Abstract
Context:
Neonatal severe hyperparathyroidism (NSHPT) is a severe form of familial hypocalciuric hypercalcemia characterized by severe hypercalcemia and skeletal demineralization. In most cases, NSHPT is due to biallelic loss-of-function mutations in the CASR gene encoding the calcium-sensing receptor (CaSR), but some patients have heterozygous mutations. Conventional treatment consists of iv saline, bisphosphonates, and parathyroidectomy.
Objective:
The aim of this project was to characterize the molecular basis for NSHPT in an affected newborn and to describe the response to monotherapy with cinacalcet.
Methods:
Clinical and biochemical features were monitored as cinacalcet therapy was initiated and maintained. Genomic DNA was obtained from the proband and parents. The CASR gene was amplified by PCR and sequenced directly.
Results:
The patient was a full-term male who developed hypotonia and respiratory failure soon after birth. He was found to have multiple fractures and diffuse bone demineralization, with a marked elevation in serum ionized calcium (1.99 mmol/L) and elevated serum levels of intact PTH (1154 pg/mL); serum 25-hydroxyvitamin D was low, and fractional excretion of calcium was reduced. The serum calcium level was not reduced by iv saline infusion. Based on an extensive family history of autosomal dominant hypercalcemia, a diagnosis of NSHPT was made, and cinacalcet therapy was initiated with a robust and durable effect. Molecular studies revealed a heterozygous R185Q missense mutation in the CASR in the patient and his father, whereas normal sequences for the CASR gene were present in the patient's mother.
Conclusions:
We describe the first use of cinacalcet as monotherapy for severe hypercalcemia in a newborn with NSHPT. The rapid and durable response to cinacalcet suggests that a trial of calcimimetic therapy should be considered early in the course of NSHPT.
Neonatal severe hyperparathyroidism (NSHPT) is a life-threatening form of familial hypocalciuric hypercalcemia (FHH) and is characterized by severe hypercalcemia, skeletal demineralization, low fractional excretion of urinary calcium, and elevated serum levels of PTH. NSHPT manifests within the first few days of life and is also associated with hypotonia, intestinal dysmotility, and failure to thrive; without appropriate treatment, NSHPT has a mortality rate that is greater than 50% (1, 2).
Loss-of-function mutations in the CASR gene (3) located at 3p13.3 (OMIM 601199) are the most common cause of FHH and NSHPT. The CASR gene encodes the calcium-sensing receptor (CaSR), a G protein-coupled receptor that is highly expressed in the parathyroid and kidney. More than 200 mutations have been described for the CASR gene (http://www.casrdb.mcgill.ca), and most of them are inactivating mutations that are located within the calcium binding site of the receptor (4–7). In most cases, these mutations reduce the sensitivity of the CaSR to extracellular calcium, with consequent increased parathyroid secretion of PTH and decreased renal excretion of calcium. FHH (OMIM 145980) typically results from heterozygous CASR mutations and in some cases biallelic mutations (3), and it is generally asymptomatic. By contrast, NSHPT (OMIM 239200) is characterized by a severe phenotype that manifests very early in life. Although NSHPT typically results from homozygous CASR mutations, in some cases only a single abnormal allele is present (1, 4–9).
Because NSHPT is associated with life-threatening hypercalcemia, urgent treatment is necessary. The most commonly used therapies for NSHPT include iv saline, iv bisphosphonates, and parathyroidectomy (2, 10). Intravenous saline results in only modest and temporary improvements in serum calcium such that bisphosphonates and/or parathyroidectomy are usually necessary. In vitro characterization of parathyroid cells from some patients with NSHPT (11) as well as studies of cell lines expressing heterologous mutant CaSRs (11, 12) have shown that many abnormal CaSRs respond to high levels of extracellular calcium. Moreover, calcium responsiveness is amplified by cotreatment of cells with type 2 calcimimetics (13). Cinacalcet, a type 2 calcimimetic agent, has been shown to be an effective treatment for adult patients with hypercalcemia due to primary and tertiary hyperparathyroidism as well as parathyroid carcinoma (11, 14, 15); it has also been attempted in some cases of adult CASR mutations (16). Given our knowledge of the molecular defect in NSHPT, these studies indicate that cinacalcet might reduce PTH secretion in at least some patients with NSHPT. Support for this hypothesis comes from two recent case reports that described the successful use of cinacalcet as adjunctive therapy in patients with NSHPT who were first treated with a bisphosphonate (17, 18). Here we describe the first case of NSHPT that has been successfully treated with cinacalcet alone.
Subjects and Methods
Subjects
The index case and his parents were included in the study. All subjects underwent clinical, biochemical, and genetic evaluation after providing written informed consent. Blood and urine samples for biochemical analyses were obtained in the fasting state. Genetic studies were approved by the institutional review board of The Children's Hospital of Philadelphia. The family provided written informed consent for use of cinacalcet as an experimental treatment.
Laboratory methods
Intact PTH was measured by the Immulite 2000 immunoassay (Siemens). Serum concentrations of 25(OH)D were measured by liquid chromatography-tandem mass spectrometry, and serum concentrations of 1,25(OH)2 D were measured by RIA (DiaSorin). GenomicDNA was isolated from peripheral blood leukocytes by standard techniques. The coding exons 2 through 7 and the flanking intronic sequences of the CASR gene were amplified by PCR, and each amplicon was subjected to direct sequencing as previously described (3). Sequencing was performed on both strands.
Case description
The patient was 2 days old at the time of presentation; he was the product of a full-term gestation to a nulligravid, healthy female. Pregnancy was complicated by oligohydramnios, but was otherwise unremarkable. The child appeared well initially, but became lethargic, hypotonic, and then apneic on his second day of life. He was transferred to the newborn intensive care unit where he was intubated and a sepsis evaluation was enacted. On examination his vital signs were normal apart from periodic bradycardic episodes; he had no facial dysmorphology or obvious musculoskeletal abnormalities. He was notably hypotonic on examination, but the remainder of his examination was unremarkable.
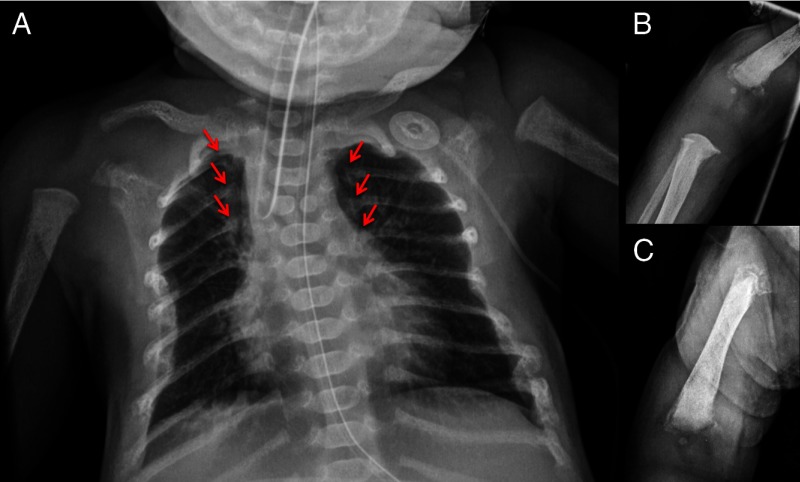
A chest radiograph revealed diffuse demineralization and multiple healing rib fractures (Figure 1). Laboratory evaluation revealed a markedly elevated serum ionized calcium of 1.99 mmol/L (normal, 1 to 1.17 mmol/L) with a serum level of total calcium of 3.23 mmol/L (normal, 2.33 to 2.74 mmol/L) and elevated serum magnesium concentration (1.1 mmol/L; normal, 0.62 to 1.03 mmol/L), whereas the serum concentration of phosphorus was reduced (0.94 mmol/L; normal, 1.55 to 2.65 mmol/L). The serum concentration of 25-hydroxyvitamin D [25(OH)D] was low at 10.9 nmol/L (normal, 32 to 80 nmol/L), and serum concentrations of 1,25-dihydroxyvitamin D [1,25(OH)2D] (280.8 pmol/L; normal, 39 to 195 pmol/L) and intact PTH (1154 pg/mL; normal, <60 pg/mL) were markedly increased. The fractional excretion of calcium was 0.003 (normal, >0.01). A skeletal survey demonstrated multiple rib fractures, diffuse bone demineralization, and chondrodystrophy in the distal humeri and femora.
Figure 1.
Radiographs of the patient demonstrating diffuse demineralization (A), multiple rib fractures (arrows), and chondrodystrophy of the distal humerus (B) and femur (C). A butterfly vertebrae was also noted on the chest radiograph.
The proband's father, paternal grandfather, paternal aunt, and at least one paternal cousin also had a history of hypercalcemia, although none required aggressive interventions during early childhood. The serum calcium of the patient's mother was 2.35 mmol/L during pregnancy, and his father's serum calcium was found to be elevated at 3.23 mmol/L. There was no history of consanguinity.
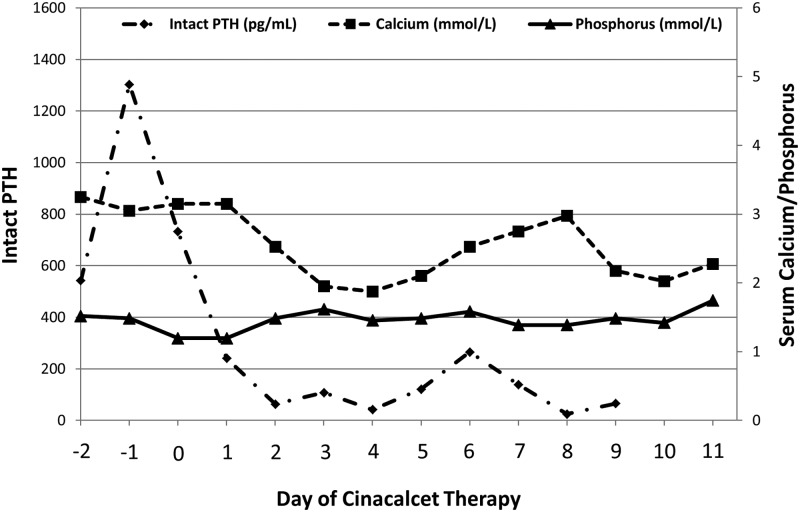
The patient was initially treated with iv saline without significant effect on the serum calcium level. Potassium phosphate (2 mmol/kg/d) was administered to normalize the serum phosphorus concentration without effect on hypercalcemia. Cholecalciferol was administered at standard doses (400 IU/d), resulting in a normalization of 25(OH)D to 51.6 nmol/mL by the 36th day of life. Because the family expressed reservations about medical treatment with iv bisphosphonates and surgical parathyroidectomy, cinacalcet was offered as an experimental alternative. After obtaining informed consent, cinacalcet therapy was begun at 0.4 mg/kg/d (6 mg/m2/d) divided in two oral doses per day. Cinacalcet treatment led to marked reductions in serum concentrations of ionized calcium, 1,25(OH)2D, and intact PTH over the next 4 days (Figure 2); the fractional excretion of calcium remained low. As the patient's serum calcium level normalized, his muscle tone and overall clinical condition improved significantly; the response was so effective that the dose was briefly held due to concern for developing hypocalcemia. The medication was resumed and then continued until the calcium levels stabilized, and he was then discharged home at 21 days of life.
Figure 2.
Serum levels of intact PTH (normal, <60 pg/mL), calcium (normal, 2.33 to 2.74 mmol/L), and phosphorus (normal, 1.55 to 2.65 mmol/L) during the initiation of cinacalcet. Cinacalcet was initiated on day zero and, because of the robust response, it was briefly held on day 4 and restarted on day 5.
At 3 months of age, the patient was diagnosed with severe gastroenteritis due to rotavirus and was unable to tolerate oral medications. Subsequently, his serum calcium level increased to 3.63 mmol/L as his intact PTH level increased to 78.8 pg/mL; the serum phosphorus was 1.55 mmol/L. In this context, the patient again developed hypotonia along with periodic apnea and bradycardia. Because cinacalcet could not be tolerated, repeat trials of iv saline as well as escalating doses of calcitonin were used, but had no effect on his elevated serum calcium levels. Within days he had recovered sufficiently to tolerate oral feeding, and we were able to restart cinacalcet therapy, which again led to rapid reduction in serum calcium levels and resolution of irritability, hypotonia, and episodic apnea and bradycardia.
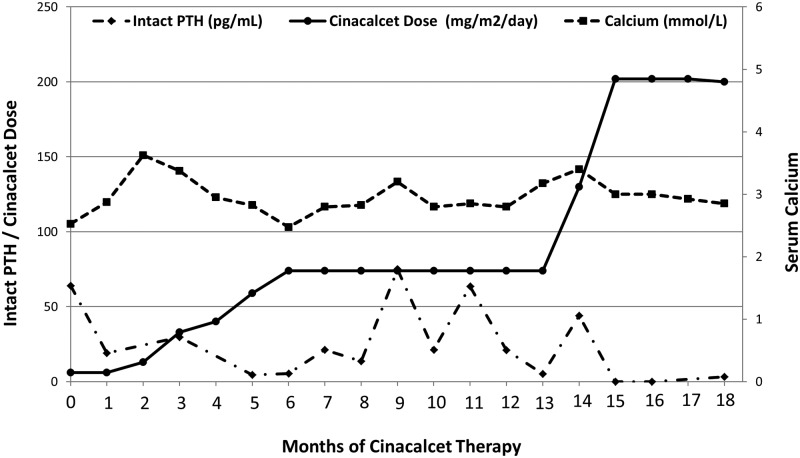
The patient was successfully managed with cinacalcet through the first 18 months of his life; he never required bisphosphonate therapy or surgical intervention. During the course of this treatment, his serum calcium levels were intermittently elevated despite reasonable suppression of intact PTH (Figure 3), but the serum levels of 1,25(OH)2D remained elevated. The initial effective dose of cinacalcet was 0.4 mg/kg/d (6 mg/m2/d) divided twice per day—a dose selected due to efficacy in young children treated for secondary hyperparathyroidism (19). This dose was gradually increased through the course of follow-up to a final dose of 9.6 mg/kg/d (202 mg/m2/d) divided three times per day in an attempt to normalize his lab values. Follow-up radiographs demonstrated improvement in bone mineralization and no further fractures.
Figure 3.
Serum levels of intact PTH (normal, <60 pg/mL), calcium (normal, 2.33 to 2.74 mmol/L), and phosphorus (normal, 1.55 to 2.65 mmol/L) over 18 months of therapy with cinacalcet.
Results
Sequence analysis of CASR gene
Genetic sequencing identified that the proband and his father were heterozygous for a known guanine to adenine substitution at nucleotide position 554 (c.554G>A) in exon 4 that led to replacement of a conserved arginine (CGA) by glutamine (CAA) at codon 185 (R185Q) (18). Exon 4, which encodes part of the extracellular domain of the CaSR, is the most common site of mutation in FHH (http://www.casrdb.mcgill.ca); this particular amino acid substitution, R185Q, is located in the extracellular domain of the CaSR and has been shown to behave as a dominant inhibitor of wild-type CaSR protein (20). The proband's mother had wild-type CASR genotype.
Discussion
It is well established that most children and adults with heterozygous CASR mutations will manifest lifelong mild hypercalcemia that is expected to cause little or no morbidity. However, in some cases heterozygous mutations can produce the more severe phenotype of NSHPT (1). The R185Q mutation that we report here is among the most potent (18, 20) heterozygous mutations to be associated with severe hypercalcemia and/or NSHPT, which likely reflects its very unusual dominant inhibitor properties.
NSHPT can result in death if hypercalcemia is not treated effectively, and most children with this disorder are treated with bisphosphonate therapy and/or parathyroid surgery as life-saving interventions. Here we describe the first patient with NSHPT to be treated with cinacalcet monotherapy. This patient did not respond to iv saline, and although conventional therapy would have been iv bisphosphonates, the parents rejected this option and sought an alternative. Hence, we utilized cinacalcet in a manner that had been previously shown efficacious as an adjunctive therapy (17, 18) and found that cinacalcet was an effective monotherapy that allowed us to avoid surgical intervention. Repletion of 25(OH)D levels was also achieved with cholecalciferol, but this did not significantly impact serum calcium levels.
Although cinacalcet did reduce serum levels of calcium and PTH and facilitate healing of the skeletal lesions, the infant's serum calcium levels remained mildly elevated through the course of the year despite increasing doses of cinacalcet. Use of cinacalcet generally relieved symptoms of hypercalcemia that emerged in this child each time serum calcium levels exceeded 3.125 mmol/L. Serum levels of PTH were low or undetectable when checked 1 to 2 hours after a dose of cinacalcet, but at later times the PTH level was normal or even mildly elevated. By contrast, serum levels of 1,25(OH)2D were consistently at the upper limit of normal or frankly elevated, and correlated with hypercalcemia. Although a broad range of cinacalcet doses and dosing intervals were utilized, we were unsuccessful in fully suppressing this patient's 1,25(OH)2D level and achieving a sustained “normal” serum calcium level. Despite this persistence of mild hypercalcemia after the first few months of life, the patient did not manifest symptoms of hypercalcemia and apparently achieved a clinical status that was similar to that of his asymptomatic affected father. Curiously, due to persistence of hypercalcemia and periodic symptomatic hypercalcemia despite high doses of cinacalcet, we have not yet been able to withdraw the medication and are uncertain when a withdrawal will be tolerated.
Our inability to normalize the serum concentration of calcium completely in this infant with cinacalcet is similar to the experiences reported by others (17, 18) who used cinacalcet in children with R185Q CASR mutations, although the doses that we have used are much higher. These results contrast with the effects of cinacalcet in adults with primary hyperparathyroidism (22–25) and children with FHH (M.A.L., unpublished observations), in whom serum calcium levels are typically normalized with much lower doses of cinacalcet. We suspect that the principal obstacle to achieving eucalcemia in patients with the R185Q mutation is the dominant inhibitor effect of the abnormal CaSR protein, which markedly reduces the sensitivity of diverse cells to extracellular calcium. Although we were able to reduce PTH levels transiently after each dose of cinacalcet, PTH levels consistently increased over the next several hours. This, as well as impaired CaSR signaling in the kidney, may have mediated the persistently elevated serum levels of 1,25(OH)2D and subsequent mild hypercalcemia in this patient. Finally, the loss of CaSR signaling in the skeleton, and possibly on calcitonin secretion (26), in patients with the R185Q mutation may also have contributed to the hypercalcemia. Alternatively, we cannot exclude the possibility that absorption of the drug was inefficient in our patient, or an unexpected effect of age on metabolism of cinacalcet, because it is well established that children may metabolize medications at a different rate than do adults. In this case, we found that dosing three times per day was more successful than twice per day, but the final dose required in our young patient was far in excess of what is required in adults with primary hyperparathyroidism. An additional possibility is that this child may have had an additional difference in other alleles that we did not evaluate. Recent studies have identified that mutations in AP2S1 and GNA11 may also impact calcium sensing, and these could have resulted in a more severe phenotype for this child (27, 28).
Although we and others have reported cinacalcet to be effective in select patients with NSHPT, it is likely that not all patients would be expected to respond to this therapy. This will be most evident in cases where biallelic mutations completely abrogate production of cell surface CaSRs (29). A recent case report clearly demonstrated the inefficacy of cinacalcet in a child with a severe homozygous mutation (30). Therefore, we conclude that a response to cinacalcet should not be expected in all cases, and its effect should be monitored closely. We expect that clinical responsiveness should be quickly determined by examining the effect of a test dose of cinacalcet on the level of PTH. Because cinacalcet has a dose-dependent effect on modulating PTH, it is important to monitor serum calcium levels closely and avoid hypocalcemia, which may be life-threatening. This is particularly salient considering the death of a young person during a clinical trial involving cinacalcet therapy earlier this year; as a result of this incident, clinical trials of cinacalcet use in children have been placed on hold (21).
In conclusion, the rapid and durable clinical and biochemical response to cinacalcet in patients with NSHPT justifies consideration of a trial of calcimimetic therapy in patients with NSHPT to avoid parathyroidectomy and to minimize the need for repeated administration of iv bisphosphonates.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 2R01DK079970 and T32DK063688-10.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CaSR
- calcium-sensing receptor
- FHH
- familial hypocalciuric hypercalcemia
- NSHPT
- neonatal severe hyperparathyroidism
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- 25(OH)D
- 25-hydroxyvitamin D.
References
- 1. Egbuna OI, Brown EM. Hypercalcaemic and hypocalcaemic conditions due to calcium-sensing receptor mutations. Best Pract Res Clin Rheumatol. 2008;22:129–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pearce S, Steinmann B. Casting new light on the clinical spectrum of neonatal severe hyperparathyroidism. Clin Endocrinol (Oxf). 1999;50:691–693 [DOI] [PubMed] [Google Scholar]
- 3. Lietman SA, Tenenbaum-Rakover Y, Jap TS, et al. A novel loss-of-function mutation, Gln459Arg, of the calcium-sensing receptor gene associated with apparent autosomal recessive inheritance of familial hypocalciuric hypercalcemia. J Clin Endocrinol Metab. 2009;94:4372–4379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakker RV. Diseases associated with the extracellular calcium-sensing receptor. Cell Calcium. 2004;35:275–282 [DOI] [PubMed] [Google Scholar]
- 5. Gunn IR, Gaffney D. Clinical and laboratory features of calcium-sensing receptor disorders: a systematic review. Ann Clin Biochem. 2004;41:441–458 [DOI] [PubMed] [Google Scholar]
- 6. Bouschet T, Henley JM. Calcium as an extracellular signalling molecule: perspectives on the calcium sensing receptor in the brain. C R Biol. 2005;328:691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cole De, Forsythe CR, Dooley JM, Grantmyre EB, Salisbury SR. Primary neonatal hyperparathyroidism: a devastating neurodevelopmental disorder if left untreated. J Craniofac Genet Dev Biol. 1990;10:205–214 [PubMed] [Google Scholar]
- 8. Brown EM. Clinical lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3:122–133 [DOI] [PubMed] [Google Scholar]
- 9. Brown EM. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem. 2007;45:139–167 [DOI] [PubMed] [Google Scholar]
- 10. Waller S, Kurzawinski T, Spitz L, et al. Neonatal severe hyperparathyroidism: genotype/phenotype correlation and the use of pamidronate as rescue therapy. Eur J Pediatr. 2004;163:589–594 [DOI] [PubMed] [Google Scholar]
- 11. Brown EM. Clinical utility of calcimimetics targeting the extracellular calcium-sensing receptor (CaSR). Biochem Pharmacol. 2010;80:297–307 [DOI] [PubMed] [Google Scholar]
- 12. Zajickova K, Vrbikova J, Canaff L, Pawelek PD, Goltzman D, Hendy GN. Identification and functional characterization of a novel mutation in the calcium-sensing receptor gene in familial hypocalciuric hypercalcemia: modulation of clinical severity by vitamin D status. J Clin Endocrinol Metab. 2007;92:2616–2623 [DOI] [PubMed] [Google Scholar]
- 13. Lu JY, Yang Y, Gnacadja G, Christopoulos A, Reagan JD. Effect of the calcimimetic R-568 [3-(2-chlorophenyl)-N-((1R)-1-(3-methoxyphenyl)ethyl)-1-propanamine] on correcting inactivating mutations in the human calcium-sensing receptor. J Pharmacol Exp Ther. 2009;331:775–786 [DOI] [PubMed] [Google Scholar]
- 14. Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:135–141 [DOI] [PubMed] [Google Scholar]
- 15. Li D, Shao L, Zhou H, Jiang W, Zhang W, Xu Y. The efficacy of cinacalcet combined with conventional therapy on bone and mineral metabolism in dialysis patients with secondary hyperparathyroidism: a meta-analysis. Endocrine. 2013;43:68–77 [DOI] [PubMed] [Google Scholar]
- 16. Festen-Spanjer B, Haring CM, Koster JB, Mudde AH. Correction of hypercalcaemia by cinacalcet in familial hypocalciuric hypercalcaemia. Clin Endocrinol (Oxf). 2008;68:324–325 [DOI] [PubMed] [Google Scholar]
- 17. Wilhelm-Bals A, Parvex P, Magdelaine C, Girardin E. Successful use of bisphosphonate and calcimimetic in neonatal severe primary hyperparathyroidism. Pediatrics. 2012;129:e812–e816 [DOI] [PubMed] [Google Scholar]
- 18. Reh CM, Hendy GN, Cole DE, Jeandron DD. Neonatal hyperparathyroidism with a heterozygous calcium-sensing receptor (CASR) R185Q mutation: clinical benefit from cinacalcet. J Clin Endocrinol Metab. 2011;96:E707–E712 [DOI] [PubMed] [Google Scholar]
- 19. Platt C, Inward C, McGraw M, et al. Middle-term use of Cinacalcet in paediatric dialysis patients. Pediatr Nephrol. 2010;25:143–148 [DOI] [PubMed] [Google Scholar]
- 20. Obermannova B, Banghova K, Sumník Z, et al. Unusually severe phenotype of neonatal primary hyperparathyroidism due to a heterozygous inactivating mutation in the CASR gene. Eur J Pediatr. 2009;168:569–573 [DOI] [PubMed] [Google Scholar]
- 21. U.S. Food and Drug Administration Sensipar (cinacalcet hydrochloride): Drug Safety Communication. FDA suspends pediatric clinical trials after report of death. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm341255.htm. Published February 26, 2013 Accessed November 14, 2013
- 22. Marcocci C, Cetani F. Update on the use of cinacalcet in the management of primary hyperparathyroidism. J Endocrinol Invest. 2012;35:90–95 [DOI] [PubMed] [Google Scholar]
- 23. Dillon ML, Frazee LA. Cinacalcet for the treatment of primary hyperparathyroidism. Am J Ther. 2011;18:313–322 [DOI] [PubMed] [Google Scholar]
- 24. Khan A, Grey A, Shoback D. Medical management of asymptomatic primary hyperparathyroidism: proceedings of the Third International Workshop. J Clin Endocrinol Metab. 2009;94:373–381 [DOI] [PubMed] [Google Scholar]
- 25. Marcocci C, Chanson P, Shoback D, et al. Cinacalcet reduces serum calcium concentrations in patients with intractable primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:2766–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kantham L, Quinn SJ, Egbuna OI, et al. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab. 2009;297:E915–E923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nesbit MA, Hannan FM, Howles SA, et al. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368:2476–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nesbit MA, Hannan FM, Howles SA, et al. Mutations in AP2S1 cause familial hypocalciuric hypercalcemia type 3. Nat Genet. 2013;45:93–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rus R, Haag C, Bumke-Vogt C, et al. Novel inactivating mutations of the calcium-sensing receptor: the calcimimetic NPS R-568 improves signal transduction of mutant receptors. J Clin Endocrinol Metab. 2008;93:4797–4803 [DOI] [PubMed] [Google Scholar]
- 30. García Soblechero E, Ferrer Castillo MT, Jiménez Crespo B, Domínguez Quintero ML, González Fuentes C. Neonatal hypercalcemia due to a homozygous mutation in the calcium-sensing receptor: failure of cinacalcet. Neonatology. 2013;104:104–108 [DOI] [PubMed] [Google Scholar]