Abstract
Context:
Racial/ethnic minorities suffer disproportionate morbidity and mortality from chronic diseases.
Objective:
Our objective was to assess racial and socioeconomic status (SES) disparities in well-differentiated thyroid cancer (WDTC) patients.
Design and Participants:
We conducted a retrospective cohort study on 25 945 patients with WDTC (1999–2008) from the California Cancer Registry (57% white, 4% black, 24% Hispanic, and 15% Asian-Pacific Islander [API]).
Main Outcomes:
We evaluated effect of race and SES variables on stage of cancer presentation and overall/disease-specific survival.
Results:
Significant differences in stage of presentation between all racial groups were found (P < .001), with minority groups presenting with a higher percentage of metastatic disease as compared with white patients (black, odds ratio [OR] = 1.36 with confidence interval [CI] 1.01–1.84; Hispanic, OR = 1.89 [CI, 1.62–2.21], API, OR = 1.82 [CI, 1.54–2.15]). Hispanic (OR = 1.59, [CI, 1.48–1.72]) and API (OR = 1.32 [1.22–1.44]) patients also presented with higher odds of regional disease. Patients with the lowest SES presented with metastatic disease more often than those with the highest SES (OR = 1.45 [CI, 1.16–1.82]). Those that were poor/uninsured and/or with Medicaid insurance had higher odds of presenting with metastatic disease as compared with those with private insurance (OR = 2.41, [CI, 2.10–2.77]). Unadjusted overall survival rates were higher among API and Hispanic patients and lower among black patients (P < .001 vs white patients). Adjusted overall survival also showed a survival disadvantage for black patients (hazard ratio = 1.4, [CI, 1.10–1.73]) and survival advantage for API patients (hazard ratio = 0.83, [CI, 0.71–0.97]). In disease-specific survival analyses, when only those patients with metastatic disease were analyzed separately, black patients again had the lowest survival rates, and Hispanic/API patients had the highest survival rates (P < .04).
Conclusion:
Black patients and those with low SES have worse outcomes for thyroid cancer. API and Hispanic patients may have a protective effect on survival despite presenting with more advanced disease.
Racial/ethnic minorities and those in a lower socioeconomic class suffer a disproportionate morbidity and mortality from a large variety of chronic diseases, including cancer, heart disease, diabetes, and stroke (1). Studies have attributed these healthcare inequalities to differences in access to care and/or to living in resource-poor neighborhoods. Others propose a difference in disease biology and genetic variance that contribute to disparities in disease presentation and outcomes (2). Still others suggest that providers may have inherent biases in their treatment of different races despite equal access to care (1, 3). There have also been implications of external sources affecting disease incidence and severity, including environmental exposures or less healthy lifestyles. Therefore, the cause of healthcare disparities is likely multifactorial.
There has been a modest amount of research on thyroid cancer disparities in the past few years (4). This issue is timely because the incidence of thyroid cancer has been rapidly increasing over the past 30 years, only partially explained by incidentally discovered thyroid nodules due to enhanced detection by imaging modalities to diagnose other disease processes (5–7). Other explanations that have been thought to contribute include obesity (7) or environmental impacts such as radiation and environmental chemicals (6). Recently, racial and socioeconomic status (SES) disparities have also emerged as potential contributors to the rapid rise of thyroid cancer in the United States.
The Endocrine Society, which is the leading society for endocrine science and medicine, has recently published a statement focusing on healthcare disparities in endocrine diseases, indicating this topic is an important one to the organization. In this statement, they specifically noted the correlation of advanced stage of thyroid cancer presentation with low SES as well as lower access to high-volume thyroid surgeons in minority racial populations (4). Recent reviews of the Surveillance Epidemiology and End Results registry show different incidence trends of thyroid cancer among racial/ethnic groups as well as an increasing incidence of thyroid cancer in those with higher access to care (8, 9). Most of these studies, however, were limited by their population databases and fall short of being comprehensive. We are fortunate to have access to the California Cancer registry (CCR), which is California's statewide population-based cancer surveillance system and one of the leading cancer registries in the world. It captures information on all patients diagnosed with cancer within the state of California, one of the most ethnically, economically, and racially diverse populations in the United States.
We hypothesize that there are inherent racial disparities in the presentation and outcomes of thyroid cancer patients, which may be only partially explained by their SES and/or access to care. The CCR data offer us the ability to more completely characterize these differences over time. Here we investigate the effect of race and SES on outcomes in well-differentiated thyroid cancer (WDTC) in the state of California.
Patients and Methods
Patients with a new diagnosis of WDTC were abstracted from the CCR (1999–2008). We included the following ICD-0–3 histology codes to identify all patients with WDTC as well as their subtype and variants: 8050, 8260, 8330, 8331, 8332, 8335, 8337, 8340, 8341, 8342, 8343, 8344, and 8350. We excluded patients with non-WDTCs (n = 2698), those with unknown stage (n = 434), and those with Native American and/or unknown races (due to too small a sample size, n = 284). Those excluded with unknown stage were equally distributed among races. Dual entries were excluded (n = 46) (Supplemental Figure 1, published on The Endocrine Society's Journals Online website at http://jcem.endojournals.org).
Racial groups were defined as Non-Hispanic white, Non-Hispanic black, Hispanic, and Asian/Pacific Islander (API). Our race variable is derived from the CCR variable RACE08. This variable is derived from a combination of self-report, birthplace, North American Association of Central Cancer Registries Hispanic/Latino Identification Algorithm, Indian Health Services linkage, and surnames.
Staging was defined as localized, regional, and remote/metastatic. Regional disease was grouped as follows: regional by direct extension, regional by lymph nodes, or regional by direct extension and lymph nodes. Comorbidty was scored using the Charlson comorbidity scoring system (10). Age was dichotomized at 45 years (ie, > 45 vs <= 45). SES score was coded as the quintiles of Yost's index of SES level based on a principal components analysis where the lowest SES score was 1 and the highest SES score was 5 (11).
Health insurance type was categorical with 3 levels: private, poor/uninsured, government/military. Private insurance included the following subtypes: managed care/Health Maintenance organization/preferred provider organization, fee-for-service, Medicare administered through a managed-care plan or private supplementation, and insurance/not otherwise specified. Poor/uninsured subtypes included Medicaid, Medicaid through managed care or with Medicare supplement, and county-funded not otherwise specified. Governmental insurance included TRICARE, Military, and Veterans Affairs.
The primary predictor of the multinomial logistic regression model was race for which Non-Hispanic white was treated as the reference. The primary outcome variable was thyroid cancer stage at diagnosis. Multinomial regression analyses controlled for age, SES, sex, and insurance type. Multinomial regression analysis was used in lieu of assuming proportional odds because the score test revealed that the proportional odds assumption was violated. Comparison of race groups was performed using ANOVA for normally distributed continuous data, Kruskal-Wallis test for nonnormal continuous variables, and χ2 test for categorical variables.
The overall survival functions were estimated using the Kaplan-Meier method within each race. The survival time was calculated as the time from diagnosis until death or last follow-up (censored). The curves were compared across race groups using the log-rank test. Multivariable analysis was further conducted via Cox regression models with race as the primary predictor, adjusting for stage, age, gender, and comorbidity. Competing risks analyses and cumulative incidence functions evaluated racial differences in thyroid cancer-specific survival, where death due to other causes was regarded as a competing risk. Cause-specific hazard regression was adjusted for stage, age, gender, and comorbidity via the Cox model. Similar overall and disease-specific survival analyses were performed for remote/metastatic patients only.
Overall median follow-up is 68.1 (range 0–156.1) months and by racial group (in months) was white 70.5 (0–156.1), black 64.7 (0–151.5), Hispanic 64.2 (0–154.8), and Asian 66.5 (0–155.6) (P < .001). Those with no follow-up were excluded from survival analyses (n = 143 total).
Competing risks analysis was conducted using the R statistical package “Cmprsk” (12). All other analyses were performed using SAS release 9.2 (SAS Institute, Inc).
Model evaluations
To assess model diagnostics and fit and to prove that membership in one category is not related to membership in another, the Hausman-McFadden test for the independence of irrelevant alternatives assumption was performed. In this test, the assumption that membership in one category is not related to the membership in another was not violated. The P values of the test were .99 and .93 for comparison of the model estimates leaving out either stage 2 or 3 vs those from the full model.
To investigate model fit and the percent variation of the outcome variable explained by the model, we used the Cox-Snell pseudo R2 measure. Because the regression techniques we used in this paper (multinomial logit regression and Cox regression) do not have an R2 measure as in linear regression, we used the Cox-Snell pseudo-R2 (max-rescaled) and C-index for multinomial logit regression and Cox regression, respectively, as a substitute. The Cox-Snell pseudo-R2 increased from 0.018 to 0.066 after including covariates other than race into the model for stage (see Table 3), and the C-index increased from 0.63 to 0.71 after including covariates other than race into the Cox model for overall survival.
Table 3.
Multivariable Regression: Effect of Race on Stage Presentation in WDTCa
| Stage | Parameter | OR Estimates |
|||
|---|---|---|---|---|---|
| Point Estimate | 95% CI | P Value | |||
| Regional | Race: NH black vs NH white | 0.61 | 0.51 | 0.74 | <.001 |
| Remote/metastatic | Race: NH black vs NH white | 1.36 | 1.01 | 1.84 | .042 |
| Regional | Race: Hispanic vs NH white | 1.60 | 1.48 | 1.72 | <.001 |
| Remote/metastatic | Race: Hispanic vs NH white | 1.89 | 1.62 | 2.21 | <.001 |
| Regional | Race: API vs NH white | 1.32 | 1.22 | 1.44 | <.001 |
| Remote/metastatic | Race: API vs NH white | 1.82 | 1.54 | 2.15 | <.001 |
| Regional | Age: >45 vs ≤45 y | 0.70 | 0.65 | 0.73 | <.001 |
| Remote/metastatic | Age: >45 vs ≤45 y | 2.05 | 1.79 | 2.35 | <.001 |
| Regional | Sex: 1 vs 0 | 1.74 | 1.62 | 1.86 | <.001 |
| Remote/metastatic | Sex: 1 vs 0 | 2.35 | 2.06 | 2.68 | <.001 |
| Regional | SES: 1 vs 5 | 0.93 | 0.84 | 1.04 | .20 |
| Remote/metastatic | SES: 1 vs 5 | 1.45 | 1.16 | 1.82 | <.001 |
| Regional | SES: 2 vs 5 | 0.94 | 0.86 | 1.04 | .22 |
| Remote/metastatic | SES: 2 vs 5 | 1.42 | 1.15 | 1.74 | <.001 |
| Regional | SES: 3 vs 5 | 0.95 | 0.87 | 1.04 | .26 |
| Remote/metastatic | SES: 3 vs 5 | 1.47 | 1.21 | 1.79 | <.001 |
| Regional | SES: 4 vs 5 | 0.90 | 0.83 | 0.98 | .012 |
| Remote/metastatic | SES: 4 vs 5 | 1.39 | 1.15 | 1.68 | <.001 |
| Regional | Insurance: poor/Medicaid (vs private) | 1.19 | 1.09 | 1.29 | <.001 |
| Remote/metastatic | Insurance: poor/Medicaid (vs private) | 2.41 | 2.10 | 2.77 | <.001 |
| Regional | Insurance: government (vs private) | 0.65 | 0.53 | 0.79 | <.001 |
| Remote/metastatic | Insurance: government (vs private) | 0.82 | 0.56 | 1.22 | .34 |
Abbreviation: NH, non-Hispanic
For sex, 0 is female and 1 is male. For stage, localized is the reference group. For race, white is the reference group. SES values are Yost socioeconomic status quintiles; 5 is the highest SES, and 1 is the lowest.
We tested the proportional hazards assumption in the Cox regression model by testing the significance of interaction between each covariate and log(time). The goodness of fit was evaluated using the C-index. The test of proportional hazards showed no violation of the model assumptions.
This study was approved by the University of California, Los Angeles, and the CCR Institutional Review Boards.
Results
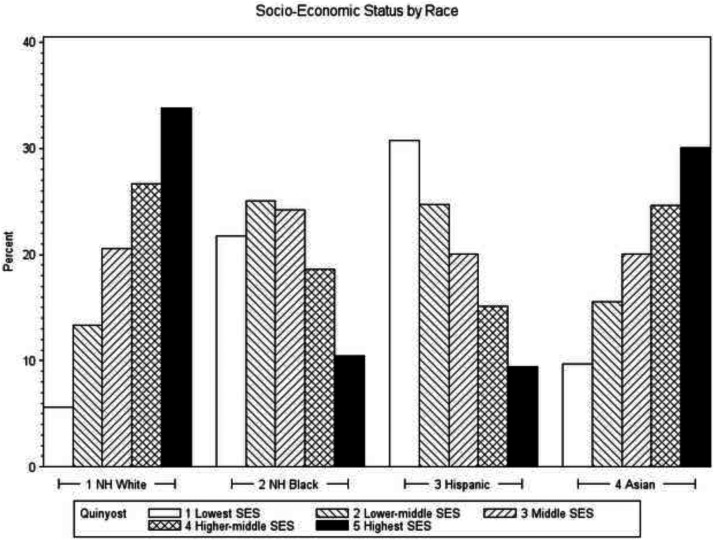
The final study cohort comprised 25 945 patients with WDTC, including 14 802 non-Hispanic white patients (57%); 939 non-Hispanic black patients (4%); 6303 Hispanic patients (24%); and 3901 API patients (15%) (Table 1). Black patients were on average older at the time of diagnosis and had higher comorbidity scores. There were fewer male patients in the Hispanic population. More black and Hispanic patients were in the lower SES categories, whereas more white/Asian patients were in the higher SES categories (Table 1 and Figure 1). Black patients, on average, had larger tumor sizes than white patients, and there were no differences in presentation of aggressive subtypes of WDTC among racial groups.
Table 1.
Demographics
| Non-Hispanic White | Non-Hispanic Black | Hispanic | API | P Value† | |
|---|---|---|---|---|---|
| n (%) | 14802 (57.1) | 939 (3.6) | 6303 (24.3) | 3901 (15.0) | |
| Age at diagnosis (years), mean ± SD | 49.7 ± 15.8 | 49.6 ± 15.3 | 44.0 ± 15.5 | 48.3 ± 15.4 | <.001a |
| Age >45 y, % | 8682 (58.7) | 553 (58.9) | 2737 (43.4) | 2166 (55.5) | <.001b |
| Male, n (%) | 3896 (26.3) | 208 (22.2) | 1116 (17.7) | 753 (19.3) | <.001b |
| Comorbidity (no cancer-related items included), median (min, max) | 0 (0, 12) | 0 (0, 10) | 0 (0, 12) | 0 (0, 11) | <.001c |
| Comorbidity (no cancer-related items included) >0 (%) | 3552 (24.0) | 329 (35.0) | 1421 (22.5) | 781 (20.0) | <.001b |
| SES status, n (%)d | <.001b | ||||
| 1 | 831 (5.6) | 204 (21.7) | 1937 (30.7) | 379 (9.7) | |
| 2 | 1973 (13.3) | 235 (25.0) | 1557 (24.7) | 607 (15.6) | |
| 3 | 3043 (20.6) | 227 (24.2) | 1263 (20.0) | 782 (20.1) | |
| 4 | 3948 (26.7) | 175 (18.6) | 952 (15.1) | 960 (24.6) | |
| 5 | 5007 (33.8) | 98 (10.4) | 594 (9.4) | 1173 (30.1) | |
| Tumor (mm), median (min, max) | 15 (0, 125) | 19 (0, 130) | 20 (0, 130) | 17 (0, 130) | <.001c |
| Aggressive tumor, n (%) | 82 (0.6) | 5 (0.5) | 47 (0.8) | 22 (0.6) | .40b |
P values from a ANOVA; b χ2 test; c Kruskal-Wallis test.
Yost socioeconomic status quintiles; 5 is the highest SES, and 1 is the lowest (11).
Figure 1.
Socioeconomic categories within races in WDTC patients of California.
Significant differences in stage of presentation between all racial groups were found, with minority groups presenting with a higher percentage of remote/metastatic disease as compared with white patients (P < .001). (Table 2) Unadjusted regression analysis confirmed that minority groups had a higher odds of presenting with remote/metastatic disease as compared with white patients (black, odds ratio [OR] = 1.56 with confidence interval [CI] of 1.2–2.04; Hispanic, OR = 1.85 [CI, 1.62–2.11]; API, OR = 1.81 [CI, 1.19–1.39]). After adjusting for age, sex, SES, and insurance type, minority groups continued to have an increased odds of presenting with remote/metastatic disease as compared with white (black, OR = 1.36 [CI, 1.01–1.84]; Hispanic, OR = 1.89 [CI, 1.62–2.21]; API, OR = 1.82 [CI, 1.54–2.15]) (Table 3). Hispanic (OR = 1.59 [CI, 1.47–1.71]) and API (OR = 1.34 [CI, 1.22–1.44]) patients also presented with higher odds of locoregional disease than white patients (Table 3).
Table 2.
Univariate Association: Effect of Race on Stage Presentation in WDTCa
| Race/Ethnicity | Stage, n (%) |
||
|---|---|---|---|
| Localized | Regional | Remote/Metastatic | |
| Non-Hispanic white | 10279 (69.5) | 3899 (26.3) | 624 (4.2) |
| Non-Hispanic black | 714 (76.0) | 157 (16.7) | 68 (7.3) |
| Hispanic | 3651 (57.9) | 2242 (35.6) | 410 (6.5) |
| API | 2443 (62.6) | 1189 (30.5) | 269 (6.9) |
| Total | 17087 (65.9) | 7487 (28.9) | 1371 (5.2) |
For χ2 association, P < .001.
Not surprisingly, as with previous studies on SES and its relation to cancer outcomes, patients with the lowest SES presented with remote/metastatic disease more often than those with the highest SES (OR = 1.45 [CI, 1.16–1.82]) (13, 14). As SES increased, there seemed to be decreasing odds of presenting with remote/metastatic disease. Additionally, those that were poor/uninsured and/or with Medicaid insurance had a higher odds of presenting with remote/metastatic disease as compared with those with private insurance (OR = 2.41 [CI, 2.10–2.77]). There was no significant difference in remote/metastatic disease presentation between those with government insurance and those with private insurance.
Additionally, a patient with age >45 years was more likely to present with remote/metastatic disease than those in the younger age group (OR = 2.05 [CI, 1.79–2.35]). Men were more likely to present with locoregional disease (OR = 1.74 [CI, 1.62–1.86]) and remote/metastatic disease (OR = 2.35 [CI, 2.06–2.85]) as compared with women.
When the same multivariable analysis was done within each racial group, SES and insurance type had an effect on stage of presentation in white patients only. In fact, within the white race, there was a pattern of presenting with later-stage disease as SES status declined (Supplemental Table 1). Interestingly, all other races showed no effect of SES status and/or insurance type on stage of presentation when evaluated within their race alone. Age >45 years remained significant for all races, however, for presenting with remote/metastatic disease (older whites, OR = 1.7 [CI, 1.38–2.09]; older blacks, OR = 2.96 [CI, 1.49–5.88]; older Hispanics, OR = 2.34 [CI, 1.84–2.96]).
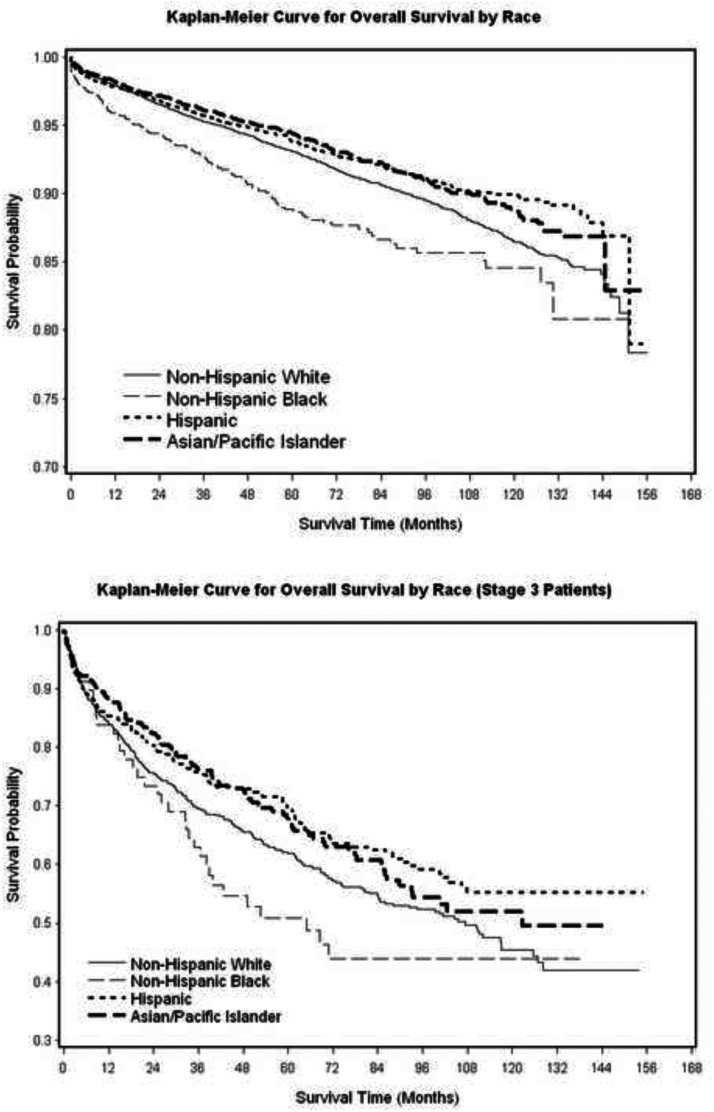
Despite presenting with more advanced disease, unadjusted overall survival rates were higher among API and Hispanic patients and lower among black patients (vs white patients, P < .001). After adjusting for stage, age, sex, comorbidities, and treatment variables, Cox regression analysis of overall survival showed a persistent survival advantage for Asian patients (hazard ratio = 0.83, [CI0.71–0.97]) and survival disadvantage for black patients (hazard ratio = of 1.4, [CI, 1.10–1.73]) (Figure 2 and Table 4). Male sex and regional and remote stages all had increased mortality, whereas radioactive iodine treatment had a protective effect on survival. When only those patients with remote/metastatic disease were analyzed separately, black patients still had the lowest overall and disease-specific survival rates, and Hispanic/API patients had the highest overall and disease-specific survival rates (P < .04) (Supplemental Figure 2).
Figure 2.
Overall survival (total and remote/metastatic patients only).
Table 4.
Cox Regression Analysis of Overall Survivala
| Parameter | Hazard Ratio | 95% Hazard Ratio | Confidence Limits | P value |
|---|---|---|---|---|
| NH black (vs NH white) | 1.38 | 1.10 | 1.73 | .006 |
| Hispanic (vs NH white) | 0.97 | 0.85 | 1.10 | .65 |
| API (vs NH white) | 0.83 | 0.71 | 0.97 | .016 |
| Regional stage | 1.90 | 1.68 | 2.15 | <.001 |
| Remote/metastatic stage | 4.93 | 4.32 | 5.63 | <.001 |
| Age, y | 1.07 | 1.06 | 1.07 | <.001 |
| Sex (male vs female) | 1.60 | 1.45 | 1.77 | <.001 |
| Charlson comorbidity score >0 vs 0 (no cancer-related items included) | 2.40 | 2.16 | 2.67 | <.001 |
| Treatment with lobectomy (vs total thyroidectomy) | 1.06 | 0.93 | 1.20 | .37 |
| RAI (yes vs no) | 0.63 | 0.57 | 0.70 | <.001 |
Abbreviations: NH, non-Hispanic; RAI, radioactive iodine.
For sex, 0 is female 1 is male.
We tested the interaction effect between race and gender for multivariable regression models presented in Tables 3 (P = .25) and 4 (P = .19). Because the interactions are not significant, the effect of race on stage at diagnosis is essentially the same between gender groups. This indicates that we do not need to stratify the results by gender.
Discussion
Our paper has shown that both racial and SES factors have a significant influence on the presentation and outcomes of WDTC patients. To our knowledge, this is the largest comprehensive study of racial and SES differences among thyroid cancer patients.
We show that racial minorities (blacks, Hispanics, and Asians) present with more advanced WDTCs as compared with their white counterparts. These racial differences cannot be fully explained by their socioeconomic factors, age, sex, or insurance type. Those with low SES have more advanced disease than those with higher SES. Black patients consistently present with later disease and have worse survival over any other racial group. Interestingly, there seems to be a persistent protective survival advantage of API and Hispanic patients despite presenting with later-stage disease and despite having a lower percentage of males.
Previous studies on this topic have been limited. Single-institution studies are restricted by their power, and other large database studies are limited by their comprehensiveness (9, 15). The National Surveillance Epidemiology and End Results registry comprises disease information collected from individual national cities, only 2 of which are in California. Although this registry attempts to capture a representational proportion of the U.S. population, it falls short in its comprehensiveness and detail. Established in 1947, the CCR is one of the most complete cancer registries in the country and is the cornerstone of a substantial amount of research on cancer in the California population. The amount of comprehensive detail and follow-up in the CCR is unparalleled by any other state in the country. To date, the CCR has collected detailed information on over 3.4 million cases of cancer among Californians diagnosed from 1988 forward, and more than 162 000 new cases are added annually (16). The CCR data give us a unique and complete perspective to study racial and SES differences in disease.
Numerous studies of other chronic diseases have shown that those in a lower socioeconomic class or in a minority class tend to suffer a disproportionate morbidity and mortality. For example, prostate cancer and hypertension are more prevalent and more aggressive in African Americans (17, 18). There is also decreased cancer-specific survival in those with low SES with endometrial cancer (19). We have also shown that there are disparities in presentation and outcomes among thyroid cancer patients in different SES and racial groups.
Earlier we suggested that the causes of these disparities must be multifactorial. Below we review those potential etiologies as they pertain to our outcomes.
Access to care
Some of the most discussed and persuasive etiologies of healthcare disparities are inequality in access to care and/or living in resource-poor neighborhoods (1, 3). There is a well-established perspective that racial discrimination and poverty are the major contributors to unequal health status (20, 21). However, although some disparities can be explained by underlying differences in healthcare access among individuals within vulnerable and less vulnerable groups, such differences do not completely explain persistent gaps in health (22).
There has been an established increasing incidence of thyroid cancer in those with higher access to care; thus, it is not unexpected that in our study we saw that within the white patient population, those with higher SES had a lower chance of presenting with later-stage disease (9). However, once SES status was adjusted for in the multivariable regression (which in theory adjusts for their access to care), the other races had persistent differences in their stage presentation. This suggests that within minority groups, their SES is not the only cause contributing to the differences in presentation and outcomes we have presented here.
In California, racial subsets tend to live in clustered communities of the same racial and socioeconomic demographic. These patient clusters may differ widely on where they live in relation to where they were diagnosed or treated. Thus, those with the same insurance type may have differences in access to care based on where they live. A subsequent study is required to assess this clustering effect.
Difference in disease biology
Inherent differences in tumor biology and/or genetic variances have also been proposed as possible explanations of racial disease disparities. Other diseases have shown differences in disease predilection, such as sickle cell anemia, which is more prevalent among those of sub-Saharan ancestry and in Latin-Americans (23, 24). Also, African Americans present with more prevalent and advanced breast cancer. Some studies suggest this can be partially explained by increased prevalence of obesity and diabetes in that subpopulation (increasing total body adiponectin) as well as differences in progesterone receptor genes (25, 26).
It is also possible that differences in tumor biology or genetic variance explains some of the more advanced thyroid cancers seen in our study's minority populations. It might explain why the API and Hispanic populations seem to have a survival advantage despite presenting with later disease. As an attempt to look at the thyroid cancer tumor biology, we looked at differences in tumor size. Black patients have a larger tumor size on average than other races, which may partially explain their worse stage of presentation and mortality. We found no differences in aggressive WDTC tumor types among races; however, this may have been underpowered because this subtype is likely undercoded.
Our group recently published a study showing obesity as an independent risk factor for more advanced thyroid cancer (7). Obesity is very prevalent in both the black and Hispanic races and could explain some of the more advanced stage differences within those populations. We cannot assess the BMI of the patients in our study, and thus, that is one of this study's limitations. Obesity, however, is not as prevalent in Asian communities, so it could not fully explain the racial disparities observed in that population.
Gender is a strong predictor for thyroid cancer aggressiveness and plays an important role in thyroid cancer prognosis. Males tend to present with later stage and more aggressive thyroid cancers than females, and their hazard ratios are higher for mortality than females (28). This is well documented in the literature and is why sex was included both in mortality and stage regressions (28, 29). Our regressions confirm more aggressive disease and higher mortality in men. This was consistent across all races.
It is also possible that a genetic variation exists among races that could contribute to some of the disparities in presentation that we have shown in this study. Clear evidence of an easily discernible and well-understood genetic contribution to common diseases has provided valuable insights for health management, as in the cases of some breast and colon cancers (20). One study showed differences in thyroid cancer presentation within Asian groups in the state of California, solely based on their country of origin, suggesting that genetic variants might also exist within the same race (30).
Although genes undoubtedly make some contribution to disparities in aggregate group health status, the potential genetic contribution is unknown (20). In fact, many publications have stated that too much emphasis is placed on genetic variations to explain healthcare disparities and suggest that more important are the disparities in SES and environmental impacts on disease prevalence and aggressiveness (20).
Unfortunately, we cannot comprehensively study tumor or genetic differences with this database. Further study on this is warranted.
External sources (provider bias, environmental exposure, and lifestyle)
Another possible contributor to the differences in thyroid cancer outcomes among racial and SES groups is an inherent provider bias when treating those with equal access to care. Substantial evidence indicates that disparities in health status in the United States result largely from longstanding pervasive racial and ethnic discrimination, which can manifest by offering dissimilar diagnostic procedures and/or therapies for the same disease process (20). General experiences with racial/ethnic discrimination are associated with a variety of adverse health outcomes including higher mortality, lower use of cancer screening, elevated blood pressure, and increased depressive symptoms (1). One study noted racially based differences among Medicare beneficiaries in access to and quality of care for colorectal cancer (3). Although we cannot assess provider discrimination in our database, it should be a consideration when interpreting our results.
Differences in environmental exposures may also contribute to the racial and SES disparities observed in our study. We know that minorities are more likely to be exposed to environmental hazards within their own homes from lead paint-coated walls and from indoor pesticides used to control cockroach and rat infestations (20, 31). Jobs open to minorities also often pose serious health risks through exposure to toxic chemicals used in manufacturing or agriculture (20). A link between environmental toxins/pesticides and thyroid cancer has been suggested, but no study large enough to assess that implication has yet been done (32).
An unhealthy lifestyle has been suggested as another possible cause of worse healthcare outcomes and increased risk of cancer (33, 34). Due to longstanding discrimination, ethnic minorities are only recently moving into modern lifestyles and rapidly acquiring lifestyle diseases, such as obesity, cardiovascular disease, and type 2 diabetes, as well as physical, social, and mental disorders linked to misuse of alcohol and of other drugs (35). One paper suggested a dietary impact affecting the prevalence of thyroid nodules and in thyroid cancer among Asian women in northern California (30). Sleep disturbances are also much more prevalent in minority racial groups and have been linked to an increased incidence of thyroid cancer (27, 36).
Summary
Our study has several limitations. The first is that in a study of this magnitude, there can be false-positive findings just by statistical chance alone. However, our CIs are short, which means there is higher precision and accuracy in the estimation of effects. In addition, our survival data are limited by the number of years followed. We had to censor many patients and assume a competed risk strategy when assessing disease-specific survival. We were also somewhat limited in our regression analysis from factors that might explain the racial differences but could not be included, such as obesity, family history, exposure to radiation/carcinogens, and lifestyle. We did not have this information from the registry, unfortunately, so we could not assess whether these factors had an impact on the adjusted analyses. However, a family history of thyroid cancer and a history of radiation exposure are both so rare that we do not think it would matter much in the ultimate analysis, even if they were included. Lastly, we feel that the CCR reflects the thyroid cancer population of the United States very well. However, it may not reflect the worldwide population because there are environmental and genetic impacts that can alter disease presentation between countries.
Conclusion
Racial and socioeconomic factors have a significant influence on presentation and outcomes in WDTC. Black patients and those with low SES have worse outcomes. API and Hispanic patients may have a protective effect on survival despite presenting with more advanced disease.
Further studies are warranted to investigate the mutifactorial etiologies for the disparities noted here as well as to determine necessary interventions to provide for better patient outcomes. We suggest that physicians who diagnose and treat thyroid cancer be aware of the aggressive nature of these thyroid tumors in different racial minorities and be more vigilant with their screening practices.
Acknowledgments
This work was supported in part by NIH/NCRR/NCATS UCLA CTSI Grant UL1TR000124. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
This work was presented as an oral podium presentation at The National Endocrine Society meeting in San Francisco, June 2013.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- API
- Asian/Pacific Islander
- CCR
- California Cancer registry
- CI
- confidence interval
- OR
- odds ratio
- SES
- socioeconomic status
- WDTC
- well-differentiated thyroid cancer.
References
- 1. Shavers VL, Fagan P, Jones D, et al. The state of research on racial/ethnic discrimination in the receipt of health care. Am J Public Health. 2012;102:953–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooper GS, Yuan Z, Landefeld CS, Rimm AA. Surgery for colorectal cancer: race-related differences in rates and survival among Medicare beneficiaries. Am J Public Health. 1996;86:582–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors–an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97:E1579–E1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167 [DOI] [PubMed] [Google Scholar]
- 6. Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the US: review of incidence trends by socioeconomic status within the Surveillance, Epidemiology, and End Results registry, 1980–2008. Thyroid. 2013;23:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harari A, Endo B, Nishimoto S, Ituarte PH, Yeh MW. Risk of advanced papillary thyroid cancer in obese patients. Arch Surg. 2012;147:805–811 [DOI] [PubMed] [Google Scholar]
- 8. Yu GP, Li JC, Branovan D, McCormick S, Schantz SP. Thyroid cancer incidence and survival in the national cancer institute surveillance, epidemiology, and end results race/ethnicity groups. Thyroid. 2010;20:465–473 [DOI] [PubMed] [Google Scholar]
- 9. Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23:885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 11. Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711 [DOI] [PubMed] [Google Scholar]
- 12. cmprsk: Subdistribution analysis of competing risks [computer program]. Version 2.2–6 Vienna, Austria: R Foundation for Statistical Computing; 2013. http://cran.r-project.org/web/packages/cmprsk/index.html [Google Scholar]
- 13. Lim II, Hochman T, Blumberg SN, Patel KN, Heller KS, Ogilvie JB. Disparities in the initial presentation of differentiated thyroid cancer in a large public hospital and adjoining university teaching hospital. Thyroid. 2012;22:269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akinyemiju TF, Soliman AS, Copeland G, Banerjee M, Schwartz K, Merajver SD. Trends in breast cancer stage and mortality in Michigan (1992–2009) by race, socioeconomic status, and area healthcare resources. PLoS One. 2013;8:e61879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, Angelos P, Grogan RH. The Acceleration in Papillary Thyroid Cancer Incidence Rates is Similar Among Racial and Ethnic Groups in the United States. Ann Surg Oncol. 2013;20:2746–2753 [DOI] [PubMed] [Google Scholar]
- 16. California Department of Public Health California Cancer Registry. California Department of Public Health website. http://www.ccrcal.org/ Accessed October 2013
- 17. Ritch CR, Morrison BF, Hruby G, et al. Pathological outcome and biochemical recurrence-free survival after radical prostatectomy in African-American, Afro-Caribbean (Jamaican) and Caucasian-American men: an international comparison. BJU Int. 2013;111(4 Pt B):E186–E190 [DOI] [PubMed] [Google Scholar]
- 18. Flack JM, Okwuosa T, Sudhakar R, Ference B, Levy P. Should African Americans Have a Lower Blood Pressure Goal than Other Ethnic Groups to Prevent Organ Damage? Curr Cardiol Rep. 2012;14:660–666 [DOI] [PubMed] [Google Scholar]
- 19. Long B, Liu FW, Bristow RE. Disparities in uterine cancer epidemiology, treatment, and survival among African Americans in the United States. Gynecol Oncol. 2013;130:652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sankar P, Cho MK, Condit CM, et al. Genetic research and health disparities. JAMA. 2004;291:2985–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davis K. Inequality and access to health care. Milbank Q. 1991;69:253–273 [PubMed] [Google Scholar]
- 22. Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, Fine MJ. Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health. 2006;96:2113–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koopmans J, Ross LF. Identification and management of sickle cell trait by young physicians. J Natl Med Assoc. 2012;104:299–304 [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Kennedy J, Caggana M, et al. Sickle cell disease incidence among newborns in New York State by maternal race/ethnicity and nativity. Genet Med. 2013;15:222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaklamani VG, Hoffmann TJ, Thornton TA, et al. Adiponectin pathway polymorphisms and risk of breast cancer in African Americans and Hispanics in the Women's Health Initiative. Breast Cancer Res Treat. 2013;139:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gabriel CA, Mitra N, Demichele A, Rebbeck T. Association of progesterone receptor gene (PGR) variants and breast cancer risk in African American women. Breast Cancer Res Treat. 2013;139:833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baldwin CM, Ervin AM, Mays MZ, et al. Sleep disturbances, quality of life, and ethnicity: the Sleep Heart Health Study. J Clin Sleep Med. 2010;6:176–183 [PMC free article] [PubMed] [Google Scholar]
- 28. Mitchell I, Livingston EH, Chang AY, et al. Trends in thyroid cancer demographics and surgical therapy in the United States. Surgery. 2007;142:823–828; discussion 828.e1 [DOI] [PubMed] [Google Scholar]
- 29. Salvesen H, Njølstad PR, Akslen LA, Albrektsen G, Søreide O, Varhaug JE. Papillary thyroid carcinoma: a multivariate analysis of prognostic factors including an evaluation of the p-TNM staging system. Eur J Surg. 1992;158:583–589 [PubMed] [Google Scholar]
- 30. Haselkorn T, Stewart SL, Horn-Ross PL. Why are thyroid cancer rates so high in southeast asian women living in the United States? The bay area thyroid cancer study. Cancer Epidemiol Biomarkers Prev. 2003;12:144–150 [PubMed] [Google Scholar]
- 31. Berkowitz GS, Obel J, Deych E, et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leux C, Guénel P. Risk factors of thyroid tumors: role of environmental and occupational exposures to chemical pollutants. Rev Epidemiol Sante Publique. 2010;58:359–367 [DOI] [PubMed] [Google Scholar]
- 33. Redmond N, Baer HJ, Hicks LS. Health behaviors and racial disparity in blood pressure control in the national health and nutrition examination survey. Hypertension. 2011;57:383–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vidrine JI, Stewart DW, Stuyck SC, et al. Lifestyle and cancer prevention in women: knowledge, perceptions, and compliance with recommended guidelines. J Womens Health (Larchmt). 2013;22:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet. 2009;374:65–75 [DOI] [PubMed] [Google Scholar]
- 36. Luo J, Sands M, Wactawski-Wende J, Song Y, Margolis KL. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women's Health Initiative. Am J Epidemiol. 2013;177:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]