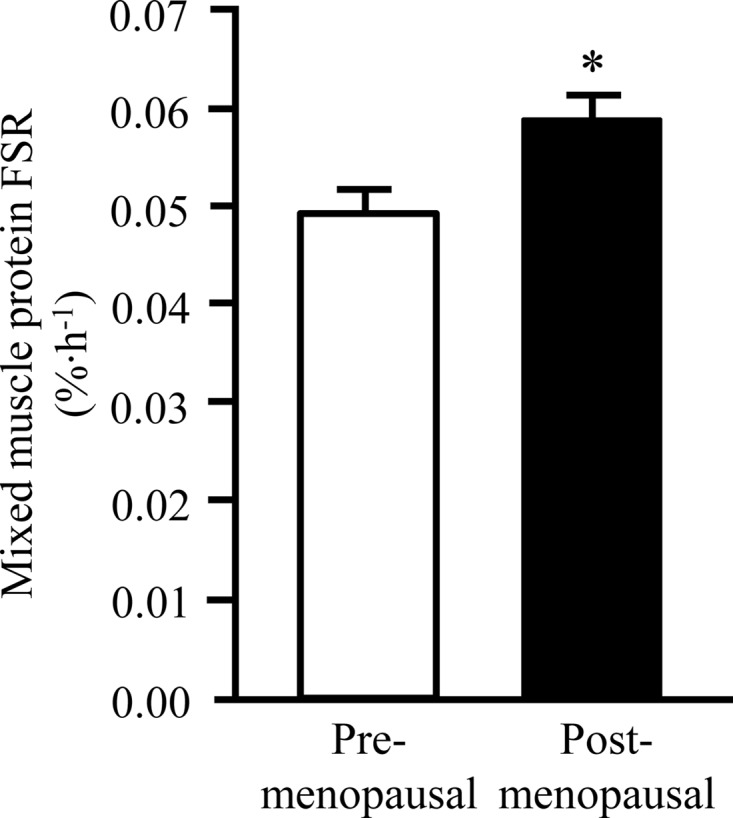
Skeletal muscle protein FSR during basal, postabsorptive conditions in pre- and postmenopausal women. Data are mean ± SEM. *, Value significantly different from value in premenopausal women (P < .05).
Figure 2.
Skeletal muscle MYOD1, FST, MSTN, and FOXO3 gene expression (relative to GAPDH) during basal, postabsorptive conditions in pre- and postmenopausal women. Data are median (central horizontal line), 25th and 75th percentiles (box), and minimum and maximum values (vertical lines). *, Value significantly different from corresponding value in premenopausal women (P < .05).
Effect of hormone treatments in postmenopausal women
Plasma substrate and hormone concentrations were unchanged in the control group (Table 2). In the T and progesterone groups, plasma T and progesterone concentrations, respectively, were significantly greater the day after the final treatment dose than before treatment, whereas plasma substrate and hormone concentrations were unaffected by the hormone treatments (Table 2). In the estradiol group, plasma estrogen concentration was significantly elevated 12 hours after the patch removal, and plasma triacylglycerol concentration was reduced by approximately 20% compared with pretreatment values (both P < .05; Table 2).
Table 2.
Plasma Substrate and Hormone Concentrations in Postmenopausal Women Before and After Treatment With T, Estradiol, or Progesterone or No Intervention (Control)
| Control |
T |
Estradiol |
Progesterone |
|||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Glucose, mg/dL | 97 ± 3 | 94 ± 3 | 92 ± 2 | 91 ± 1 | 96 ± 4 | 95 ± 3 | 93 ± 2 | 95 ± 2 |
| Glucose, mM | 5.36 ± 0.19 | 5.21 ± 0.17 | 5.11 ± 0.11 | 5.07 ± 0.08 | 5.33 ± 0.24 | 5.28 ± 0.15 | 5.17 ± 0.12 | 5.27 ± 0.11 |
| Insulin, mU/L | 3.1 (2.6, 4.3) | 3.3 (2.8, 4.1) | 2.8 (1.6, 4.2) | 3.2 (2.1, 3.9) | 4.0 (3.0, 5.6) | 3.3 (2.7, 4.5) | 3.2 (2.7, 3.6) | 4.0 (2.8, 4.9) |
| Insulin, pM | 22 (18, 30) | 23 (19, 28) | 19 (11, 29) | 22 (15, 27) | 28 (21, 39) | 23 (19, 31) | 22 (19, 25) | 27 (19, 34) |
| Leucine, mg/dL | 1.30 ± 0.10 | 1.27 ± 0.07 | 1.32 ± 0.13 | 1.38 ± 0.16 | 1.22 ± 0.05 | 1.25 ± 0.07 | 1.40 ± 0.10 | 1.43 ± 0.09 |
| Leucine, μM | 99 ± 7 | 97 ± 5 | 101 ± 10 | 105 ± 12 | 93 ± 4 | 95 ± 6 | 106 ± 7 | 109 ± 7 |
| Triacylglyerol, mg/dL | 102 ± 14 | 86 ± 15 | 73 ± 5 | 82 ± 8 | 132 ± 22 | 108 ± 19a | 95 ± 16 | 105 ± 23 |
| Triacylglyerol, mM | 1.15 ± 0.15 | 0.98 ± 0.17 | 0.82 ± 0.06 | 0.93 ± 0.09 | 1.49 ± 0.25 | 1.22 ± 0.22a | 1.07 ± 0.18 | 1.19 ± 0.26 |
| Total cholesterol, mg/dL | 187 ± 6 | 181 ± 6 | 198 ± 13 | 195 ± 11 | 180 ± 15 | 180 ± 12 | 192 ± 8 | 188 ± 9 |
| Total cholesterol, mM | 4.83 ± 0.15 | 4.68 ± 0.16 | 5.14 ± 0.33 | 5.06 ± 0.28 | 4.66 ± 0.40 | 4.67 ± 0.31 | 4.96 ± 0.21 | 4.86 ± 0.24 |
| HDL-cholesterol, mg/dL | 64 ± 5 | 66 ± 4 | 70 ± 8 | 64 ± 5 | 53 ± 7 | 54 ± 6 | 65 ± 8 | 54 ± 6 |
| HDL-cholesterol, mM | 1.66 ± 0.13 | 1.70 ± 0.11 | 1.81 ± 0.20 | 1.65 ± 0.13 | 1.38 ± 0.18 | 1.40 ± 0.17 | 1.68 ± 0.21 | 1.39 ± 0.16 |
| LDL-cholesterol, mg/dL | 102 ± 6 | 98 ± 5 | 114 ± 11 | 115 ± 10 | 100 ± 12 | 105 ± 8 | 113 ± 7 | 112 ± 8 |
| LDL-cholesterol, mM | 2.65 ± 0.15 | 2.53 ± 0.14 | 2.95 ± 0.28 | 2.98 ± 0.25 | 2.60 ± 0.31 | 2.72 ± 0.21 | 2.91 ± 0.18 | 2.91 ± 0.20 |
| Total T, ng/dL | 16 ± 3 | 16 ± 3 | 21 ± 6 | 241 ± 78a | 24 ± 11 | 23 ± 8 | 22 ± 5 | 22 ± 5 |
| Total T, nM | 0.55 ± 0.10 | 0.56 ± 0.12 | 0.74 ± 0.20 | 8.36 ± 2.70a | 0.82 ± 0.37 | 0.79 ± 0.27 | 0.75 ± 0.17 | 0.77 ± 0.17 |
| Bioavailable T, ng/dl | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.5 ± 0.1 | 6.7 ± 2.3a | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 |
| Bioavailable T, nM | 0.011 ± 0.001 | 0.013 ± 0.003 | 0.016 ± 0.005 | 0.232 ± 0.080a | 0.017 ± 0.007 | 0.014 ± 0.004 | 0.017 ± 0.004 | 0.014 ± 0.004 |
| E1 + E2, pg/mL | 82 ± 5 | 92 ± 14 | 91 ± 17 | 89 ± 13 | 88 ± 12 | 114 ± 18a | 77 ± 11 | 92 ± 23 |
| E1 + E2, pM | 304 ± 19 | 339 ± 53 | 337 ± 62 | 327 ± 48 | 323 ± 43 | 422 ± 66a | 283 ± 41 | 340 ± 84 |
| Progesterone, ng/mL | 0.17 ± 0.07 | 0.19 ± 0.05 | 0.12 ± 0.03 | 0.13 ± 0.03 | 0.08 ± 0.03 | 0.09 ± 0.03 | 0.25 ± 0.12 | 1.16 ± 0.42a |
| Progesterone, nM | 0.55 ± 0.21 | 0.62 ± 0.17 | 0.38 ± 0.08 | 0.43 ± 0.08 | 0.25 ± 0.08 | 0.27 ± 0.09 | 0.79 ± 0.39 | 3.68 ± 1.34a |
| SHBG, mg/L | 5.62 ± 1.02 | 5.27 ± 0.80 | 4.40 ± 0.67 | 4.41 ± 0.59 | 3.93 ± 0.66 | 5.51 ± 1.27 | 4.87 ± 1.13 | 5.00 ± 1.00 |
| SHBG, nM | 50 ± 9 | 47 ± 7 | 39 ± 6 | 39 ± 5 | 35 ± 6 | 49 ± 11 | 43 ± 10 | 44 ± 9 |
| Cortisol, ng/mL | 129 ± 17 | 110 ± 18 | 137 ± 13 | 165 ± 17 | 104 ± 18 | 141 ± 12 | 160 ± 27 | 135 ± 21 |
| Cortisol, nM | 355 ± 48 | 303 ± 49 | 378 ± 37 | 456 ± 48 | 286 ± 51 | 388 ± 33 | 443 ± 75 | 371 ± 58 |
| IGF-I, ng/mL | 84 ± 9 | 80 ± 8 | 72 ± 11 | 72 ± 15 | 79 ± 6 | 71 ± 8 | 61 ± 3 | 72 ± 10 |
| IGF-I, nM | 11.0 ± 1.1 | 10.5 ± 1.0 | 9.4 ± 1.5 | 9.4 ± 2.0 | 10.4 ± 0.8 | 9.3 ± 1.1 | 8.0 ± 0.4 | 9.4 ± 1.3 |
| IGF-BP3, ng/mL | 1883 ± 164 | 1873 ± 152 | 1856 ± 173 | 1898 ± 121 | 1703 ± 168 | 1476 ± 146 | 1651 ± 200 | 1428 ± 148 |
| IGF-BP3, nM | 68 ± 6 | 67 ± 5 | 67 ± 6 | 68 ± 4 | 61 ± 6 | 53 ± 5 | 59 ± 7 | 51 ± 5 |
Data are expressed as mean ± SEM or median (quartiles) for normally distributed and skewed data sets, respectively, after values were obtained 12 hours after the last dose of T or progesterone administration or removal of the last estradiol patch (see text for details).
Value significantly different (P < .05) from corresponding preintervention value.
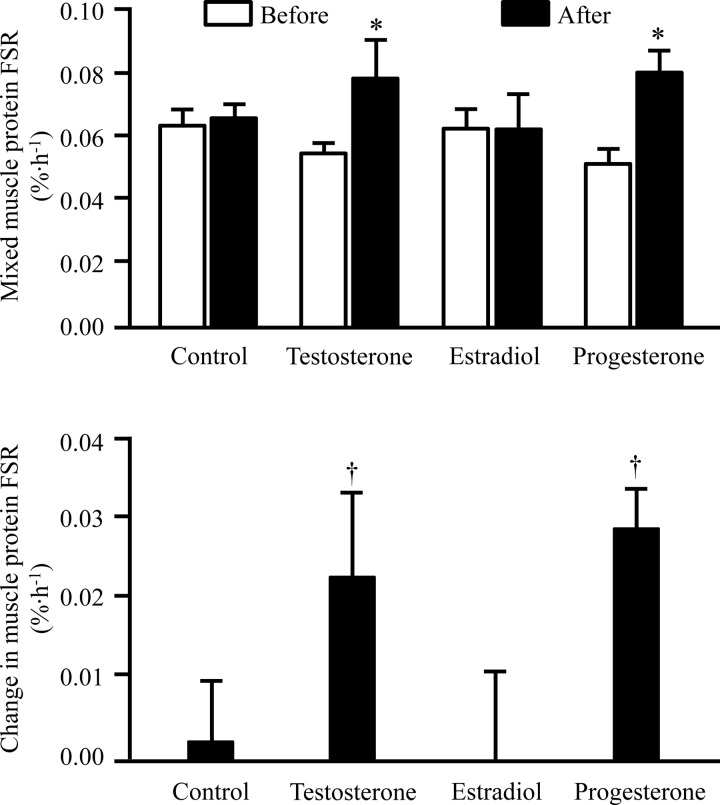
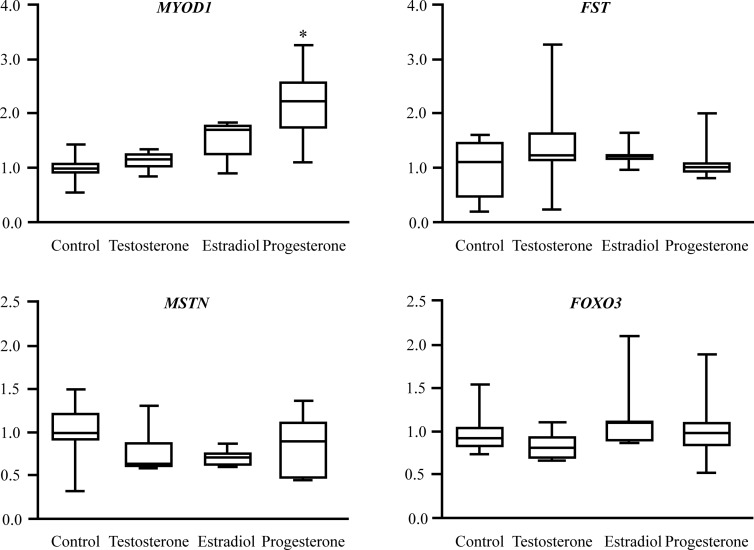
The average muscle protein FSR was unchanged in the control group (Figure 3), and the time between the two studies (31–78 d) did not correlate with the change in muscle protein FSR (r = 0.06, P = .91). Estradiol treatment did not affect the muscle protein FSR, whereas both T and progesterone treatment increased it by approximately 50% (both P < .01; Figure 3). T and estradiol treatment had no effect on skeletal muscle MYOD1, MSTN, FST, and FOXO3 mRNA expression; progesterone treatment significantly increased MYOD1 mRNA expression (P < .05) but had no effect on MSTN, FST, and FOXO3 mRNA expression (Figure 4).
Figure 3.
Skeletal muscle protein FSR in postmenopausal women during basal, postabsorptive conditions before and after no intervention (control) or treatment with T, estradiol, or progesterone (top) and the treatment-induced changes in FSR in each group (bottom). Data are mean ± SEM. *, Value significantly different from corresponding value before treatment (P < .05); †, value significantly different from corresponding value in the control group (P < .05).
Figure 4.
Sex hormone treatment-induced changes in muscle MYOD1, FST, MSTN, and FOXO3 gene expression (relative to the average prepost change in the control group) in postmenopausal women. Data are median (central horizontal line), 25th and 75th percentiles (box), and minimum and maximum values (vertical lines). *, Significantly different from corresponding value in the control group (P < .05).
Discussion
Our study confirms previous observations by our own and other research groups concerning the anabolic effect of T (3–7) and an age-related up-regulation of both stimulatory and inhibitory muscle growth regulatory genes and accelerated basal muscle protein turnover in women (14, 43, 44), which might be indicative of increased skeletal muscle remodeling in post- compared with premenopausal women. In addition, it provides several novel findings regarding female sex hormone action on muscle protein metabolism. We have demonstrated that estradiol has no effect on muscle protein synthesis or the expression of genes involved in the regulation of muscle mass, whereas progesterone has potent stimulatory effects on muscle protein synthesis and MYOD1 mRNA expression. It is therefore unlikely that the greater rate of muscle protein synthesis and overexpression of muscle growth-regulatory genes in older, postmenopausal compared with younger, premenopausal women in the present and other studies (14, 43, 44) are due solely to age- and menopause-associated changes in the plasma sex steroid milieu.
The lack of effect of estradiol on muscle growth-regulatory gene expression in our study is consistent with the results from studies conducted in rodents. Several studies have demonstrated that estradiol has no effect on basal muscle growth-stimulatory factor expression in ovariectomized female and orchidetcomized male mice and rats (45–49). However, estradiol treatment augments muscle MYOD1 expression during recovery from muscle injury in ovariectomized rats (45, 46, 49) and prevents the orchidetcomy-induced decrease in FBXO32 expression (47). Similar studies evaluating the effect of progesterone have, to our knowledge, not been performed. The results from previous studies concerning the effects of female sex steroids on muscle protein turnover are inconsistent. A study conducted in ovariectomized rats demonstrated that progesterone and estradiol suppress the rate of muscle protein synthesis (13), whereas a study conducted in growing steer found that estradiol increases the rate of muscle protein synthesis (50). The results from earlier studies conducted in postmenopausal women suggest that the basal rate of muscle protein synthesis is less in women who received estrogen replacement therapy after hysterectomy compared with age-matched postmenopausal women (15) and less in women who use oral, combination birth control preparations than those who do not (16).
The interpretation of these results, however, is complicated by the following: 1) the overiectomy-induced changes in physical activity and body weight in rats (51, 52), 2) the fact that muscle protein synthesis was not directly measured (but estimated based on indirect measures of the muscle protein breakdown rate and net muscle protein mass changes) in growing steer (50), 3) the cross-sectional nature of the human studies, and 4) the effect of orally delivered sex steroids on anabolic and catabolic plasma hormone (eg, IGF-I, T, and cortisol) concentrations (20–22). We, in contrast, used a longitudinal, randomized, controlled study design and carefully selected our treatments to mimic as closely as possible normal physiological conditions; ie, we chose to provide 17β-estradiol and micronized progesterone in replacement doses and delivered them to tissues through the systemic circulation to avoid these confounding influences; in fact, none of our hormone treatments affected the concentrations of insulin, IGF-I, IGF-BP3, or cortisol or resulted in nonspecific sex hormone concentration changes.
The mechanism(s) responsible for the progesterone-induced increase in muscle protein synthesis are unclear. However, our data suggest that the mechanism(s) responsible for the anabolic effect of progesterone is different from that of T and therefore most likely the result of progesterone receptor rather than androgen receptor activation, which is consistent with the results from studies performed on rodent cardiac muscle (53) demonstrating that blocking the progesterone receptor also blocks the stimulatory effect of progesterone on cardiac muscle protein synthesis. Progesterone treatment induced a significant increase in MYOD1 mRNA expression, whereas congruent with the results from studies conducted by other investigators (54, 55), T did not affect MYOD1 or MSTN mRNA expression. Increased progesterone-induced MYOD1 expression suggests increased satellite cell activation may play a role in this observation.
It is unlikely that the lack of an effect of estradiol was due to lack of compliance or insufficient dosing. We chose a dosing regimen that raises plasma estrogen to concentrations comparable with those in young healthy women during the mid- to late-follicular phase of the menstrual cycle (33), and plasma estrogen concentration was still significantly increased 12 hours after patch removal in our subjects. Furthermore, transdermal estradiol treatment produced the well-known hypotriglyceridemic effect in our women (56). It is also unlikely that the duration of estradiol treatment in the present study was too short to have an effect on the rate of muscle protein synthesis. Both estradiol and progesterone were given for the same amount of time, and progesterone increased the rate of muscle protein synthesis. Furthermore, T and other known anabolic steroids (4, 5, 57, 58) have been shown to increase the rate of muscle protein synthesis within 5–28 days of treatment. It is conceivable (although we consider it unlikely) that our study was underpowered to detect a change in muscle protein FSR after estradiol treatment. In fact, the pre- and posttreatment difference in muscle protein FSR in the estradiol group was so small (0.0003% ± 0.011%/h) that we would have needed more than 50 000 subjects to detect it with a sufficiently small probability of type I error (α < .05) and sufficient power (≥ .80) to reject the null hypothesis. Thus, we conclude that even if estradiol did affect muscle protein synthesis but we were not able to detect it, the effect was likely to be negligible. We recognize the limitations of our study and the fact that we are not able to determine whether larger, pharmacological doses of estradiol, or more prolonged treatment, or estradiol and progesterone combination therapy would result in different outcomes; we will attempt to answer these questions in future studies.
We did not measure muscle protein breakdown rates or changes in muscle mass in response to the different sex hormone treatments in this study. Therefore, we are unable to tell whether the progesterone-induced increase in muscle protein synthesis will ultimately result in net muscle protein gain and increased muscle mass or whether the lack of an effect of transdermally delivered estradiol on muscle protein synthesis rules out this treatment as a potential muscle net growth agent in postmenopausal women. To our knowledge, only two studies have evaluated the effect of transdermal estradiol therapy on lean body (but not muscle) mass. One reported no estradiol-induced change in lean body mass (55) and the other reported an increase (56) but lacked a placebo control group to rigorously judge the effect. Therefore, the effect of systemically delivered estradiol on muscle mass remains unknown. We are not aware of any study that evaluated the effect of progesterone on muscle mass. On the other hand, our data from young, premenopausal vs older, postmenopausal women (greater synthesis rate in old compared with young women) and the well-established decline in muscle mass with aging (59–61) indicate that older women must (by deduction) also have an increased rate of muscle protein breakdown. We are not aware of any study that reports muscle protein breakdown rates in old compared with young women. However, we (present study) and others (44) have observed an age-related up-regulation of catabolic genes (ie, FOXO3 and MuRF1 mRNA expression) in muscle that supports this concept and helps explain the age-associated loss of muscle mass in the face of an increased muscle protein synthesis rate. Furthermore, the concomitant up-regulation of both muscle protein synthesis and breakdown in old compared with young women might further be indicative of increased skeletal muscle remodeling in post- compared with premenopausal women.
In summary, we report an up-regulation of both stimulatory and inhibitory muscle growth regulatory genes and a faster muscle protein FSR in older, postmenopausal compared with carefully matched, younger, premenopausal women. These age-related differences in basal muscle protein turnover in women do not appear to be explained by the age- and menopause-related changes in the plasma sex hormone milieu because we found that both testosterone (21 d of continuous delivery) and progesterone replacement (given for the equivalent of approximately three menstrual cycles) stimulate muscle protein synthesis, whereas estradiol replacement (given for the equivalent amount of time) does not affect the rate of muscle protein synthesis. The exact mechanisms by which progesterone stimulates muscle protein turnover remain to be elucidated but may be related to increased satellite cell activation as suggested by the increased MYOD1 expression.
Acknowledgments
We thank Sophie Julliand for help in subject recruitment, Dr Adewole Okunade and Jennifer Shew for their technical assistance, the staff of the Clinical Research Unit for their help in performing the studies, and the study subjects for their participation.
This study was registered in clinicaltrials.gov as trial number NCT00805207.
This work was supported by National Institutes of Health Grants HD 57796, DK 94483, RR024994, and DK 56341 (to the Nutrition and Obesity Research Center), Grant RR024992 (to the Washington University School of Medicine Clinical Translational Science Award) and Grant RR-00954 (to the Biomedical Mass Spectrometry Resource).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- E1
- estrone
- E2
- 17β-estradiol
- FOXO3
- forkhead box O3
- FSR
- fractional synthesis rate
- FST
- follistatin
- HDL
- high-density lipoprotein
- IGF-BP3
- IGF-binding protein 3
- LDL
- low-density lipoprotein
- MSTN
- myostatin
- MYOD1
- myogenic differentiation 1
- OGTT
- oral glucose tolerance test
- TTR
- tracer to tracee ratio.
References
- 1. Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1970;19:653–663 [DOI] [PubMed] [Google Scholar]
- 2. Lee SJ, Janssen I, Heymsfield SB, Ross R. Relation between whole-body and regional measures of human skeletal muscle. Am J Clin Nutr. 2004;80:1215–1221 [DOI] [PubMed] [Google Scholar]
- 3. Griggs RC, Halliday D, Kingston W, Moxley RT., 3rd Effect of testosterone on muscle protein synthesis in myotonic dystrophy. Ann Neurol. 1986;20:590–596 [DOI] [PubMed] [Google Scholar]
- 4. Urban RJ, Bodenburg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–E826 [DOI] [PubMed] [Google Scholar]
- 5. Ferrando AA, Tipton KD, Doyle D, Phillips SM, Cortiella J, Wolfe RR. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol. 1998;275:E864–E871 [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Smith GI, Patterson BW, Reeds DN, Kampelman J, Magkos F, Mittendorfer B. Testosterone increases the muscle protein synthesis rate but does not affect very-low-density lipoprotein metabolism in obese premenopausal women. Am J Physiol Endocrinol Metab. 2012;302:E740–E746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol. 1989;66:498–503 [DOI] [PubMed] [Google Scholar]
- 8. Isidori AM, Giannetta E, Greco EA, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63:280–293 [DOI] [PubMed] [Google Scholar]
- 9. Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci. 2003;58:618–625 [DOI] [PubMed] [Google Scholar]
- 10. Snyder PJ, Peachey H, Berlin JA, et al. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677 [DOI] [PubMed] [Google Scholar]
- 11. Wang C, Cunningham G, Dobs A, et al. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085–2098 [DOI] [PubMed] [Google Scholar]
- 12. Loomba-Albrecht LA, Styne DM. Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes. 2009;16:10–15 [DOI] [PubMed] [Google Scholar]
- 13. Toth MJ, Poehlman ET, Matthews DE, Tchernof A, MacCoss MJ. Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am J Physiol Endocrinol Metab. 2001;280:E496–E501 [DOI] [PubMed] [Google Scholar]
- 14. Smith GI, Reeds DN, Hall AM, Chambers KT, Finck BN, Mittendorfer B. Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol Sex Differ. 2012;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen M, Skovgaard D, Reitelseder S, Holm L, Langbjerg H, Kjaer M. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005–1013 [DOI] [PubMed] [Google Scholar]
- 16. Hansen M, Langberg H, Holm L, et al. Effect of administration of oral contraceptives on the synthesis and breakdown of myofibrillar proteins in young women. Scand J Med Sci Sports. 2011;21:62–72 [DOI] [PubMed] [Google Scholar]
- 17. Chevalier S, Goulet ED, Burgos SA, Wykes LJ, Morais JA. Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci. 2011;66:681–688 [DOI] [PubMed] [Google Scholar]
- 18. Miller BF, Hansen M, Olesen JL, et al. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290:E163–E168 [DOI] [PubMed] [Google Scholar]
- 19. Guillet C, Masgrau A, Boirie Y. Is protein metabolism changed with obesity? Curr Opin Clin Nutr Metab Care. 2011;14:89–92 [DOI] [PubMed] [Google Scholar]
- 20. Qureshi AC, Bahri A, Breen LA, Barnes SC, Powrie JK, Thomas SM, Carroll PV. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin Endocrinol (Oxf). 2007;66:632–635 [DOI] [PubMed] [Google Scholar]
- 21. Sonnet E, Lacut K, Roudaut N, Mottier D, Kerlan V, Oger E. Effects of the route of oestrogen administration on IGF-1 and IGFBP-3 in healthy postmenopausal women: results from a randomized placebo-controlled study. Clin Endocrinol (Oxf). 2007;66:626–631 [DOI] [PubMed] [Google Scholar]
- 22. Shifren JL, Desindes S, McIlwain M, Doros G, Mazer NA. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985–994 [DOI] [PubMed] [Google Scholar]
- 23. Legerlotz K, Smith HK. Role of MyoD in denervated, disused, and exercised muscle. Muscle Nerve. 2008;38:1087–1100 [DOI] [PubMed] [Google Scholar]
- 24. Amirouche A, Durieux AC, Banzet S, et al. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology. 2009;150:286–294 [DOI] [PubMed] [Google Scholar]
- 25. Welle S, Bhatt K, Pinkert CA. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab. 2006;290:E409–E415 [DOI] [PubMed] [Google Scholar]
- 26. Welle S, Burgess K, Mehta S. Stimulation of skeletal muscle myofibrillar protein synthesis, p70 S6 kinase phosphorylation, and ribosomal protein S6 phosphorylation by inhibition of myostatin in mature mice. Am J Physiol Endocrinol Metab. 2009;296:E567–E572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haidet AM, Rizo L, Handy C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA. 2008;105:4318–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phelan N, O'Connor A, Kyaw-Tun T, et al. Lipoprotein subclass patterns in women with polycystic ovary syndrome (PCOS) compared with equally insulin-resistant women without PCOS. J Clin Endocrinol Metab. 2010;95:3933–3939 [DOI] [PubMed] [Google Scholar]
- 31. Valkenburg O, Steegers-Theunissen RP, Smedts HP, et al. A more atherogenic serum lipoprotein profile is present in women with polycystic ovary syndrome: a case-control study. J Clin Endocrinol Metab. 2008;93:470–476 [DOI] [PubMed] [Google Scholar]
- 32. Goncharov NP, Katsya GV, Chagina NA, Gooren LJ. Testosterone and obesity in men under the age of 40 years. Andrologia. 2009;41:76–83 [DOI] [PubMed] [Google Scholar]
- 33. Harrison LL, Harari D. An evaluation of bioequivalence of two 7-day 17β-estradiol transdermal delivery systems by anatomical site. J Clin Pharmacol. 2002;42:1134–1141 [DOI] [PubMed] [Google Scholar]
- 34. Levy T, Gurevitch S, Bar-Hava I, et al. Pharmacokinetics of natural progesterone administered in the form of a vaginal tablet. Hum Reprod. 1999;14:606–610 [DOI] [PubMed] [Google Scholar]
- 35. Wheeler MJ. The determination of bio-available testosterone. Ann Clin Biochem. 1995;32(Pt 4):345–357 [DOI] [PubMed] [Google Scholar]
- 36. Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543 [DOI] [PubMed] [Google Scholar]
- 37. Smith GI, Villareal DT, Mittendorfer B. Measurement of human mixed muscle protein fractional synthesis rate depends on the choice of amino acid tracer. Am J Physiol Endocrinol Metab. 2007;293:E666–E671 [DOI] [PubMed] [Google Scholar]
- 38. Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism. 1997;46:943–948 [DOI] [PubMed] [Google Scholar]
- 39. Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics. 2005;21:389–395 [DOI] [PubMed] [Google Scholar]
- 40. Touchberry CD, Wacker MJ, Richmond SR, Whitman SA, Godard MP. Age-related changes in relative expression of real-time PCR housekeeping genes in human skeletal muscle. J Biomol Tech. 2006;17:157–162 [PMC free article] [PubMed] [Google Scholar]
- 41. Watt PW, Lindsay Y, Scrimgeour CM, et al. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: Use in studies of human tissue protein synthesis. Proc Natl Acad Sci USA. 1991;88:5892–5896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 43. Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol. 2006;101:53–59 [DOI] [PubMed] [Google Scholar]
- 44. Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62:1407–1412 [DOI] [PubMed] [Google Scholar]
- 45. Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf). 2008;194:81–93 [DOI] [PubMed] [Google Scholar]
- 46. Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J. 2012;26:1909–1920 [DOI] [PubMed] [Google Scholar]
- 47. Svensson J, Moverare-Skrtic S, Windahl S, Swanson C, Sjogren K. Stimulation of both estrogen and androgen receptors maintains skeletal muscle mass in gonadectomized male mice but mainly via different pathways. J Mol Endocrinol. 2010;45:45–57 [DOI] [PubMed] [Google Scholar]
- 48. Tsai WJ, McCormick KM, Brazeau DA, Brazeau GA. Estrogen effects on skeletal muscle insulin-like growth factor 1 and myostatin in ovariectomized rats. Exp Biol Med (Maywood). 2007;232:1314–1325 [DOI] [PubMed] [Google Scholar]
- 49. Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol. 2008;104:347–353 [DOI] [PubMed] [Google Scholar]
- 50. Hayden JM, Bergen WG, Merkel RA. Skeletal muscle protein metabolism and serum growth hormone, insulin, and cortisol concentrations in growing steers implanted with estradiol-17β, trenbolone acetate, or estradiol-17β plus trenbolone acetate. J Anim Sci. 1992;70:2109–2119 [DOI] [PubMed] [Google Scholar]
- 51. Fonseca H, Powers SK, Goncalves D, Santos A, Mota MP, Duarte JA. Physical inactivity is a major contributor to ovariectomy-induced sarcopenia. Int J Sports Med. 2012;33:268–278 [DOI] [PubMed] [Google Scholar]
- 52. Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol. 2011;46:685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goldstein J, Sites CK, Toth MJ. Progesterone stimulates cardiac muscle protein synthesis via receptor-dependent pathway. Fertil Steril. 2004;82:430–436 [DOI] [PubMed] [Google Scholar]
- 54. Lewis MI, Fournier M, Storer TW, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol. 2007;103:1299–1310 [DOI] [PubMed] [Google Scholar]
- 55. Kvorning T, Andersen M, Brixen K, Schjerling P, Suetta C, Madsen K. Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF, myostatin or androgen receptor post strength training in humans. J Physiol. 2007;578:579–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. J Clin Endocrinol Metab. 2011;96:885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sheffield-Moore M, Paddon-Jones D, Casperson SL, et al. Androgen therapy induces muscle protein anabolism in older women. J Clin Endocrinol Metab. 2006;91:3844–3849 [DOI] [PubMed] [Google Scholar]
- 58. Sheffield-Moore M, Urban RJ, Wolf SE, et al. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84:2705–2711 [DOI] [PubMed] [Google Scholar]
- 59. Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001;55:663–672 [DOI] [PubMed] [Google Scholar]
- 60. Gallagher D, Ruts E, Visser M, Baumgartner RN, et al. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–E375 [DOI] [PubMed] [Google Scholar]
- 61. Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition. 2001;17:534–541 [DOI] [PubMed] [Google Scholar]