Abstract
Context:
Few studies have assessed the relationship between GH and mitochondrial function.
Objective:
The objective of this study was to determine the effects of improving IGF-I using a GHRH analog, tesamorelin, on mitochondrial function assessed by phosphocreatine (PCr) recovery using 31P magnetic resonance spectroscopy in obese adults with reduced GH.
Design:
A total of 39 obese men and women with reduced GH secretion as determined by GHRH-arginine stimulation tests underwent magnetic resonance spectroscopy as part of a 12-month, double-blind, randomized, placebo-controlled trial comparing tesamorelin vs placebo. PCr recovery after submaximal exercise was assessed at baseline and at 12 months.
Results:
At baseline, there were no differences in age, sex, race/ethnicity, and GH or PCr parameters between tesamorelin and placebo. After 12 months, tesamorelin treatment led to a significantly greater increase in IGF-I than did placebo treatment (change, 102.9 ± 31.8 μg/L vs 22.8 ± 8.9 μg/L, tesamorelin vs placebo; P = .02). We demonstrated a significant positive relationship between increases in IGF-I and improvements in PCr recovery represented as ViPCr (R = 0.56; P = .01). The association between IGF-I and PCr recovery was even stronger among subjects treated with tesamorelin only (ViPCr: R = 0.71; P = .03). This association remained significant after controlling for age, sex, race, ethnicity, and parameters of body composition and insulin sensitivity (all P < .05).
Conclusions:
Increases in IGF-I from 12 months of treatment with tesamorelin were significantly associated with improvements in PCr recovery parameters in obese men and women with reduced GH secretion, suggestive of improvements in mitochondrial function.
Obesity is associated with reduced GH secretion (1–4), and this reduction in GH is associated with increased cardiometabolic disease risk (5–8). Our previous study suggested the reduced GH secretion in obesity may also be associated with reduced mitochondrial function as represented by delayed phosphocreatine (PCr) recovery on 31P magnetic resonance spectroscopy (MRS) (9). MRS is a noninvasive technique that has been used for several decades to study high-energy phosphate metabolism in vivo. During exercise, PCr serves as a high-energy phosphate pool to buffer hydrolysis of intracellular ATP and therefore decreases dramatically. However, PCr is resynthesized during recovery, and this dynamic recovery of PCr after exercise has been used to predict skeletal muscle oxidative capacity, as a surrogate marker for skeletal muscle mitochondrial function (10, 11).
To further explore the relationship between GH and PCr recovery, we performed MRS as part of a recently completed 12-month, double-blind, randomized, placebo-controlled, intervention trial with a synthetic GHRH analog, tesamorelin, in obese subjects with reduced GH secretion. In this prior study, tesamorelin improved body composition, triglycerides, C-reactive protein, and carotid intima-media thickness (8). In the current study, we hypothesized that stimulation of the GH axis with GHRH would enhance skeletal muscle mitochondrial function. This study was designed to increase our understanding of the mechanisms by which GHRH improves cardiometabolic indices in obesity and has broad relevance to many conditions of GH insufficiency.
Materials and Methods
Study subjects
Obese subjects with reduced GH secretion were recruited at the Massachusetts General Hospital between June 2008 and November 2010 for a double-blind, randomized, placebo-controlled interventional study using tesamorelin. Data from these subjects were published previously in a study evaluating the effects of tesamorelin on visceral adipose tissue (VAT) and cardiovascular disease risk markers (8). The effects of tesamorelin on PCr recovery have yet to be reported. Of the 60 subjects enrolled in the original study, 39 subjects underwent the MRS protocol for assessment of PCr recovery. One subject was excluded based on an increased IGF-I at baseline before any intervention. Inclusion and exclusion criteria were described in detail previously (8). In brief, 18- to 55-year-old men and women with body mass index (BMI) ≥ 30 kg/m2, waist circumference ≥ 102 cm (men) and 88 cm (women), and peak stimulated GH levels of ≤9 μg/L after a GHRH-arginine stimulation test were included. Of the 39 subjects, 18 had peak stimulated GH levels of ≤4.2 μg/L. All participants were otherwise healthy without known endocrine dysfunction. Participants had no known diagnosis of diabetes mellitus, and subjects using hormonal medication including estrogen, hormone replacement therapy, oral contraceptives, testosterone, glucocorticoids, anabolic steroids, GHRH, GH, or IGF-I within 3 months of enrollment were excluded from the study. The current study was approved by the Partners Institutional Review Board. Written informed consent was obtained from all participants.
Study design
Subjects were randomized in a 1:1 fashion to tesamorelin vs placebo. Subjects performed SC injections of 2 mg of tesamorelin vs placebo once daily for 12 months. IGF-I levels were monitored by an independent physician and a dose adjustment algorithm was incorporated to maintain IGF-I levels within the normal range for the subject's age. Subjects were evaluated for PCr recovery at baseline and again after 12 months of treatment.
31P MRS Protocol
Mitochondrial function was determined using 31P MRS to assess PCr resynthesis after submaximal exercise as reported previously (9, 12). In brief, subjects were placed in a 60-cm-bore Siemens 3.0T Tim Trio System with an operating frequency of 49.879 MHZ for 31P MRS. Baseline resting spectra were obtained, after which subjects were instructed to perform submaximal exercise for 3 minutes at a constant load (40% maximal voluntary contraction [MVC]) by performing bilateral quadriceps contractions at 0.5 Hz followed by a 5-minute recovery period. An 8-cm-diameter radiofrequency surface coil, tuned for 31P, was fastened proximal to the superior aspect of the patella over the anteromedial aspect of the right thigh, keeping the coil's axis perpendicular to the z-axis of the magnet. A hard pulse of 200 μs was used for excitation of the 31P signal. The amplitude of the radiofrequency pulse was optimized to obtain a maximal 31P signal in single-shot acquisitions. Spectral analysis was performed using integrated area under the curve. Mitochondrial function was determined by fitting the time course of the PCr area under the curve during recovery with an exponential curve [β3 + β2 (1 − e−(t/τ))] to generate τPCr, which describes mitochondrial phosphorylation potential. Mitochondrial function was further described by the initial rate of phosphocreatinine recovery (ViPCr) as [(60/τPCr) × PCr depletion]. ViPCr is thought to be insensitive to end-of-exercise metabolic conditions, including end-of-exercise pH and is favored by some investigators (13). ViPCr was studied as the primary endpoint with τPCr as the secondary endpoint.
Sensitivity analyses excluding lower quality scans were also performed. Quality was determined by a PCr recovery confidence interval of >40 and PCr signal to noise ratio of <15.
Biochemical assessment
GHRH-arginine testing was performed. GH was measured using the Beckman Access Ultrasensitive human GH assay, a paramagnetic particle, chemiluminescent immunoassay (Beckman Coulter). IGF-I was measured using the Immulite 2000 assay (Siemens Diagnostics). A 75-g oral glucose tolerance test (OGTT) was performed at baseline and at the 12-month visits.
Anthropometric assessment
Height and body weight were measured after an overnight fast. Total body lean and fat mass was determined by dual-energy x-ray absorptiometry (Discovery A; Hologic, Inc). Measurements of regional lean mass, specifically the right leg lean mass, using dual-energy x-ray absorptiometry were standardized (1995 User's Guide; Hologic Inc). The technique has a precision error (1 SD) of 1.5% for lean mass (14). Cross-sectional computed tomography scans to assess for abdominal VAT were also performed as per the previous studies (4, 8).
Physical activity assessment
The physical activity level in our subjects was assessed by self-reported regular structured exercise during direct interview and expressed as total hours of activity per week (metabolic equivalents) and hours of TV watching per day.
Statistical analysis
Baseline variables were compared by the χ2 test for noncontinuous variables, the Student t test for continuous variables that were normally distributed, and the Wilcoxon rank sum test for continuous variables that were not normally distributed. Normality of the data was determined by the Shapiro-Wilk test. The effects of tesamorelin were compared against those of placebo for change from 12 months to baseline using the Student t test for normally distributed variables and the Wilcoxon rank sum test for nonnormally distributed variables. Matched paired t tests were separately conducted for the placebo- and tesamorelin-treated subjects. Sensitivity analyses excluding lower quality MRS scans were performed. Univariate regression analyses using Pearson correlation coefficients were performed to assess the relationship between the change in IGF-I and the change in ViPCr and τPCr using all available data and data excluding lower quality MRS scans. Further sensitivity analyses were performed using only data from the tesamorelin treatment group. Additional univariate regression analyses were also performed between changes in ViPCr and body composition and metabolic parameters. Multivariate regression analyses using standard least squares modeling after controlling for the effects of age, sex, race, and ethnicity as well as various parameters of body composition and insulin sensitivity were performed to further evaluate the association between the change in IGF-I and the change in PCr recovery. These parameters were selected for their known association to GH and/or mitochondrial function. Statistical analysis was performed using JMP Statistical Database Software (version 10.0.0; SAS Institute, Inc). Statistical significance was determined as a value of P < .05.
Results
Baseline clinical characteristics of study subjects
A total of 20 subjects randomized to placebo and 19 subjects randomized to tesamorelin for 12 months underwent baseline MRS. Of these, 11 placebo- and 11 tesamorelin-treated subjects completed the study. The baseline clinical characteristics of the subjects who completed the study are presented in Table 1. The placebo-treated subjects were on average 39.2 ± 3.4 years old (range, 23–55 years old) and the tesamorelin-treated subjects were 40.8 ± 1.9 years old (range, 30–55 years old). The 2 groups were not significantly different in regard to age, sex, race, ethnicity, current or past tobacco use, body weight, BMI, waist circumference, lean mass, fat mass, fasting GH, peak stimulated GH, IGF-I, IGF-I standard deviation score (SDS), physical activity, or PCr recovery parameters including the MVC at baseline (all P > .10).
Table 1.
Baseline Characteristics of All Subjects Who Completed the Study (n = 22)
| Placebo | Tesamorelin | P | |
|---|---|---|---|
| No. of subjects | 11 | 11 | |
| Age, y | 39.2 ± 3.4 | 40.8 ± 1.9 | .68 |
| Male sex, n (%) | 6 (54.5) | 8 (72.7) | .38 |
| Race, n (%) | 7 (63.6) | 5 (45.5) | .39 |
| Hispanic ethnicity, n (%) | 1 (9.1) | 2 (18.2) | .53 |
| Current or past smoker, yes, n (%) | 6 (54.5) | 8 (72.7) | .38 |
| BMI, kg/m2 | 38.2 (34.3–42.7) | 36.3 (33.7–39.4) | .38 |
| Waist circumference, cm | 124 ± 3 | 119 ± 3 | .28 |
| Total lean mass, kg | 69.1 ± 3.5 | 72.8 ± 4.1 | .49 |
| Right leg lean mass, kg | 12.2 ± 0.8 | 12.9 ± 0.7 | .51 |
| Total body fat, kg | 45.5 ± 3.9 | 41.2 ± 2.4 | .35 |
| Trunk fat, kg | 24.1 ± 2.2 | 22.4 ± 1.6 | .55 |
| VAT, cm2 | 190 ± 21 | 211 ± 20 | .48 |
| Fasting GH, μg/L | 0.05 (0.03–0.10) | 0.04 (0.02–0.14) | .89 |
| Peak GH, μg/L | 4.85 ± 0.70 | 4.70 ± 0.90 | .90 |
| IGF-I, μg/L | 125 ± 12 | 113 ± 13 | .51 |
| IGF-I (SDS) | −0.08 ± 0.20 | −0.28 ± 0.22 | .51 |
| Total hours of activity/wk (metabolic equivalents) | 9.3 (5.5–123.7) | 65.0 (50.8–121.7) | .11 |
| Total hours of TV/day, h | 1.9 (1–4.5) | 2.5 (2–4) | .43 |
| ViPCr, mM/min | 18.1 (14.9–25.5) | 21.1 (19.6–32.6) | .18 |
| τPCr, s | 56.4 ± 5.3 | 49.9 ± 3.9 | .34 |
| PCr depletion, mM | 18.3 ± 1.2 | 19.3 ± 1.3 | .60 |
| pH at end of exercise | 6.68 ± 0.06 | 6.72 ± 0.08 | .69 |
| MVC, kg | 49.7 (39.7–61.3) | 52.9 (37.2–60.0) | .89 |
Parameters that are normally distributed are presented as means ± SEM, and parameters that are not normally distributed are presented as medians (interquartile range).
Effects of tesamorelin
Treatment with tesamorelin led to a significant increase in IGF-I compared with that for placebo treatment (change from baseline, 102.9 ± 31.8 μg/L vs 22.8 ± 8.9 μg/L, tesamorelin vs placebo; P = .02). IGF-I SDS also increased (change from baseline, 1.69 ± 0.52 vs 0.37 ± 0.15, tesamorelin vs placebo; P = .02). Four subjects required dose adjustments to maintain IGF-I within the physiological range, suggesting that the vast majority of IGF-I levels remained within the physiological range. Treatment with tesamorelin did not affect fasting glucose, 2-hour glucose with an OGTT, fasting insulin, or homeostasis model assessment of insulin resistance (all P > .10). A total of 20 paired (baseline and 12 months) MRS scans were available for evaluation. After treatment with tesamorelin for 12 months, the change in ViPCr was 0.01 ± 3.76 vs −1.02 ± 1.71 mM/min (tesamorelin vs placebo, P > .10). A matched paired t test evaluating placebo- and tesamorelin-treatment groups independently confirmed the above findings.
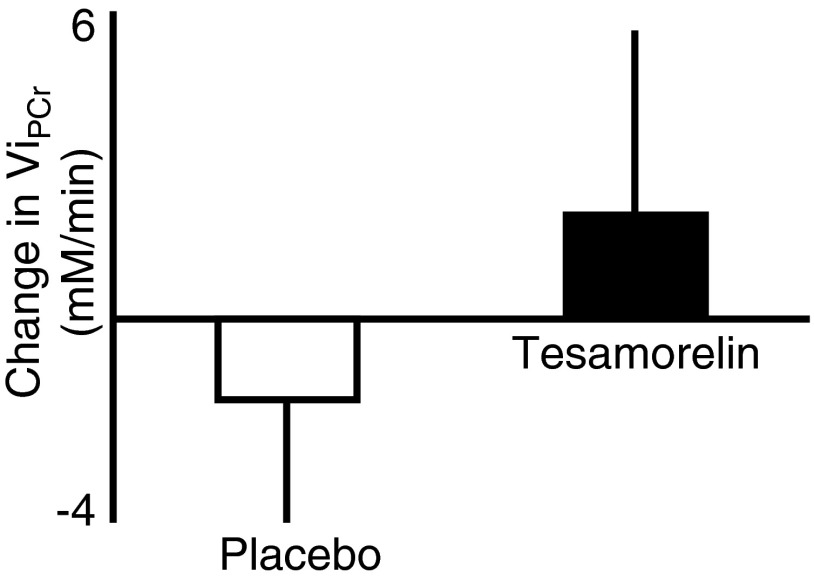
Sensitivity analyses, using stringent cutoffs to exclude lower quality scans, were performed, resulting in 15 paired scans (7 placebo and 8 tesamorelin treatment) available for evaluation. Treatment with tesamorelin in this subset of patients led to a significant increase in IGF-I compared with that for placebo (change from baseline 110.8 ± 34.0 vs 13.6 ± 12.7 μg/L, tesamorelin vs placebo; P = .04). In this analysis, tesamorelin led to a further improvement in ViPCr, relative to that for placebo, although again statistical significance was not reached because of the relatively small number of paired assessments included in the analysis (change from baseline ViPCr, 2.02 ± 3.60 mM/min vs −1.59 ± 2.40 mM/min, tesamorelin-treated subjects vs placebo-treated subjects; P > .10) (Figure 1). In this analysis, a change in the positive direction for ViPCr indicates improved mitochondrial function.
Figure 1.
Change in ViPCr over 12 months of treatment with placebo vs tesamorelin.
Univariate regression analyses
Univariate regression analysis among all evaluable paired MRS data (n = 20 pairs) revealed a significant positive relationship between increases in IGF-I and improvements in ViPCr (R = 0.56; P = .01) (Figure 2) and similarly, increases in IGF-I trended toward significance in relationship to improvement in τPCr (R = −0.42; P = .07). There were no associations between the change in IGF-I with the change in PCr depletion or MVC (P > .10) (Table 2).
Figure 2.
Univariate regression analysis between the change in IGF-I and the change in ViPCr over 12 months among all subjects with available MRS scans. □, placebo-treated subjects (n = 11); ●, tesamorelin-treated subjects (n = 9).
Table 2.
Univariate Regression Analyses Demonstrating Associations Between Change in IGF-I and PCr Parameters Among All Subjects Who Completed the Study
| Parameter | R | P |
|---|---|---|
| ViPCr | 0.56 | .01 |
| τPCr | −0.42 | .07 |
| PCr depletion | −0.03 | .89 |
| MVC | 0 | 1.00 |
Sensitivity analyses using higher quality scans only (n = 15 pairs) confirmed the significant association between increases in IGF-I and improvement in ViPCr (R = 0.61; P = .02) (Supplemental Figure 1A published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). In this analyses, the change in IGF-1 also trended toward significance in relationship to improvement in τPCr (R = −0.45; P = .10) (Supplemental Figure 1B).
Analyses of subjects treated with tesamorelin only (n = 9 pairs) demonstrated an even stronger statistical association between the increases in IGF-I and improvements in ViPCr (R = 0.71; P = .03) and τPCr (R = −0.80; P = .01) (Supplemental Figure 2, A and B).
The change in ViPCr was not significantly associated with changes in body composition parameters including changes in right leg lean mass or physical activity or diet (all P > 1.0).
Multivariate regression analyses
The change in IGF-I remained significantly associated with improvements in ViPCr after controlling for the effects of baseline age, sex, race, and ethnicity using multivariate regression modeling. Independent models with controls for the additional effects of body composition parameters including baseline body weight, BMI, waist circumference, total fat mass, trunk fat mass, total lean mass, and right leg lean mass continued to confirm this strong association (all models P < .05). Further independent modeling with controls for the effects of insulin sensitivity including baseline fasting glucose, 2-hour glucose on the OGTT, fasting insulin, homeostasis model assessment, and hemoglobin A1c also confirmed this strong association (all models P < .05).
Discussion
This is the first study, to our knowledge, to assess the effects of improving endogenous, pulsatile GH secretion using a synthetic GHRH analog on PCr recovery, as a measure of mitochondrial function, in obese adults with reduced GH levels. For the first time, we demonstrate a significant association between increases in IGF-I within the physiological range and improvements in PCr recovery among obese men and women with reduced GH secretion. Furthermore, this association remained significant after controlling for parameters known to affect GH and insulin sensitivity. Treatment with GHRH differs fundamentally from that with GH, because GHRH increases endogenous GH pulsatility and augments IGF-I in a more physiological fashion, without aggravating glucose, and thus these results cannot be directly generalized to treatment with GH.
These results are consistent with and expand on our prior observational study demonstrating a relationship between reduced GH secretion in obesity and delayed PCr recovery by MRS (9). The findings are also consistent with a prior acute interventional study in which GH was infused for 14 hours in healthy volunteers that showed an increase in mitochondrial ATP production rate and oxidative capacity (15) and with a more long-term 6-month interventional study of young abdominally obese men treated with exogenous GH, which demonstrated improvements in PCr recovery, using 31P MRS (16). However, Bredella et al (16) demonstrated their effect on PCr recovery after controlling for adverse effects on glucose, which was not necessary in our study. This difference may be due to the lack of adverse effect of GHRH specifically on glucose (8, 17) compared with GH.
Given the previously demonstrated role of GH/IGF-I in stimulating the peroxisome proliferator–activated receptor γ coactivator 1-alpha, a nuclear encoded mitochondrial gene important for both mitochondrial biogenesis and function, in rat soleus muscle (18), a direct effect of improving IGF-I on mitochondrial biogenesis and function can be postulated. We cannot rule out the indirect effects secondary to the positive metabolic benefits of 12 months of treatment with tesamorelin (8), but this relationship remained significant after controlling for a number of these variables, suggesting a more direct association between the change in mitochondrial function and the change in IGF-I.
This was a randomized, double-blind, placebo-controlled study that used a novel hypothalamic GHRH analog and a dose adjustment algorithm to maintain IGF-I within the age-adjusted normal range for each subject. This led to a physiological increase in IGF-I as demonstrated by an average improvement in IGF-I of 1.69 SDS. However, certain limitations exist. The high dropout rate resulted in a small sample size for PCr recovery analyses between the baseline and 12 month visits. A larger study sample may be needed to demonstrate a statistically significant effect, as is suggested in the comparison of tesamorelin vs placebo in this study, particularly among the subset with the highest quality scans. In addition, a more homogeneous population with similar age ranges may also have yielded significant results, given possible effects of age on mitochondrial function (12). Nonetheless, a statistically significant effect was noted using univariate regression analyses to determine the association between the change in IGF-I and change in PCr recovery. Although this study suggests that increasing the GH levels in obese adults may improve mitochondrial function, no study to date has addressed whether this reflects an increase in mitochondrial number, size, density, efficiency, or subcellular localization, and clearly further mechanistic physiology studies will be needed. Furthermore, additional studies are needed to evaluate the specific physiological benefits of improving mitochondrial function.
In summary, this is the first study to demonstrate a significant association between increasing IGF-I levels and improvements in PCr recovery after 12 months of treatment with a GHRH analog, tesamorelin, in obese subjects with reduced GH secretion. Larger studies using a more comprehensive evaluation of skeletal muscle mitochondria will be needed to confirm and expand on these findings. Although the clinical significance of this finding remains unknown, these data suggest a potentially important relationship between changes in IGF-I and improvement in mitochondrial function in obese subjects with relative reductions in GH secretion and may help to explain a number of the metabolic benefits of GHRH treatment in obese patients with reduced GH secretion. This study also has important implications and relevance for the numerous conditions in which insufficient GH may contribute to metabolic abnormalities and for which augmentation of GH may be indicated.
Acknowledgments
This work was supported by the National Institutes of Health (Grants K23DK087857 to H.M., R01HL085268, P30DK040561, and K24DK064545 to S.K.G.), the National Center for Research Resources (UL1RR025758, Harvard Clinical and Translational Science Center), and the Center for Functional Neuroimaging Technologies (Grant P41RR14075 to the Athinoula A. Martinos Center for Biomedical Imaging where the 31P MRS imaging studies were conducted). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Partial research funding and study drug was provided by Theratechnologies, Inc.
This study was registered with clinical trial registration number NCT00675506.
Disclosure Summary: S.K.G. has served as a consultant to Theratechnologies, Inc, Aileron Therapeutics, Inc, Alize Pharma SAS, F. Hoffmann-La Roche LTD, and EMD Serono Inc, all unrelated to this article, and has received investigator-initiated research funds from Theratechnologies, Inc. H.M. has received investigator initiated research support from Pfizer, Inc., unrelated to this article. The other authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- MRS
- magnetic resonance spectroscopy
- MVC
- maximal voluntary contraction
- OGTT
- oral glucose tolerance test
- PCr
- phosphocreatinine
- SDS
- standard deviation score
- VAT
- visceral adipose tissue
- ViPcr
- initial rate of phosphocreatinine recovery.
References
- 1. Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73:1081–1088 [DOI] [PubMed] [Google Scholar]
- 2. Pijl H, Langendonk JG, Burggraaf J, et al. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab. 2001;86:5509–5515 [DOI] [PubMed] [Google Scholar]
- 3. Bonert VS, Elashoff JD, Barnett P, Melmed S. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: diagnostic caveat for adult GH deficiency. J Clin Endocrinol Metab. 2004;89:3397–3401 [DOI] [PubMed] [Google Scholar]
- 4. Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008;93:4254–4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Utz AL, Yamamoto A, Hemphill L, Miller KK. Growth hormone deficiency by growth hormone releasing hormone-arginine testing criteria predicts increased cardiovascular risk markers in normal young overweight and obese women. J Clin Endocrinol Metab. 2008;93:2507–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Makimura H, Feldpausch MN, Stanley TL, Sun N, Grinspoon SK. Reduced growth hormone secretion in obesity is associated with smaller LDL and HDL particle size. Clin Endocrinol (Oxf). 2012;76:220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Makimura H, Stanley T, Mun D, et al. Reduced growth hormone secretion is associated with increased carotid intima-media thickness in obesity. J Clin Endocrinol Metab. 2009;94:5131–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makimura H, Feldpausch MN, Rope AM, et al. Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: a randomized controlled trial. J Clin Endocrinol Metab. 2012;97:4769–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Makimura H, Stanley TL, Sun N, Hrovat MI, Systrom DM, Grinspoon SK. The association of growth hormone parameters with skeletal muscle phosphocreatine recovery in adult men. J Clin Endocrinol Metab. 2011;96:817–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Befroy DE, Shulman GI. Magnetic resonance spectroscopy studies of human metabolism. Diabetes. 2011;60:1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perry CG, Kane DA, Lanza IR, Neufer PD. Methods for assessing mitochondrial function in diabetes. Diabetes. 2013;62:1041–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fleischman A, Makimura H, Stanley TL, et al. Skeletal muscle phosphocreatine recovery after submaximal exercise in children and young and middle-aged adults. J Clin Endocrinol Metab. 2010;95:E69–E74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roussel M, Bendahan D, Mattei JP, Le Fur Y, Cozzone PJ. 31P magnetic resonance spectroscopy study of phosphocreatine recovery kinetics in skeletal muscle: the issue of intersubject variability. Biochim Biophys Acta. 2000;1457:18–26 [DOI] [PubMed] [Google Scholar]
- 14. Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112 [DOI] [PubMed] [Google Scholar]
- 15. Short KR, Moller N, Bigelow ML, Coenen-Schimke J, Nair KS. Enhancement of muscle mitochondrial function by growth hormone. J Clin Endocrinol Metab. 2008;93:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bredella MA, Gerweck AV, Lin E, et al. Effects of GH on body composition and cardiovascular risk markers in young men with abdominal obesity. J Clin Endocrinol Metab. 2013;98:3864–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stanley TL, Chen CY, Branch KL, et al. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab. 2011;96:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vescovo G, Ravara B, Gobbo V, Angelini A, Dalla Libera L. Skeletal muscle fibres synthesis in heart failure: role of PGC-1α, calcineurin and GH. Int J Cardiol. 2005;104:298–306 [DOI] [PubMed] [Google Scholar]