Abstract
Context:
Anaplastic thyroid carcinoma (ATC) is an aggressive malignancy having no effective treatment. Laminin subunit-γ-2 (LAMC2) is an epithelial basement membrane protein involved in cell migration and tumor invasion and might represent an ideal target for the development of novel therapeutic approaches for ATC.
Objective:
The objective of the investigation was to study the role of LAMC2 in ATC tumorigenesis.
Design:
LAMC2 expression was evaluated by RT-PCR, Western blotting, and immunohistochemistry in tumor specimens, adjacent noncancerous tissues, and cell lines. The short hairpin RNA (shRNA) approach was used to investigate the effect of LAMC2 knockdown on the tumorigenesis of ATC.
Results:
LAMC2 was highly expressed in ATC samples and cell lines compared with normal thyroid tissues. Silencing LAMC2 by shRNA in ATC cells moderately inhibited cell growth in liquid culture and dramatically decreased growth in soft agar and in xenografts growing in immunodeficient mice. Silencing LAMC2 caused cell cycle arrest and significantly suppressed the migration, invasion, and wound healing of ATC cells. Rescue experiments by overexpressing LAMC2 in LAMC2 knockdown cells reversed the inhibitory effects as shown by increased cell proliferation and colony formation. Microarray data demonstrated that LAMC2 shRNA significantly altered the expression of genes associated with migration, invasion, proliferation, and survival. Immunoprecipitation studies showed that LAMC2 bound to epidermal growth factor receptor (EGFR) in the ATC cells. Silencing LAMC2 partially blocked epidermal growth factor-mediated activation of EGFR and its downstream pathway. Interestingly, cetuximab (an EGFR blocking antibody) or EGFR small interfering RNA additively enhanced the antiproliferative activity of the LAMC2 knockdown ATC cells compared with the control cells.
Conclusions:
To our knowledge, this is the first report investigating the effect of LAMC2 on cell growth, cell cycle, migration, invasion, and EGFR signaling in ATC cells, suggesting that LAMC2 may be a potential therapeutic target for the treatment of ATC.
Thyroid cancer accounts for approximately 0.5%–1% of all human malignancies and is the most common cancer of the endocrine system (1). Anaplastic thyroid cancers (ATCs) are undifferentiated tumors of the thyroid follicular epithelium and account for 1%–2% of all thyroid cancers. ATCs have a poor prognosis due to their extremely aggressive nature and resistance to treatment. Therefore, new therapeutic targets are needed to improve the clinical care of these patients. Laminins are members of a family of the basement membrane proteins implicated in a variety of biological functions such as cell adhesion, differentiation, migration, neurite outgrowth, and metastasis. Laminin-332 (previously known as laminin-5) is an essential adhesive component of epithelial basement membrane, which helps to control cell migration of epithelial cells in normal tissues (2–4). Laminin-332 is composed of nonidentical chains of laminin-α (α3), -β (β3), and -γ (γ2), resulting in a heterotrimeric glycoprotein (5). Human laminin subunit-γ2 gene (known as LAMC2) is located on chromosome 1q25-q31. A large number of immunohistochemical studies showed strong expression of LAMC2 in many human cancers including carcinomas of the pancreas (6, 7), stomach (8), tongue (9), colorectal (10, 11), lung (12), cervix (13), and esophagus (squamous) as well as melanoma. Expression of LAMC2 is especially robust at the invasive front of tumors. An elevated level of LAMC2 in human cancers is associated with a poor survival (7, 9), recurrence (14), and metastasis (15).
Signaling by the epidermal growth factor receptor (EGFR) plays an important role in the behavior of malignant cells in a variety of human tumors by increasing proliferation, decreasing apoptosis and enhancing tumor cell motility and angiogenesis. Increased expression of epidermal growth factor (EGF) and EGFR has been detected in 58%–87% of ATC when compared with normal tissue, and this pathway has been proposed to be an important driver of proliferation and metastasis of thyroid carcinoma (16–18). Preclinical investigations have shown that EGF can stimulate proliferation and enhance migration and invasiveness of thyroid cancers (19–21). Also, studies have demonstrated that laminin-332 can interact with α6 β4-integrin to promote the activation of phosphatidylinositol 3-kinase and tumor invasion (22). Domain III of LAMC2 is composed of EGF-like repeats, and binding of a recombinant DIII fragment to EGFR can stimulate downstream signaling (MAPK), resulting in cell migration in breast carcinoma (23). The present investigation reveals the dramatic role that LAMC2 has in ATC.
Materials and Methods
Patient samples
Paraffin-embedded ATC and adjacent noncancerous tissue (ANCT) were obtained from the Department of Pathology, University of California, Los Angeles (Los Angeles, California). In addition, fresh-matched ATC and ANCT were obtained from the National University Hospital (Singapore). All surgical specimens were collected after obtaining informed consent from the patients under the terms and conditions approved by the institutional ethical committee.
Cell culture and antibodies
The cell culture and antibodies are described in Supplemental Materials and Methods, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org. STR profiling of the ATC cell lines are described in Supplemental Table 1 (24).
RT-PCR analysis and quantitative real-time PCR (qRT-PCR)
RT-PCR analysis and qRT-PCR are described in Supplemental Materials and Methods. qRT-PCR primer sequences are detailed in Supplemental Table 2.
Immunofluorescence assay
HTH83, TL3, SW1736, and CAL62 ATC cells were used for the immunofluorescence assays as described earlier (25) (see Supplemental Materials and Methods).
Immunoblot analysis
Cell lysates were prepared using ProteoJET mammalian cell lysis reagent (Fermentas) and 1× protease inhibitor mixture (Roche Molecular Biochemicals). Immunoblotting was performed as described earlier (26). ImageJ software (National Institutes of Health, Bethesda, Maryland) was used for the densitometric analysis of Western blots.
Immunohistochemistry
Paraffin-embedded sections were cut at 4 μm thickness and subjected to immunohistochemical analysis as previously described (27) (see Supplemental Materials and Methods).
Short hairpin RNA (shRNA) transfection and stable transduction
shRNA is described in Supplemental Materials and Methods.
Small interfering RNA (siRNA) transfection
siRNA transfection is described in Supplemental Materials and Methods.
Cellular proliferation, colony formation assay for clonogenic growth on plastic and soft agar, cell migration, invasion, and wound-healing assay
Cellular proliferation, colony formation assay for clonogenic growth on plastic and soft agar, cell migration, invasion, and wound-healing assay are described in Supplemental Materials and Methods.
Cell cycle analysis by flow cytometry
Cultured cells were trypsinized and washed twice with cold PBS and fixed with 70% ethanol (precooled at −20°C) at 4°C overnight. The cell cycle analysis was performed as described earlier (26).
Xenograft model
The animal studies were approved by the National University of Singapore Institutional Animal Care and Use Committee, and details are described in Supplemental Materials and Methods.
Cloning of LAMC2 and coimmunoprecipitation
For cloning of full-length LAMC2 and coimmunoprecipitation, see Supplemental Materials and Methods.
Microarray and data analysis
Microarray and data analysis are described in Supplemental Materials and Methods.
Statistical analysis
All experiments were repeated at least three times. Statistical significance was determined by unpaired Student t test using statistical software package SPSS (version 16.0; SPSS Inc). We used ANOVA when comparing more than two groups of a data set. A value of P < .05 was considered statistically significant. The correlation between the expression levels of LAMC2 and EGFR was analyzed using the Pearson's R correlation test.
Results
Expression analysis using microarray database
Relative expression of LAMC2 was examined in different histotypes of thyroid carcinoma, using microarray database GSE27155 comprising 95 human thyroid cancer samples (papillary, follicular, medullary, and anaplastic thyroid carcinoma) and four normal thyroid samples downloaded from the Gene Expression Omnibus database. We observed that 97% of thyroid tumor samples showed significantly (P < .01) higher expression of LAMC2 as compared with normal thyroid specimens (Supplemental Figure 1A).
LAMC2 mRNA and protein expression in ATC cell lines and samples from patients
LAMC2 mRNA was highly expressed in both ATC cell lines and patient samples compared with ANCT (Figure 1, A and B). LAMC2 coding sequences showed no mutations in the HTH83, TL3, SW1736, and CAL62 cells by Sanger sequencing. A strong cytoplasmic localization of LAMC2 protein was observed in fixed and permeabilized ATC cells (HTH83, TL3, SW1736, and CAL62) by indirect immunofluorescence (Figure 1C). LAMC2 protein expression of these cells paralleled their RNA levels (Figure 1, D and E). Immunohistochemistry also showed positive diffuse cytoplasmic staining of LAMC2 in 70% (7 of 10) ATC tissue specimens, whereas very little or no expression was observed in ANCT tissues (0 of 10), and cells stained with control IgG failed to show reactivity (Figure 1F).
Figure 1.
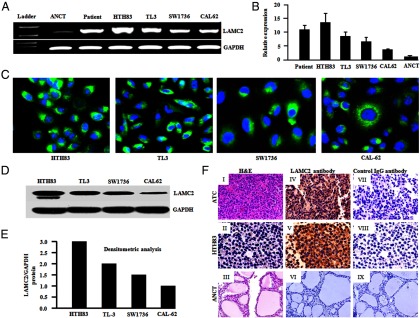
LAMC2 mRNA and protein expression in ATC cell lines and ATC tissue specimens. A, RT-PCR analysis demonstrated strong LAMC2 mRNA expression in human ATC cells (HTH83, TL3, SW1736, CAL62, and ATC351) and ATC tissue samples, whereas low LAMC2 mRNA expression was detected in the normal thyroid (ANCT) tissue sample. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. B, Real-time PCR analysis showed the relative expression of LAMC2 in ATC cell lines and ANCT. Data represent the mean ± SD of three independent experiments. C, Indirect immunofluorescence assay detected endogenous LAMC2 protein expression in fixed/permeabilized ATC cell lines; 4′,6′-diamino-2-phenylindole (DAPI) stains nuclei. D, Western blot analysis of ATC cell line demonstrated LAMC2 antibody specifically recognizes a band of 150 kDa corresponding to LAMC2 protein. E, Densitometric analysis of Western blot showed LAMC2 protein expression in ATC cell lines. F, Immunohistochemical analysis of ATC specimen and HTH83 cell line for the detection of LAMC2 protein. I, ATC sample. II, HTH83. III, ANCT: sections stained with hematoxylin and eosin (H&E). Cytoplasmic localization of the LAMC2 was observed in ATC specimen (IV) and HTH83 cells with anti-LAMC2 antibody (V), whereas ANCT tissues showed no reactivity (VI). Negligible staining was observed in the serial sections of the ATC sample (VII), HTH83 (VIII), and ANCT sample probed with control IgG (IX) (original magnification, ×200).
shRNA-mediated knockdown of LAMC2 in ATC
Three sets of LAMC2-specific shRNA in lentiviruses inhibited LAMC2 expression (Supplemental Figure 1, B and C). Among these, LAMC2 shRNA3 resulted in 80%–90% silencing of LAMC2 in HTH83, TL3, and SW1736 cells as shown by both qRT-PCR and Western blot analysis (Figure 2, A and B). ATC cells (HTH83, TL3, and SW1736) stably silenced by LAMC2 shRNA proliferated at a slower rate compared with those transduced with scramble shRNA (P < .05) (Figure 2C). Similarly, anchorage-dependent clonal growth was significantly suppressed by LAMC2 shRNA in HTH-83 cells (Figure 2D). In addition, anchorage-independent clonogenic growth of HTH83 cells stably expressing LAMC2 shRNA compared with scramble vector control HTH83 cells was decreased by 41% (from a mean 490 to 193 colonies, P < .01), respectively (Figure 2E). Moreover, the size of colonies in LAMC2 knockdown cells was smaller as compared with scramble vector control cells (Supplemental Figure 1D). In rescue experiments, HTH83 cells stably expressing LAMC2 shRNA were transiently transfected with either an expression construct of LAMC2 or an empty vector (pCDNA3). Western blot confirmed the overexpression of LAMC2 (Figure 2F). Forced expression of LAMC2 into shRNA silenced LAMC2 ATC cells (HTH83) rescued their cell proliferation in liquid culture (Figure 2G). Similarly, a complete recovery in clonogenic growth was obtained when LAMC2 was overexpressed in ATC cells (HTH83) stably expressing LAMC2 shRNA, indicating the phenotype of shRNA LAMC2-expressing cells did not represent an off-target effect of the shRNA (Figure 2H).
Figure 2.
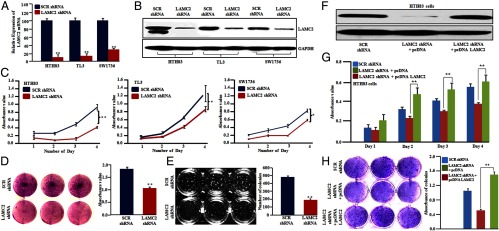
Silencing of LAMC2 decreased growth of ATC cells in both liquid culture and in a clonogenic assay in soft gel plates and forced expression of LAMC2 rescued the phenotype of LAMC2 knockdown cells. A and B, Real-time PCR analysis and Western blot showed reduced LAMC2 mRNA and protein in LAMC2 shRNA-treated cells compared with scramble (SCR) shRNA-infected HTH83, TL3, and SW1736 ATC cells. Antiglyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was used to assure equal loading controls. C–E, Reduced LAMC2 protein by silencing LAMC2 (LAMC2 shRNA) inhibited cell proliferation in liquid and soft gel culture of HTH83, TL-3, and SW1736 cells compared with SCR shRNA. F, Western blot showed the reconstitution of the expression of LAMC2 using a pcDNA LAMC2 construct in HTH83 cells stably expressing LAMC2 shRNA. G and H, Forced expression of LAMC2 in HTH83 LAMC2 stable knockdown cells rescued the cell proliferation as shown by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and colony-forming ability of LAMC2 knockdown cells as shown by a clonogenic growth assay. Data represent mean ± SD of three independent experiments done in triplicates. *, P ≤ .01; **, P ≤ .001 (Student's t test).
LAMC2 controls progression of cell cycle in ATC cells
Flow cytometric measurements showed that LAMC2 shRNA stably expressing ATC cells compared with scrambled shRNA cells induced a significant increase in the G1 phase (60% vs 36%, respectively) and a parallel decrease in the S phase (15% vs 23%, respectively) in HTH83 cells (Figure 3, A and B). Less dramatically in TL3 cells, LAMC2 shRNA induced an increase in the G1 phase (80% vs 65%, P < .01) and a decrease in the S phase as compared with scramble shRNA (Supplemental Figure 2A). Under the same experimental conditions, cyclin-dependent kinase inhibitor 1A (CDKN1A) (p21/WAF1) mRNA increased in the LAMC2 shRNA cells [shRNA HTH83 cells by 3.64-fold (P < .05) and shRNA TL3 cells by 2.4-fold] (Supplemental Figure 2B). Furthermore, we observed an increased expression of p21/WAF protein in LAMC2 shRNA-expressing HTH83 cells (Figure 3C) and TL3 cells (Supplemental Figure 2C). Furthermore, densitometric analysis of Western blot showed a 1.8- and 1.4-fold higher expression of p21 in LAMC2 shRNA-expressing HTH83 and TL3 cells, respectively (Supplemental Figure 2D).
Figure 3.
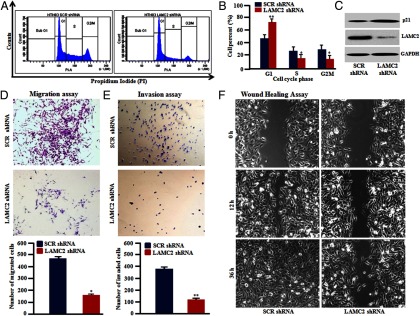
Silencing of LAMC2 in ATC cells induced cell cycle arrest at the G1 phase and decreased cell migration, invasion, and wound-healing properties in vitro. A and B, HTH83 cells stably expressing LAMC2 shRNA had increased cells in the G1 phase compared with HTH83 cells stably carrying a scrambled (SCR) shRNA as determined by flow cytometry. C, Western blot showed increased expression of p21/WAF protein in LAMC2 knockdown cells (HTH83 cells). D, Migration assay measured the number of HTH83 cells that migrated through 8-μm pores. LAMC2 shRNA reduced the number of migrated HTH83 cells compared with SCR shRNA. E, LAMC2 shRNA-expressing HTH83 cells displayed a reduced ability to invade through 8-μm pores coated with matrigel as compared with SCR shRNA. F, Wound-healing assay demonstrated that closure of the gap by HTH83 cells stably carrying a LAMC2 shRNA was not completed in 36 hours, whereas HTH83 cells stably carrying a SCR shRNA successfully closed the scratch wound within 36 hours. Cell cycle, migration, and invasion data represent mean ± SD of three independent experiments. *, P ≤ .01; **, P ≤ .001 (Student's t test).
Silencing of LAMC2 inhibits cell invasion, migration, and wound healing in vitro
Migration and invasion of tumor cells through their basement membrane is an important process in the cascade of metastasis. A significant reduction occurred in the motility of LAMC2-silenced cells [shRNA HTH83 cells by 60% (P < .05) and shRNA TL3 cells by 55% (P < .01)] through 8-μm apertures in comparison with scramble shRNA cells. Similarly, the stable inhibition of LAMC2 in HTH83 and TL3 cells significantly (P < .01) reduced the invasiveness through matrigel [shRNA HTH83, 71%, and shRNA TL3, 63%] compared with scrambled shRNA-expressing cells (Figure 3, D and E; TL3 data are shown in Supplemental Figure 3, A and B). Furthermore, a wound-healing assay measured cell motility. It was greatly decreased (delay in wound closure even at 36 h) in stable LAMC2 knockdown cells, whereas scramble shRNA cells completed the wound healing by 36 hours in shRNA HTH83 and shRNA TL3 cells (Figure 3F; TL3 data are shown in Supplemental Figure 3C).
LAMC2 knockdown reduced in vivo ATC tumor growth in nude mice
A xenograft model was developed using immunodeficient mice carrying HTH83 LAMC2-silenced cells (injected on the right flank) and an equal number of HTH83 cells carrying scramble shRNA (injected on the left flank). After 30 days, LAMC2 knockdown tumors had a mean 75% reduction in tumor weight compared with the scramble knockdown tumors (P = .006) (Figure 4, A and B). As anticipated, approximately 90% inhibition of the LAMC2 transcripts and protein expression occurred in the LAMC2 knockdown tumors compared with the scramble knockdown tumors (Supplemental Figure 4, A and B). Immunohistochemistry showed decreased immunoreactivity of LAMC2 and Ki-67 stained nuclei (proliferation marker) as well as fewer blood vessels (CD31) in the LAMC2 knockdown tumors compared with the scramble shRNA tumors (Figure 4C).
Figure 4.
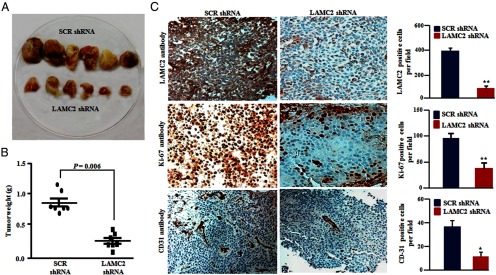
Stable knockdown of LAMC2 in HTH83 cells inhibited tumor growth in a nude murine model. HTH83 cell (2 × 106) stably transfected with either SCR shRNA or LAMC2 shRNA were injected sc into the right and left flanks, respectively, of 5-week-old female nude mice in matrigel [total volume 200 μL (100 μl matrigel and 100 μL medium)], and the animals were subsequently monitored for tumor growth. A, Representative photograph showing smaller tumor sizes in the LAMC2 shRNA group compared with tumors carrying SCR shRNA in the nude mice. B, Weight of individual tumors in each group. C, Tumor sections from nude mice in both groups were subjected to immunohistochemical analysis. Tumors with SCR shRNA revealed strong staining for LAMC2 protein, proliferative marker Ki-67, and blood vessel marker CD31 compared with SCR shRNA. Columns represent mean ± SD of three independent experiments. *, P ≤ .01; **, P ≤ .001 (Student's t test). Original magnification, ×200; objective, ×20.
LAMC2 interacted with EGFR and influenced its downstream pathway
Immunocytochemistry experiments showed colocalization of LAMC2 with EGFR on the cell membrane in both HTH83 (Figure 5A) and TL3 cells (Supplemental Figure 4C). To further explore the interactions between LAMC2 and EGFR, LAMC2 was transfected into 293T cells; protein lysates were immunoprecipitated with monoclonal anti-LAMC2 antibody and probed with monoclonal anti-EGFR. Endogenous EGFR was present in a complex with the ectopically expressed LAMC2 (Supplemental Figure 4D). No interaction was observed with control vector. In further experiments using lysates of either HTH-83 or TL3 cells, endogenous EGFR coimmunoprecipitated with endogenously expressed LAMC2 or vice versa (Figure 5, B–D). However, no interaction was observed using control IgG. In addition, analysis of the GSE27155 microarray database showed that EGFR is expressed more highly in most of the thyroid cancers samples compared with the normal thyroid specimens (Figure 5E). Furthermore, the Pearson correlation test showed significant correlation between LAMC2 and EGFR expression (P = .0054). Additional experiments demonstrated that knockdown of LAMC2 decreased EGF-stimulated phosphorylation of EGFR, ERK1/2, and AKT as compared with the same cells carrying scramble shRNA (Figure 5F).
Figure 5.
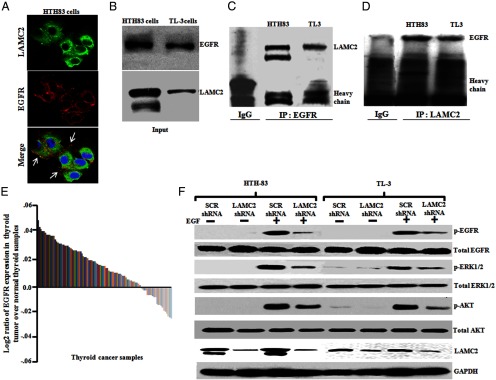
LAMC2 can bind to EGFR, and LAMC2 shRNA affected phosphorylation of EGFR and its downstream signaling in human ATC cells (HTH83 and TL3). A, HTH83 cells were fixed, permeablized, and immunostained, and images were captured using a confocal microscope. Alexa 488 (green fluorescence) and Alexa 680 (red fluorescence) were used for LAMC2 and EGFR staining, respectively. The images were merged for the colocalization of LAMC2 and EGFR. Arrows depict the colocalization. 4′,6′-Diamino-2-phenylindole was used to stain the nuclei of the cells. Images are representative of at least four independent experiments yielding similar results. Original magnification, ×600; objective, ×60. B, Western blot (WB) analysis showed endogenous levels of LAMC2 and EGFR protein in ATC cells (HTH83 and TL3). C, Cell lysates were subjected to immunoprecipitation using EGFR monoclonal antibody, and immunoprecipitates were Western blotted with LAMC2 monoclonal antibody. D, LAMC2 antibody was used for immunoprecipitation, and the immunoprecipitates were immunoblotted with EGFR monoclonal antibody. E, EGFR expression in thyroid cancer samples using microarray database (GSE27155). F, ATC cells stably expressing either LAMC2 shRNA or SCR shRNA were serum starved for 24 hours and then treated with EGF (50 ng/mL) for 20 minutes and phosphorylation of EGFR, ERK1/2, and AKT was analyzed by Western blot using phosphorylated (p)-EGFR, p-ERK1/2, and p-AKT antibodies. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was used for equal loading control.
EGF stimulation leads to an increased in LAMC2 mRNA and protein expression in ATC cells
We examined the effect of the EGF ligand on the induction of LAMC2 in both HTH83 and TL3 cells. Cells were serum deprived for 24 hours and then stimulated with EGF ligand (50 ng/mL) for either 24 or 48 hours, harvested, and levels of LAMC2 mRNA measured by qRT-PCR. The increase in LAMC2 mRNA expression was 2.5- to 4.2-fold (P < .05) for HTH83 cells (Supplemental Figure 4E) and 2.0- to 3.1-fold (P < .01, P < .05) for TL3 cells (Supplemental Figure 4F) at 24 and 48 hours, respectively, compared with unstimulated control cells. Under the same experimental conditions, EGF stimulation resulted in an increase in LAMC2 protein. Moreover, densitometric measurements of Western blots showed an increase in LAMC2 protein ranging from 2.0- and 3.5-fold for HTH83 cells to 2.2- to 1.8-fold for TL3 cells after EGF treatment for 24 and 48 hours, respectively, compared with untreated control cells (Figure 6, A and B). These results suggest that EGF stimulates LAMC2 expression in ATC cells.
Figure 6.
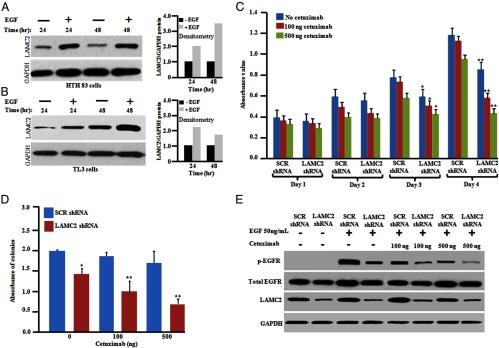
Induction of LAMC2 expression by EGF. Silencing LAMC2 enhanced the antiproliferative activity of ATC cells in combination with cetuximab. A and B, HTH83 and TL3 cells (106) cells were seeded into 10-cm culture dishes and serum starved for 24 hours followed by the addition of fresh. After serum starvation, cells were incubated either with fresh medium containing either EGF (50 ng/mL) or diluent control. The cells were harvested at the indicated time points for Western blotting. Densitometry analysis showed fold change in LAMC2 expression upon EGF treatment. C, HTH83 cells stably expressing either LAMC2 shRNA or SCR shRNA were seeded into 96-well plates and cetuximab (100 or 500 ng/mL) was added. The rate of proliferation was measured every 24 hours by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. D, HTH83 cells (2 × 103) stably expressing either LAMC2 shRNA or SCR shRNA were used for a clonogenic growth assay in the presence of cetuximab (100 or 500 ng/mL). For panels C and D, results represent the mean ± SD of three independent experiments. *, P ≤ .01; **, P ≤ .001 (Student's t test). E, Western blot (WB) analysis. HTH83 LAMC2 shRNA cells and HTH83 SCR shRNA cells were treated with cetuximab (100 or 500 ng/mL) for 24 hours and then serum starved for 24 hours followed by the addition of EGF (50 ng/mL) for 20 minutes, and phosphorylation (p) of EGFR was analyzed by Western blot using antibodies against p-EGFR, EGFR, LAMC2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; loading control).
Silencing of LAMC2 enhanced the antiproliferative activity of ATC cells in combination with either EGFR siRNA or cetuximab
EGFR-specific siRNA resulted in approximately 90%–95% silencing of EGFR in HTH83 and TL3 cells both at the mRNA and protein levels compared with control siRNA after 48 hours of transfection (Supplemental Figure 5, A and C). To examine the effect of EGFR inhibition, HTH83 cells stably expressing either LAMC2 shRNA or scramble shRNA were transiently transfected with either an EGFR siRNA or negative control or scramble siRNA. Cell growth assays showed an additive growth inhibition in LAMC2 knockdown HTH83/TL3 cell lines in combination with EGFR siRNA as compared with similarly treated scramble shRNA HTH83 or TL3 cells (Supplemental Figure 5, B and D). In addition, we also used cetuximab, which is a monoclonal blocking antibody against EGFR. Treatment of HTH83 scramble shRNA cells with cetuximab (100 or 500 ng/mL) did not result in significant antiproliferative effects. In contrast, HTH83 LAMC2 knockdown cells treated with cetuximab (100 or 500 ng/mL) had a greater antiproliferative effect compared with similarly treated control cells (Figure 6C). To ascertain the additive or synergistic nature of these modified and treated cells, a combination index was calculated using CompuSyn software (ComboSyn, Inc). The results showed that the combined inhibition of proliferation was additive because the combination index (0.90) was close to 1. A similar response was observed when examining the clonogenic growth of LAMC2-silenced HTH83 cells plus cetuximab compared with control cells (Figure 6D). Furthermore, the combination of silencing of LAMC2 plus cetuximab suppressed levels of EGF-stimulated p-EGFR greater than similar experiments with control cells (Figure 6E).
Gene expression profiling in LAMC2 knockdown cells
To determine global changes in the transcriptome resulting from the silencing of LAMC2, gene expression profiles were analyzed in LAMC2 shRNA-transfected cells by microarray. Data analysis identified 580 genes differentially expressed in LAMC2-silenced HTH83 and TL3 cell lines: 221 were down-regulated and 359 were up-regulated. As anticipated, LAMC2 was one of the top five down-regulated genes in LAMC2 shRNA cells (fold change: −4.9 in TL3 and −3.3 in HTH83, P < .05).
Differentially expressed genes were associated with cellular proliferation, cell cycle, migration, and invasion as well as focal adhesion. For example, the Kyoto Encyclopedia of Genes and Genomes Pathway and Wiki Pathway analysis showed that the top-ranked pathways related to LAMC2 were MAPK, EGFR, Janus kinase-signal transducer and activator of transcription, Wnt, cell cycle control, focal adhesion, ECM receptor, and p53 pathways. Both software analyses observed consistent enrichment of the EGFR and MAPK signaling pathways [Kyoto Encyclopedia of Genes and Genomes Pathway Analysis (P = 1.93 × 10−7] and [Wiki Pathway (P = 6.32 × 10−6)] (Supplemental Figure 6). These data suggested that the silencing of LAMC2 altered the expression of genes in the EGFR pathway. The fidelity of the microarray results was confirmed by quantitative RT-PCR validating expression of 12 genes. Congruently with the array data, expression levels of matrix metalloproteinases (MMP)-1, MMP3, bone morphogenetic protein 2, A disintegrin and metalloproteinase with thrombospondin-like repeats 1, and SNAL2 mRNA were significantly decreased, whereas levels of HMOX1 (heme oxygenase 1), DNA damage inducible transcript 3 (DDIT3), activating transcription factor 3 (ATF3), CDKN1A (cyclin-dependent kinase inhibitor 1A), HSPA1A (heat shock 70kDa protein 1A), HSPA1B, and CMTM8 (CKLF-like MARVEL transmembrane domain containing 8) were significantly increased in cells stably carrying LAMC2 shRNA compared with scramble shRNA (Supplemental Figure 6).
Discussion
The incidence of thyroid malignancy has been increasing by 5%–6% every year;, and among these, ATCs constitute approximately 1%–2% of these cancers (28, 29). The prognosis for anaplastic carcinoma is dismal, with overall survival from diagnosis of 6 months or less, often with extensive local invasion and distant metastasis at diagnosis. The treatment is surgery, external beam radiation, chemotherapy, chemoradiotherapy, and experimental targeted therapies (30). Understanding specific mechanisms involved in the deregulation of cell growth, migration, and invasion of thyroid cancer is necessary for developing new therapies.
Laminin-5 (laminin-332) is one of the primary factors that stimulates invasion and metastasis of several types of tumors, and LAMC2 (a major component of laminin-5) is highly expressed in several types of invasive tumors (8, 32). To the best of our knowledge, the present study is the first to note overexpression of LAMC2 mRNA and protein in ATC patient samples and cell lines. In addition, shRNA-mediated silencing of LAMC2 expression in ATC cells significantly decreased their proliferation in vitro and in vivo as measured by clonogenic and liquid culture assays, tumor size, and percentage Ki-67-positive cells. In addition, silencing of LAMC2 decreased migration and invasion associated with cell cycle arrest at the G1/S transition and induced CDKN1A (p21/WAF) in the ATC cells. Of note, tumors composed of the LAMC2 knockdown cells had fewer blood vessels than tumors composed of vector control ATC cells. Moreover, we performed rescue experiments by transfecting LAMC2 knockdown cells with a LAMC2 expression construct, which reversed the decreased cell proliferation and clonogenic growth. Taken together, these results provide evidence that LAMC2 shRNA is having on-target effects and thus validates that LAMC2 has an important role in ATC growth and may be a potential therapeutic target for the treatment of ATC.
Consistent with these results, our microarray and quantitative PCR data revealed that LAMC2 has profound effects on the expression of genes related to cellular proliferation, cell cycle, cell migration, and invasion. For example, the silencing of LAMC2 resulted in a significant down-regulation of MMP1 and MMP3 as well as ERK1/2 compared with scramble vector-containing cells. Prior studies have shown that activation of ERK1/2 can promote the production of MMPs, such as MMP1 and MMP3, which are important for cell proliferation and invasion (33, 34). Thus, LAMC2 may increase ATC cell invasion by stimulating the production of MMPs through the activation of ERK1/2. In addition, silencing LAMC2 produced an up-regulation of genes related to decreased cell proliferation or survival, including many transcription regulators such as ATF3, DDIT3 (CCAAT/enhancer-binding protein homologous protein), HSPA1A, and HMOX1 genes, consistent with our findings (Supplemental Figure 7). A recent study showed that an increased expression of ATF3 and DDIT3 (CCAAT/enhancer-binding protein homologous protein) is associated with decreased cell proliferation and the induction of apoptosis (35). Further analysis of the GSE27155 microarray database showed that MMP1 and MMP3 are up-regulated, whereas ATF3 and HSPA1A are down-regulated in most of the thyroid cancers samples compared with the normal thyroid specimens (Supplemental Figure. 8). A Pearson correlation coefficient analysis showed a significant correlation between LAMC2 expression and ATF3 (P = .00151) as well as HSPA1A (P = .02) within these patient samples. Taken together, these finding suggest that LAMC2 may be important for thyroid carcinogenesis by increasing the expression of the genes associated with tumor growth, migration, and invasion and decreasing the expression of tumor suppressor genes.
EGFR is a major tyrosine kinase growth factor receptor that regulates various pathways important for a variety of cellular process including proliferation, apoptosis, and cytoskeleton changes (36). Prior studies in bladder cancer using immunohistochemistry noted coexpression of LAMC2 and EGFR on tumor tissues (31). In our immunocytochemistry experiments, LAMC2 colocalized with EGFR in ATC cells. Moreover, we observed significant and consistent enrichment of the EGFR pathway in our microarray data. Furthermore, LAMC2 and EGFR proteins were coimmunoprecipitated, and knockdown of expression of LAMC2 in ATC cells decreased phosphorylation of EGFR and its downstream targets (ERK and AKT) in vitro. Taken together, these results provide evidence that LAMC2 enhances the EGFR signaling pathway in ATC cells In addition, stimulation of ATC cells with EGF enhanced transcription and translation of LAMC2. Cetuximab (human-murine chimeric monoclonal antibody to EGFR) is Food and Drug Administration approved for the treatment of the human colon, head and neck, and pancreatic carcinomas because it inhibits the growth of these EGFR-expressing tumors. Between 58% and 87% of ATC tumors overexpress EGFR compared with normal thyroid tissue. Taken together, these observations formed our rationale to use the combination of cetuximab with LAMC2 shRNA. In combination, they significantly decreased the liquid and clonogenic growth of ATC cells. This was associated with a greater reduction of phosphorylation of EGFR than either treatment alone. Furthermore, knockdown of both EGFR and LAMC2 showed additive effects on the antiproliferation of ATC cells.
In summary, we found robust expression of LAMC2 mRNA and protein in cell lines and tissue samples of ATC but not in ANCT tissue samples. Our finding showed that silencing of LAMC2 in ATC cells reduced their cell growth, migration, and invasion and impaired the cell cycle by arresting them at the G1 to S phase transition of the cell cycle. Our experiments suggest that LAMC2 enhances the activation of EGFR and its downstream pathway in ATC cells (Supplemental Figure 9). Furthermore, simultaneous silencing of LAMC2 combined with either cetuximab or silencing of EGFR enhanced the antiproliferative effects compared with either alone. Our studies provide the foundation for further investigation of LAMC2 as a promising target for treatment of ATC.
Acknowledgments
We thank Professor Dean G. Tang (University of Texas M. D. Anderson Cancer Center, Houston, Texas) for the gift of the pGIPZ-scrambled shRNA vector. We are grateful to Dr Eng Chon Boon, Dr Rajeev Singh, and their team for providing the total RNA of adjacent noncancerous tissue and anaplastic thyroid tumor samples from the National University Hospital-National University of Singapore Tissue Repository.
This work was supported by grants from National Institutes of Health; National Medical Research Council, Singapore; the National Research Foundation Singapore; and the Singapore Ministry of Education.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATC
- anaplastic thyroid carcinoma
- ANCT
- adjacent noncancerous tissue
- ATF3
- activating transcription factor 3
- DDIT3
- DNA damage-inducible transcript 3
- LAMC2
- laminin-5-γ2
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- MMP
- matrix metalloproteinase
- qRT-PCR
- quantitative real-time-PCR
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA.
References
- 1. Sherman SI. Thyroid carcinoma. Lancet. 2003;361:501–511 [DOI] [PubMed] [Google Scholar]
- 2. Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new adhesion ligand for integrin a3b1 in epithelial basement membranes. Cell. 1991;65:599–619 [DOI] [PubMed] [Google Scholar]
- 3. Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryan MC, Christiano AM, Engvall E, et al. The functions of laminins: lessons from in vivo studies. Matrix Biol. 1996;15:369–381 [DOI] [PubMed] [Google Scholar]
- 5. Marinkovich MP. Laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–380 [DOI] [PubMed] [Google Scholar]
- 6. Soini Y, Maatta M, Salo S, Tryggvason K, Autio-Harmainen H. Expression of the laminin γ2 chain in pancreatic adenocarcinoma. J Pathol. 1996;180:290–294 [DOI] [PubMed] [Google Scholar]
- 7. Takahashi S, Hasebe T, Oda T, et al. Cytoplasmic expression of laminin γ2 chain correlates with postoperative hepatic metastasis and poor prognosis in patients with pancreatic ductal adenocarcinoma. Cancer. 2002;94:1894–1901 [DOI] [PubMed] [Google Scholar]
- 8. Koshikawa N, Moriyama K, Takamura H, et al. Overexpression of laminin γ2 chain monomer in invading gastric carcinoma cells. Cancer Res. 1999;59:5596–5601 [PubMed] [Google Scholar]
- 9. Ono Y, Nakanishi Y, Ino Y, et al. Clinicopathologic significance of laminin-5 γ 2 chain expression in squamous cell carcinoma of the tongue: immunohistochemical analysis of 67 lesions. Cancer. 1999;85:2315–2321 [PubMed] [Google Scholar]
- 10. Sordat I, Bosman FT, Rousselle P, et al. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J Pathol. 1998;185:44–52 [DOI] [PubMed] [Google Scholar]
- 11. Hlubek F, Jung A, Kotzor N, Kirchner T, Brabletz T. Expression of the invasion factor laminin γ 2 in colorectal carcinomas is regulated by β-catenin. Cancer Res. 2001;61:8089–8093 [PubMed] [Google Scholar]
- 12. Kagesato Y, Mizushima H, Koshikawa N, et al. Sole expression of laminin γ 2 chain in invading tumor cells and its association with stromal fibrosis in lung adenocarcinomas. Japan J Cancer Res. 2001;92:184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skyldberg B, Salo S, Eriksson E, et al. Laminin-5 as a marker of invasiveness in cervical lesions. J Natl Cancer Inst. 1999;91:1882–1887 [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto H, Itoh F, Iku S, Hosokawa M, Imai K. Expression of the γ 2 chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human oesophageal squamous cell carcinoma. Clin Cancer Res. 2001;7:896–900 [PubMed] [Google Scholar]
- 15. Gasparoni A, Della Casa M, Milillo L, et al. Prognostic value of differential expression of laminin-5 γ2 in oral squamous cell carcinomas: correlation with survival. Oncol Rep. 2007;18:793–800 [PubMed] [Google Scholar]
- 16. Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee DH, Lee GK, Kong SY, et al. Epidermal growth factor receptor status in anaplastic thyroid carcinoma. J Clin Pathol. 2007;60:881–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ensinger C, Spizzo G, Moser P, et al. Epidermal growth factor receptor as a novel therapeutic target in anaplastic thyroid carcinomas. Ann NY Acad Sci. 2004;1030:69–77 [DOI] [PubMed] [Google Scholar]
- 19. Van der Laan BF, Freeman JL, Asa SL. Expression of growth factors and growth factor receptors in normal and tumorous human thyroid tissues. Thyroid. 1995;5:67–73 [DOI] [PubMed] [Google Scholar]
- 20. Hoelting T, Siperstein AE, Clark OH, Duh QY. Epidermal growth factor enhances proliferation, migration, and invasion of follicular and papillary thyroid cancer in vitro and in vivo. J Clin Endocrinol Metab. 1994;79:401–408 [DOI] [PubMed] [Google Scholar]
- 21. Bechtner G, Schopohl D, Rafferzeder M, Gartner R, Welsch U. Stimulation of thyroid cell proliferation by epidermal growth factor is different from cell growth induced by thyrotropin or insulin-like growth factor I. Eur J Endocrinol. 1996;134:639–648 [DOI] [PubMed] [Google Scholar]
- 22. Waterman EA, Sakai N, Nguyen NT, et al. A laminin-collagen complex drives human epidermal carcinogenesis through phosphoinositol-3-kinase activation. Cancer Res. 2007;67:4264–4270 [DOI] [PubMed] [Google Scholar]
- 23. Schenk S, Hintermann E, Bilban M, et al. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garg M, Kanojia D, Suri S, Gupta S, Gupta A, Suri A. Sperm associated antigen 9: a novel diagnostic marker for thyroid cancer. J Clin Endocrinol Metab. 2009;94:4613–4618 [DOI] [PubMed] [Google Scholar]
- 26. Hayano T, Garg M, Yin D, Sudo M, et al. SOX7 is down-regulated in lung cancer. J Exp Clin Cancer Res. 2013;32:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sudo M, Chin TM, Akashi M, Koeffler HP, et al. Inhibiting proliferation of gefitinib-resistant, non-small lung cancer. Cancer Chemother Pharmacol. 2013;71:1325–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428 [DOI] [PubMed] [Google Scholar]
- 29. Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021 [DOI] [PubMed] [Google Scholar]
- 30. Vaysburd M. Anaplastic thyroid carcinoma. In: Braunstein GD, ed. Thyroid Cancer. New York: Springer; 2012:189–200 [Google Scholar]
- 31. Kiyoshima K, Oda Y, Kinukawa N, Naito S, Tsuneyoshi M. Overexpression of laminin-5 γ2 chain and its prognostic significance in urothelial carcinoma of urinary bladder: association with expression of cyclooxygenase 2, epidermal growth factor receptor and human epidermal growth factor receptor. Hum Pathol. 2005;36:522–530 [DOI] [PubMed] [Google Scholar]
- 32. Pyke C, Salo S, Ralfkiaer E, Romer J, Dano K, Tryggvason K. Laminin-5 is a marker of invading cancer cells in some human carcinomas and is coexpressed with the receptor for urokinase plasminogen activator in budding cancer cells in colon adenocarcinomas. Cancer Res. 1995;55:4132–4139 [PubMed] [Google Scholar]
- 33. Westermarck J, Holstorm TH, Ahonen M, Eriksson JE, Kahari VM. Enhancement of fibroblast collagenase-1 (MMP-1) gene expression by tumor promoter okadaic acid is mediated by stress-activated protein kinases Jun N-terminal kinase and p38. Matrix Biol. 1998;17:547–557 [DOI] [PubMed] [Google Scholar]
- 34. Reunanen N, Li SP, Ahonen M, Foschi M, Han J, Kahari VM. Activation of p38-a MAPK enhances collagenase-1 (matrix metalloproteinase (MMP)-1) and stromelysin-1 (MMP-3) expression by mRNA stabilization. J Biol Chem. 2002;277:32360–32368 [DOI] [PubMed] [Google Scholar]
- 35. Bottone FG, Jr, Moon Y, Kim JS, Alston-Mills B, Ishibashi M, Eling TE. The anti-invasive activity of cyclooxygenase inhibitors is regulated by the transcription factor ATF3 (activating transcription factor 3). Mol Cancer Ther. 2005;4:693–703 [DOI] [PubMed] [Google Scholar]
- 36. Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31 [DOI] [PubMed] [Google Scholar]


