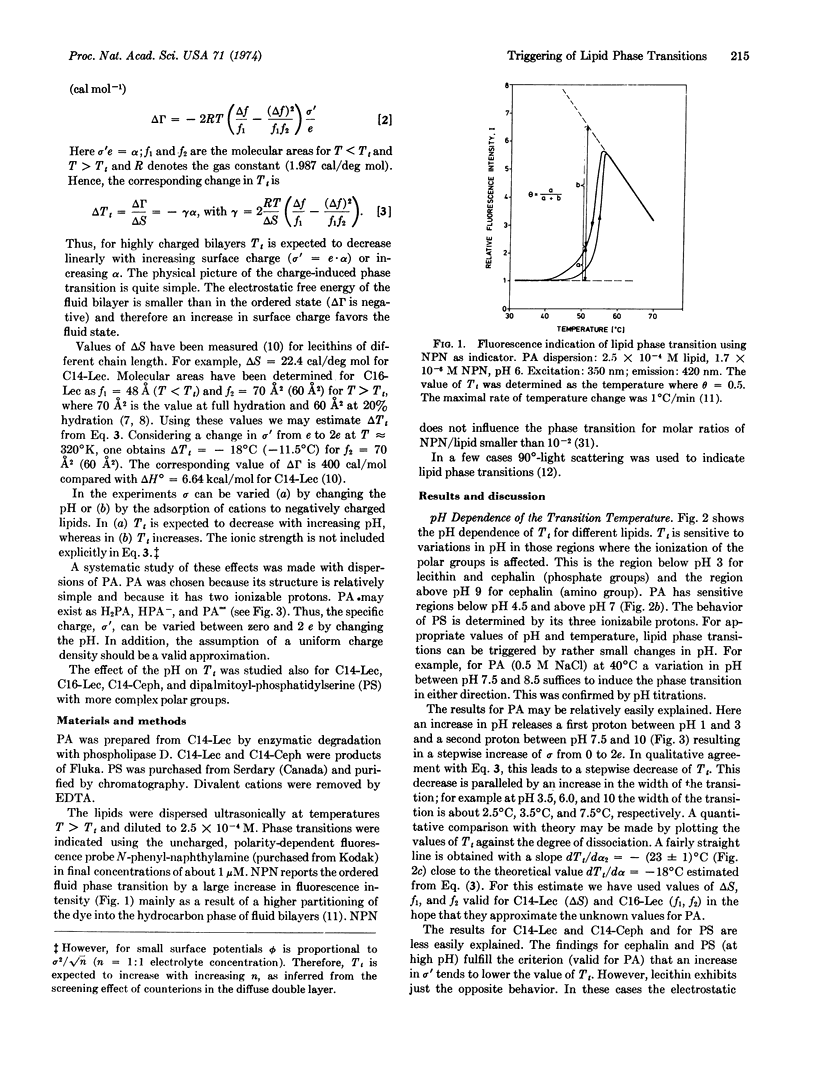
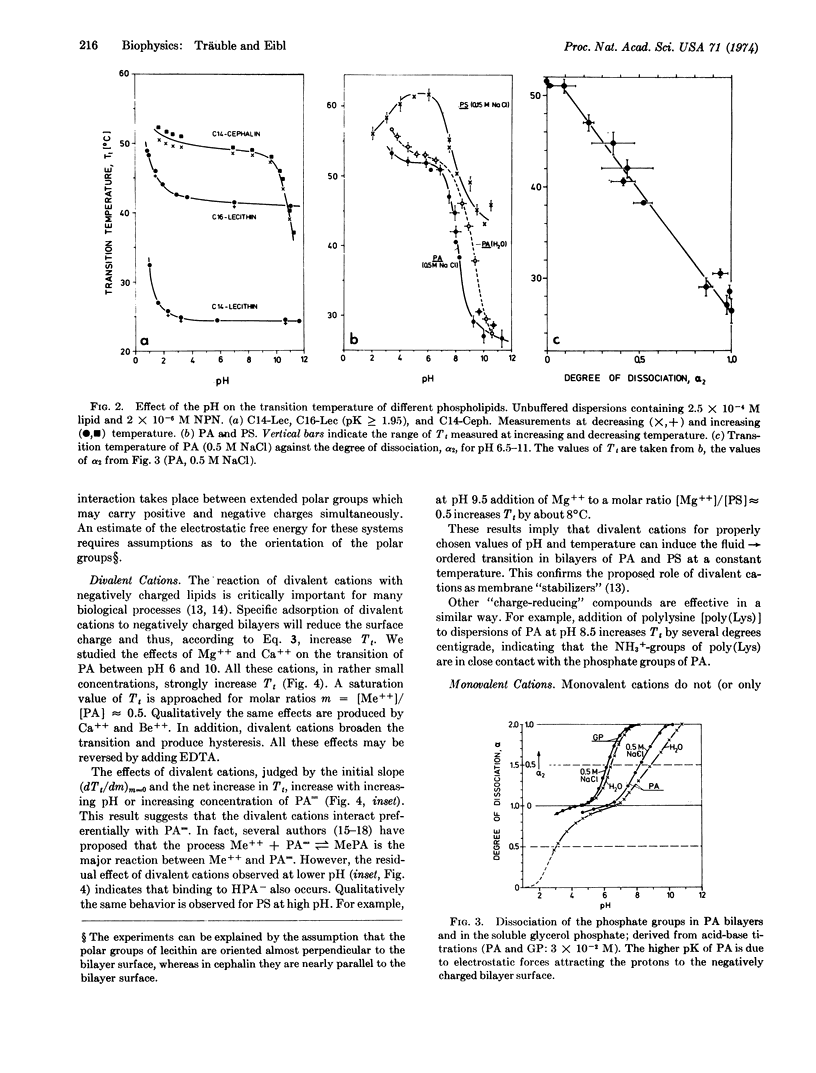
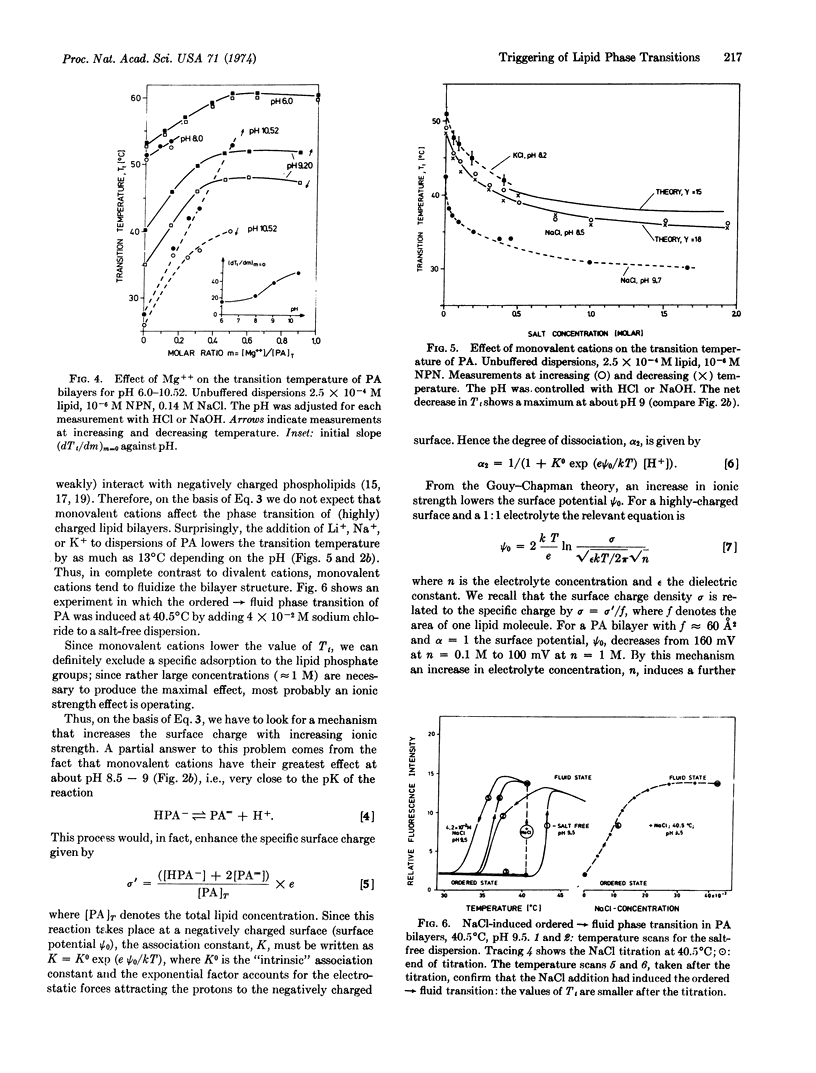
Abstract
Ordered → fluid phase transitions in bilayers of charged lipids are accompanied by a decrease in electrostatic free energy mainly as a result of bilayer expansion. For uniform charge distribution the Gouy-Chapman theory of the electrical double layer predicts a decrease of the transition temperature with increasing charge density. We studied the effects of pH and mono- and divalent cations on the phase transition of lecithin, cephalin, phosphatidylserine, and phosphatidic acid bilayers. Phosphatidic acid with two ionizable protons was selected for a systematic investigation. A change in pH from 7 to 9 increases the charge per polar group from one to two elementary charges. This lowers the transition temperature by about 20°C in agreement with the theory. In this pH region rather small changes in pH suffice to induce the phase transition at constant temperature. Divalent cations (Mg++ and Ca++) increase the transition temperature by charge neutralization and thus can be used to induce the fluid → ordered transition at constant temperature. In contrast, monovalent cations (Li+, Na+, K+) lower the transition temperature, or fluidize the bilayer structure at a given temperature. Rather small changes in ionic environment can induce gross alterations in bilayer structure; divalent and monovalent cations have antagonistic effects. This result parallels current theories on nerve excitation and sensory transduction where cation-induced structural changes in biomembranes are invoked.
Keywords: triggering factors, cation binding, pH-dependence, ionic strength, double layer energy
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson M. B., Katzman R., Curci R. Turbidimetric studies of the interaction of aqueous micelles of phosphatidic acid with cations. J Colloid Sci. 1965 Sep;20(7):777–787. doi: 10.1016/0095-8522(65)90051-6. [DOI] [PubMed] [Google Scholar]
- Camejo G., Villegas G. M., Barnola F. V., Villegas R. Characterization of two different membrane fractions isolated from the first stellar nerves of the squid Dosidicus gigas. Biochim Biophys Acta. 1969;193(2):247–259. doi: 10.1016/0005-2736(69)90186-2. [DOI] [PubMed] [Google Scholar]
- Demel R. A., Kinsky S. C., Kinsky C. B., van Deenen L. L. Effects of temperature and cholesterol on the glucose permeability of liposomes prepared with natural and synthetic lecithins. Biochim Biophys Acta. 1968 Jun 11;150(4):655–665. doi: 10.1016/0005-2736(68)90055-2. [DOI] [PubMed] [Google Scholar]
- Devaux P., McConnell H. M. Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J Am Chem Soc. 1972 Jun 28;94(13):4475–4481. doi: 10.1021/ja00768a600. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hauser H., Dawson R. M. The binding of calcium at lipid-water interfaces. Eur J Biochem. 1967 Mar;1(1):61–69. doi: 10.1007/978-3-662-25813-2_11. [DOI] [PubMed] [Google Scholar]
- Inoue I., Kobatake Y., Tasaki I. Excitability, instability and phase transitions in squid axon membrane under internal perfusion with dilute salt solutions. Biochim Biophys Acta. 1973 May 25;307(3):471–477. doi: 10.1016/0005-2736(73)90294-0. [DOI] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Birdsall N. J., Lee A. G., Metcalfe J. C. 13 C nuclear magnetic resonance relaxation measurements of synthetic lecithins and the effect of spin-labeled lipids. Biochemistry. 1972 Apr 11;11(8):1416–1421. doi: 10.1021/bi00758a014. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Peticolas W. L. Raman active vibrations in long-chain fatty acids and phospholipid sonicates. Biochim Biophys Acta. 1972 Sep 1;282(1):8–17. doi: 10.1016/0005-2736(72)90306-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Szabo G., Eisenman G. Divalent ions and the surface potential of charged phospholipid membranes. J Gen Physiol. 1971 Dec;58(6):667–687. doi: 10.1085/jgp.58.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Surface properties of acidic phospholipids: interaction of monolayers and hydrated liquid crystals with uni- and bi-valent metal ions. Biochim Biophys Acta. 1968 Sep 17;163(2):240–254. doi: 10.1016/0005-2736(68)90103-x. [DOI] [PubMed] [Google Scholar]
- Phillips M. C., Chapman D. Monolayer characteristics of saturated 1,2,-diacyl phosphatidylcholines (lecithins) and phosphatidylethanolamines at the air-water interface. Biochim Biophys Acta. 1968 Nov 5;163(3):301–313. doi: 10.1016/0005-2736(68)90115-6. [DOI] [PubMed] [Google Scholar]
- Seimiya T., Ohki S. Ionic structure of phospholipid membranes, and binding of calcium ions. Biochim Biophys Acta. 1973 Mar 29;298(3):546–561. doi: 10.1016/0005-2736(73)90073-4. [DOI] [PubMed] [Google Scholar]
- Spyropoulos C. S. Ion-exchange properties of nerve membrane. Fed Proc. 1968 Nov-Dec;27(6):1252–1262. [PubMed] [Google Scholar]
- Stark G., Benz R., Pohl G. W., Janko K. Valinomycin as a probe for the study of structural changes of black lipid membranes. Biochim Biophys Acta. 1972 Jun 20;266(3):603–612. doi: 10.1016/0006-3002(72)90004-2. [DOI] [PubMed] [Google Scholar]
- TOBIAS J. M. A CHEMICALLY SPECIFIED MOLECULAR MECHANISM UNDERLYING EXCITATION IN NERVE: A HYPOTHESIS. Nature. 1964 Jul 4;203:13–17. doi: 10.1038/203013a0. [DOI] [PubMed] [Google Scholar]
- Träuble H., Overath P. The structure of Escherichia coli membranes studied by fluorescence measurements of lipid phase transitions. Biochim Biophys Acta. 1973 May 25;307(3):491–512. doi: 10.1016/0005-2736(73)90296-4. [DOI] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]
- Wilson G., Rose S. P., Fox C. F. The effect of membrane lipid unsaturation on glycoside transport. Biochem Biophys Res Commun. 1970 Feb 20;38(4):617–623. doi: 10.1016/0006-291x(70)90625-x. [DOI] [PubMed] [Google Scholar]



