Abstract
Context:
SHBG concentrations correlate inconsistently with metabolic parameters.
Hypothesis:
SHBG assay platforms contribute to nonuniformities according to the literature.
Design:
The design of the study was a noninterventional quantification of SHBG by two immuno- and two mass spectrometric assays and abdominal visceral fat by computed tomography scan.
Setting:
The study was conducted at the Center for Translational Science Activities.
Participants:
Healthy men (n = 120) aged 18–80 years with a body mass index of 20–43 kg/m2 participated I the study.
Outcomes:
Outcomes of the study included a correlation of log SHBG with age, metabolic surrogates [body mass index, albumin, glucose, insulin, abdominal (total and visceral) fat, homeostasis model assessment insulin resistance index], sex steroids (estrone, 17β-estradiol, T, and dihydrotestosterone by mass spectrometry), and adipocytokines (IL-1β, IL-6, IL-8, IL-10 and IL-12, TNF-α, and adiponectin).
Results:
By univariate regression, age (P < 10−4), dihydrotestosterone (P < 10−4), T (P ≤ .00022), and adiponectin (P ≤ .0084) were positive correlates, and insulin and homeostasis model assessment insulin resistance index were negative correlates (P ≤ .0060) of SHBG in all four assays. Stepwise multivariate analysis unveiled that age and T together could explain 38.1%–52.5% of the statistical variance in SHBG in all assays (P < 10−11). Multivariate regression without sex steroids unveiled that age (P < 10−5) and insulin (P < 10−3) are jointly associated with SHBG levels in the four assays with overall R2 = 0.215–0.293 and P < 10−6. In one immunological SHBG assay each, abdominal visceral fat and adiponectin were weak multivariates also.
Conclusion:
Immunological and mass spectrometric SHBG assays yield both consistent and inconsistent correlations with key metabolic variables in healthy men, thereby potentially explaining earlier inconsistencies in the literature.
SHBG) is produced in the liver, brain, placenta, and other tissues (1). SHBG is postulated to transport, potentiate, or sequester sex steroid hormones, thus putatively influencing androgen or estrogen action on muscle mass, bone mass, brain function, body composition, neoplastic growth, and cardiovascular risk (2–5). Membrane-bound SHBG can also modulate certain target-tissue responses (6). Consequently, regulation of SHBG concentrations provides a potentially powerful mechanism for pleiotropic physiological control.
In addition to geographic, ethnic, and genetic factors (7–9), gender, age, exercise, and endocrine factors seem to influence SHBG concentrations in health and disease (5, 10–18). In many but not other studies, estrogen and nonaromatizable androgens respectively stimulate and suppress plasma levels and hepatocyte synthesis of SHBG (10, 16, 17). GH, IGF-I, insulin, and glucose appear to repress SHBG production in other clinical and animal models (19, 20). Less consistent candidate regulators are amount and/or distribution of body fat, leptin, triglycerides, lipoproteins, IGF binding proteins, proinflammatory cytokines, like IL-6, IL-1α, and TNF-α (21–24), or antiinflammatory cytokines, like IL-10 and adiponectin (13, 25). However, reported associations have not always been reproducible.
The degree to which age, body mass index (BMI), insulin, glucose, abdominal visceral fat (AVF), sex steroids, and/or cytokines individually and collectively determine SHBG concentrations is uncertain. Uncertainty is due to the following facts: 1) most clinical studies used only one SHBG assay platform, thus raising the question of outcome reproducibility in other systems; 2) usually only one or two of the key sex steroids, T, dihydrotestosterone (DHT), 17β-estradiol (E2) or estrone (E1), were measured as correlates and then not by definitive mass spectrometry; and 3) immunological rather than direct mass estimates of SHBG concentrations were used. These issues are relevant because immunologically based assays of sex steroids and SHBG may manifest (marked) heterogeneity of the results (26–30).
The present clinical investigation combines mass spectrometric measures of T, DHT, E2, and E1 with immunological and mass spectrometric estimates of SHBG concentrations in 120 men aged 18–80 years to appraise reproducible correlations among SHBG and sex steroids, body composition, cytokines, insulin, and/or glucose.
Materials and Methods
Subjects
One hundred twenty healthy, independently living men were recruited from Olmsted County, Minnesota, by local advertisements under institutional review board approval. Subjects agreed to provide 50 mL blood (25 mL for serum, 25 mL for EDTA-plasma) fasting at 0800 hours and undergo a single-slice computed tomography scan at L2-L3 to estimate abdominal visceral fat. Inclusion criteria comprised mental competence; BMI less than 19 kg/m2 to less than 45 kg/m2; prestudy hemoglobin greater than 13 g/dL or hematocrit greater than 36%; and provision of written informed consent. Exclusion criteria were outside the BMI range cited above; anemia; any recent (five biological half-lives) use of psycho- or neuroactive drugs or sex hormones; active psychiatric illness (eg, psychosis, depression, mania); drug or alcohol abuse; acute or chronic inflammatory, metabolic, organ-level, or systemic illness; hypothalamo-pituitary disease; recent transmeridian travel (exceeding three time zones within 10 d); acute weight loss or gain (>2 kg change in 3 wk); or failure to provide voluntary, written, witnessed institutional review board-approved informed consent.
Sex steroid assays
T, E2, DHT, and E1 were measured in 8:00 am serum samples by published validated mass spectrometry in the Mayo Laboratory, exactly as described (31–33). Bioavailable and free T were calculated as reported earlier (34). Albumin, fasting plasma glucose, and serum insulin levels were assayed, as cited (35). Homeostasis model assessment insulin resistance index (HOMA-IR) was calculated as glucose (milligrams per deciliter) × insulin (microunits per milliliter)/405, as referenced in the Supplemental Materials, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Proinflammatory cytokine multiplex assay
The human proinflammatory 7-Plex ultrasensitive kit from Mesoscale Discovery (K15008C) was used on EDTA-plasma samples. The ELISA kit provides a 96-well microtiter plate with spatially distributed capture antibodies against seven analytes: adiponectin, TNF-α, IL-1β, IL-6, IL-8, IL-10, and IL-12. Captured analytes are detected by a cocktail of detection antibodies specific to each analyte forming an antibody-analyte-antibody sandwich. The detection antibodies are coupled to an electrochemiluminescent compound, which is activated by an electrical charge applied to each well of the plate. The light signal generated is captured by a cooled coupled detector and interrogated against a standard curve. Quality controls at three different levels of each analyte, purchased from R&D Systems (QC-01), were included with each plate.
SHBG assays
Serum SHBG concentrations were quantified in each subject by four methods.
Immulite 2000
The Immulite 2000 SHBG test (L2SH2; Siemens Healthcare Diagnostics) is a solid-phase, two-site chemiluminescent immunoassay. A polystyrene bead coated with a monoclonal antibody specific for SHBG binds to SHBG in the serum sample, and then alkaline phosphatase-conjugated anti-SHBG (polyclonal) is added to an antibody sandwich complex. After washing, a chemiluminescent substrate (phosphate ester of adamantyl dioxetane) is added, and hydrolysis produces emission of light. The photon output is proportional to the concentration of SHBG in the sample. The method is standardized against the first International Standard for SHBG from the National Institute for Biological Standards and Control (NIBSC) code 95/560. The measurement range is from 2.0 nmol/L to180 nmol/L. Intraassay coefficients of variation (CVs) are 2.7% and 3.1% at 5.5 and 96 nmol/L, respectively. Interassay CVs are 4.0% at 5.4 and 5.9% at 74 nmol/L.
COBAS e411
The COBAS e411 SHBG assay (Roche Diagnostics) is an automated immunochemiluminescence assay based on the sandwich principle. A biotinylated monoclonal SHBG-specific antibody and a monoclonal SHBG-specific antibody labeled with a ruthenium complex form a sandwich complex with SHBG in the sample. During the second incubation, streptavidin-coated microparticles are added and microparticles are magnetically captured onto the surface of the electrode. Application of a voltage to the electrode then induces chemiluminescent emission, which is measured by a photomultiplier. The method is standardized against NIBSC code 95/560. Measuring range is from 0.800 to 200 nmol/L. Intraassay CVs are 2.1%, 2.4%, and 2.7% at 14, 44, and 204 nmol/L, respectively. Interassay CVs are 2.7% at 14 nmol/L and 5.6% at 204 nmol/L.
Mayo Laboratory mass spectrometry: IAL SHBG assay
This assay requires immunoextraction of SHBG, trypin digestion into peptides, and mass spectrometric (MS) quantitation of the IALGGLLFPASNLR (IAL) peptide. The immune extraction of SHBG is performed by using 20 μL of serum mixed with 8 μg of antihuman SHBG monoclonal antibody (GenWay) conjugated to 100 μL of magnetic beads. A stable isotope of the peptide is added to each reaction to adjust for recovery. Three transitions are monitored on the MS (721.92+/657.4, 721.92+/804.4, 721.92+/917.5). The assay was standardized against NIBSC code 95/560, using seven calibrators prepared by spiking purified SHBG into depleted serum. Absolute quantification is performed by liquid chromatography-tandem mass spectrometry using an API 5000 triple quadrupole mass spectrometer (Applied Biosystems). They assay has CVs of 9.0%, 9.5%, and 5.5% at the control levels of 10.7, 56.2, and 147.6 nmol/L. Full assay details are given in the Supplemental Materials. A schematic outline of the two MS SHBG assay procedures, which differ in the sequence of trypsinization, is given in Supplemental Figure 1.
Mayo Laboratory mass spectrometry: SISCAPA SHBG assay
The method proceeds via trypsin digestion of whole serum, immune extraction of IAL peptide, and MS quantitation of the IAL peptide. The assay uses 20 μL of EDTA plasma that is trypsin digested. The IAL peptide is immune extracted using a polyclonal rabbit anti-SHBG peptide antisera conjugated to protein G magnetic beads. Extraction uses an automated Thermo KingFisher flex magnetic particle processor. The assay is also standardized to NIBCS code 95/560, using seven calibrators prepared by spiking purified SHBG into depleted serum. Absolute quantification is performed by liquid chromatography-tandem mass spectrometry using an API 5000 triple quadrupole mass spectrometer (Applied Biosystems). They assay has CVs of 15.3%, 5.3%, and 3.1% at the control levels of 10.9, 55.7, and 141 nmol/L. Further assay details are given in Supplemental Materials.
AVF and TAF
A single-slice computed tomography scan at L3-L4 was used to estimate AVF and total (AVF plus sc) abdominal fat (TAF), as defined previously (36).
Statistical methods
The Matlab statistical toolbox (Mathworks, 2012 version) was applied to regress log SHBG estimates on epidemiological, metabolic, and cytokine parameters. The dependent variable was natural logarithm transformed to reduce heteroskedasticity of residuals. Univariate significance was construed at P < .01 to protect against false positives due to multiple comparisons. Thus, univariate 0.01 less than P < .05 was considered a trend. Slopes (β) and their 95% confidence intervals were estimated along with R2 values (square of correlation coefficient). Experiment-wise P < .05 was used to test the significance of the final stepwise backward-elimination multivariate regression analysis model variables.
Results
Epidemiological characteristics of the cohort of 120 men are given in Table 1. Mean (±SD), median, and absolute range are specified for each variable. By intention, age ranged from 18 to 80 years, and BMI from 20 to 43 kg/m2. AVF and TAF showed an expected wide range. Metabolic indices (albumin, glucose, insulin, adiponectin), sex steroids (T, E2, DHT, E1), cytokines, and SHBG concentrations fell within the normal range for age and gender. In one man, fasting plasma glucose was 129 mg/dL.
Table 1.
Epidemiologic, Metabolic, and Cytokine Data in Men (n = 120)
| Mean | Median | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Age, y | 48 | 48 | 18 | 18 | 80 |
| BMI, kg/m2 | 28 | 27 | 3.9 | 20 | 43 |
| AVF, cm2 | 164 | 154 | 102 | 11.3 | 440 |
| TAF, cm2 | 372 | 356 | 179 | 35.9 | 918 |
| Albumin, g/dL | 4.4 | 4.4 | 0.27 | 4 | 5.1 |
| Glucose, mg/dL | 95 | 94 | 9.0 | 77 | 129 |
| Insulin, mU/L | 6.4 | 5.2 | 4.5 | 0.8 | 26 |
| HOMA-IR | 1.36 | 1.09 | 1.04 | 0.16 | 5.92 |
| E1, pg/mL | 39 | 36 | 13 | 16 | 92 |
| E2, pg/mL | 28 | 27 | 10.6 | 3 | 65 |
| DHT, pg/mL | 395 | 384 | 158 | 56 | 850 |
| T, ng/dL | 564 | 538 | 186 | 226 | 1030 |
| Free T, ng/dLa | 13 | 12 | 7.4 | 6.7 | 40 |
| Bioavailable T, ng/dLa | 165 | 150 | 96 | 21 | 520 |
| IL-10, pg/mL | 39 | 38 | 385 | 23 | 4220 |
| IL-12, pg/mL | 107 | 41 | 1087 | 10.5 | 11 910 |
| IL-1β, pg/mL | 1.00 | 0.37 | 1.0 | 0.032 | 5.67 |
| TNF-α, pg/mL | 3.28 | 2.82 | 1.80 | 1.03 | 12.4 |
| IL-6, pg/mL | 0.83 | 0.49 | 1.32 | 0.16 | 11.6 |
| IL-8, pg/mL | 3.68 | 3.31 | 2.16 | 0.78 | 14.9 |
| Adiponectin, mg/L | 9.0 | 7.1 | 6.5 | 1.8 | 45 |
| SHBG-Immulite | 39 | 36 | 18 | 7.9 | 112 |
| SHBG-Cobas | 40 | 39 | 19 | 9.3 | 115 |
| SHBG-MS-IAL | 37 | 35 | 19 | 6.3 | 121 |
| SHBG-MS-SISCAPA | 36 | 34 | 19 | 5.0 | 122 |
Abbreviation: TAF, total abdominal fat cross-sectional area at L3-L4 interspace. Immulite used the manufacturer's in-house SHBG standard, but the other three assays all used the NIBSC code 95/560.
Calculated bioavailable T.
Interassay comparisons by simple linear correlations showed that outcomes in the four SHBG assays correlated well, as summarized in Supplemental Table 1. Slopes of linear interassay regressions varied from 0.926 to 1.05 for the six paired comparisons with R2 = 0.948–0.985 (all P < 10−4). The Cobas immunoassay (Roche Diagnostics) correlated with the two MS assays with slopes indistinguishable from unity. These three assays all used the same NIBSC code 95/560 SHBG standard. To permit visual comparisons of the assays objectively, both Bland-Altman plots (n = 6) and Passing-Bablok plots (n = 6) are presented in Supplemental Materials (Figures 2 and 3). Matching parameters were calculated. Supplemental Tables 2 and 3 summarize the comparisons and reference these methods. Interassay agreement was excellent across the range from just detectable to SHBG levels of 100 nM, above which assays may differ markedly. Upper and lower bounds for 95% confidence interval are plotted to illustrate these points.
Figure 2.
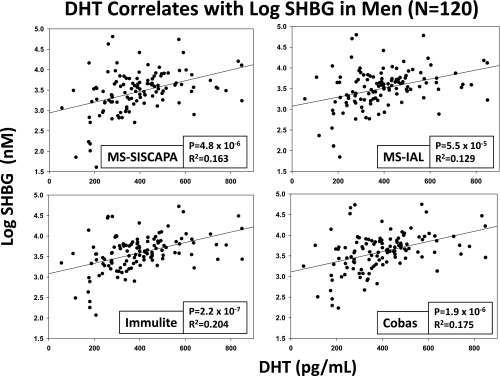
DHT predicts (log) SHBG concentrations in four assays. See Figure 1 for data format.
Figure 3.
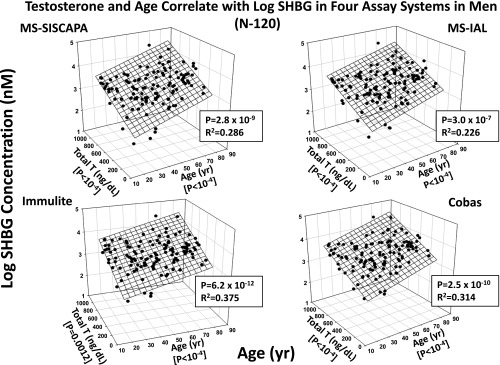
Dual determination of (log) SHBG levels by age (years) and total T (nanograms per deciliter) in each of four SHBG assays in 120 healthy men. P and R2 values are for the joint correlations.
Univariate regression of log SHBG estimates (dependent variable) on metabolic parameters (independent variables shown in Table 1) disclosed consistent SHBG associations (identified by P < .01 for all four SHBG assays) with each of age, insulin, adiponectin, total T, and DHT (see Supplemental Table 4). In particular, age was a strongly positive (P < 10−4) and insulin a strongly negative correlate (P ≤ .00076) of SHBG quantified in both immunoassays (Immulite; Siemens Healthcare Diagnostics; Cobas; Roche Diagnostics) and both MS methods (IAL; GenWay; SISCAPA). Significantly positive univariate correlates of SHBG in all four assays were adiponectin (P ≤ .0084; Figure 1), total T (P < 10−4), and DHT (P < 10−4; Figure 2). In contrast, glucose, albumin, TAF, AVF, estrogens, and cytokines were nonsignificant univariate correlates of SHBG in any of the same four assays. BMI was negatively correlated with untransformed log SHBG (see Supplemental Table 4 footnote). HOMA-IR was an even stronger negative correlate of SHBG in all four assays.
Figure 1.
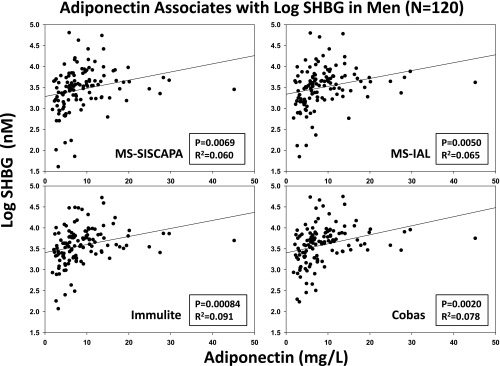
Regression of (log) SHBG concentrations on adiponectin concentrations in 120 men. Individual panels reflect the distinct SHBG assays used. P and R2 values are stated for the univariate effect.
Multivariate regression analysis, using all independent variables including sex steroids, yielded a consensus (four of four assays) SHBG correlations jointly with age (positively) and total T (positively) (Supplemental Table 5 and Figure 3). Multivariate model significance levels were P < 10−11 and 0.381 R2 or less 0.525 or less, thus explaining one third to one half of intersubject variance. In three of the four SHBG assays (the exception was MS-IAL), DHT also positively covaried with SHBG concentrations (partial P ≤ .0050). In the two immunoassays only, SHBG varied directly with albumin at a trend level (partial P ≤ .033), whereas in the two MS assays only, SHBG trended directly with free T (partial P ≤ .026). In individual assays of SHBG, there were trendwise negative associations between SHBG and either IL-1β (partial P ≤ .04) or bioavailable T (partial P = .026).
When sex steroid measurements were not included in the multivariate analysis, SHBG robustly varied with age (positively) and insulin (negatively) in all four SHBG assays. The overall value of P was < 10−6 and 0.293 R2 or greater 0.215 or greater (Figure 4 and Table 2). Individual values for the contributions of age and insulin were both partial P < .001. Adiponectin was a trend-level positive covariate in the final model in one immunological SHBG assay (partial P = .037), whereas AVF was a trend-level negative covariate in the other immunological assay (partial P = .047).
Figure 4.
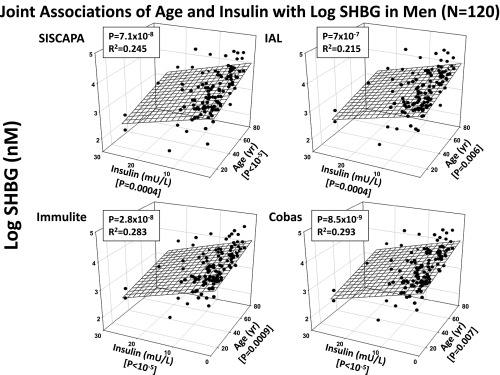
Combined prediction of (log) SHBG concentrations in four assays (vertical, z-axis) by age (x-axis) and insulin (y-axis) in 120 men. Joint R2 and P values are given in each panel.
Table 2.
Multivariate Regression of Log SHBG on (Non-Sex Steroid) Variables in Men
| Dependent Variable | Independent Variables |
Overall P and R2 Values |
||
|---|---|---|---|---|
| Age | Insulin | Other | Final Model | |
| Log SHBG | ||||
| MS-SISCAPA | P < 10−5 | P = .0004 | None | P = 7.1 × 10−8 |
| Slope = 0.011 | Slope = −0.034 | R2 = 0.245 | ||
| MS-IAL | P < 10−5 | P = .0006 | None | P = 7.0 × 10−7 |
| Slope = 0.0093 | Slope = −0.031 | R2 = 0.215 | ||
| Immulite | P < 10−5 | P = .0009 | AVF: P = 0.047 | P = 2.8 × 10−8 |
| Slope = 0.11 | Slope = −0.029 | Slope = −9.1 × 10−4 | R2 = 0.283 | |
| Cobas | P < 10−5 | P = .0007 | Adiponectin: P = 0.037 | P = 8.5 × 10−9 |
| Slope = 0.0093 | Slope = −0.029 | Slope = 1.2 × 10−5 | R2 = 0.293 | |
Discussion
In a cohort of 120 healthy men, SHBG concentrations measured in each of four assays, three of which used the identical human SHBG standard, were strongly associated at the univariate level with age (positive), insulin (negative), HOMA-IR (negative), and the adipokine and adiponectin, (positive). Contrary to our initial hypothesis, the pro- and antiinflammatory cytokines examined here did not correlate with SHBG concentrations univariately in any of the SHBG assays. The single exception was adiponectin (Figure 1), which was positively, albeit weakly, correlated with SHBG in all four SHBG assays. Positive controls were expected correlations between SHBG and total T and DHT concentrations, inasmuch as 50% or more of total plasma T and DHT is contained in the SHBG-bound fraction. However, estrogens did not correlate with SHBG. At the multivariate reductionistic level, omitting the T and DHT effects, age and insulin together explained approximately 25% of the intersubject variability in the 18- to 80-year-old men studied here (Figure 4). Inconsistent or trend-level covariates were albumin and IL-1β in one or two SHBG assays each. Accordingly, both consistent (in all four SHBG assays) and inconsistent (in one or two SHBG assays) associations with SHBG exist in healthy men, depending upon the assay platform and the biological standards used for the SHBG determinations.
Important inconsistencies were noted among assays
DHT was a positive predictor in three of the four SHBG assays; albumin, IL-1β, and free T in two of the four assays; and bioavailable T in only one of the four assays. The direction of influence, viz. whether T (or DHT) elevates SHBG, or whether T (or DHT) reflects SHBG levels because of strong binding to SHBG, cannot be determined from our data alone because correlations do not prove causation. The positive correlation of SHBG with both T and DHT tends to favor binding of T and DHT to SHBG as the basis for the association. Conversely, T and nonaromatizable androgens typically suppress SHBG synthesis (10, 16) but not always (18). Notably, when sex steroids are omitted from the set of possible independent variables, age and insulin together account for one quarter of the variability in SHBG concentrations, leaving unidentified factors to explain the remaining variance. Heredity, ethnicity, race, physical fitness, T4, and assay error are relevant other considerations in this regard (7, 9, 11, 37).
The uniformly positive effect of age on SHBG measurements might be explicable in part by the strong covariation between age and adiponectin (P ≤ .0084) because adiponectin was not only a consistently positive univariate correlate of SHBG but also tended to increase with age (present data, P = .011). In addition, in some studies adiponectin increases as T levels decrease (13, 25). However, age remained a major covariate of SHBG when the effects of adiponectin were controlled for by a multivariate analysis. Indeed, the positive correlation with adiponectin was attributed largely to five individuals with higher adiponectin and higher SHBG levels, resulting in a weak overall correlation. Other correlates of age in the present cohort of 120 men included BMI, albumin, glucose, insulin, HOMA-IR, E1, AVF and TAF, free T, and bioavailable T. Among these age-related factors, only insulin and HOMA-IR were negative covariates of SHBG across all four assays. In this regard, insulin directly represses the SHBG gene in vitro (17). However, the positive association of age with SHBG was significant after accounting for the change of insulin with age, thus indicating at least partially independent effects of age and insulin in the cohort studied here.
Insulin was an unequivocal negative univariate correlate, and strong negative joint multivariate correlate with age, of SHBG levels in all four SHBG assay platforms when sex steroids were not included in the final model. HOMA-IR behaved similarly to insulin. Primary correlates of insulin in the present cohort of men included AVF, BMI, glucose, and adiponectin, putatively reflecting more broadly insulin resistance (HOMA-IR, AVF, BMI, glucose) and insulin sensitivity (adiponectin). However, in the condensed multivariate model, AVF and adiponectin only trended to significance once age and insulin were accounted for. The combined explanatory power of age and insulin as independent variables was 21.5%–24.5% of the intersubject variability in the two MS SHBG assays and 28.3%–29.3% in the two immunological SHBG assays, when explanatory power was defined as the square of the correlation coefficient. Together these data further support the notion that low SHBG in men covaries with clinical risk of hyperinsulinism, insulin resistance, the metabolic syndrome and type 2 diabetes mellitus (38, 39).
Contrary to the a priori hypotheses, proinflammatory (IL-1β, TNF-α, IL-6, or IL-8) and antiinflammatory (IL-10, IL-12) cytokines bore no consistent univariate relationships with any of the four SHBG measurements (all P ≥ .053). By multivariate analysis, IL-1β was negatively related to SHBG in two assays only at a trend level. This was unexpected, given that certain cytokines like TNF-α can reduce SHBG production in vitro (24).
Each of the two MS assays has both advantages and disadvantages. Prior extraction of the immunologically reactive SHBG provides additional specificity because the antiprotein antibodies generally do not react with precursor forms or degradation products of mature SHBG, whereas antipeptide antibodies would extract these extra forms. This assay specificity may relate to the clinical performance differences noted. On the other hand, the extraction of the protein requires specific antisera, which generally are more difficult to develop than antipeptide antisera. Fortunately, commercial anti-SHBG antisera were available for this study. A second advantage of extracting the protein prior to trypsin digestion is the marked reduction in the amount of protein that needs to be digested. This reduced protein makes the digestion more efficient and should better assure completeness of digestion.
Caveats in this study include the need to assess longitudinal stability of the observed SHBG correlations because correlations do not establish causation; evaluate larger cohorts of healthy men; compare multiple SHBG assays with an identical SHBG standard (here three of four assays used NIBSC code 95/560) assess other unique peptide sequences in SHBG for possible MS assay targets; evaluate other immunoassays against the putatively criterion-based MS method; accomplish similar studies in women and adolescents; further assess the possible effects of cytokines; and ultimately elucidate molecular bases for reproducible pathophysiological associations with SHBG. Lastly, whereas assays generally agreed, differences were identifiable in Bland-Altman and Passing-Bablok plots at extreme SHBG levels.
In summary, quantification of SHBG in two immunoassays and two MS assays in 120 healthy men aged 18–80 years discloses consistent univariate (age, insulin, adiponectin, HOMA-IR, total T, DHT) and multivariate (age and insulin) associations with this glycoprotein in all four SHBG platforms. There were weaker inconsistent correlations of free T, bioavailable T, albumin, and IL-1β with individual SHBG assays. These outcomes may help explain nonuniformly reproduced results in earlier (single assay) studies. The MS of SHBG using unique peptide sequences has the potential to become an interinstitutional standard, although certain immunoassays appear to correlate closely with MS.
Supplemental material is available.
Acknowledgments
We thank Jill Smith for the support of the manuscript preparation; the Mayo Immunochemical Laboratory for assay assistance; and the Mayo research nursing staff for implementing the protocol.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of National Institutes of Health or the National Center for Advancing Translational Sciences.
This work was supported in part by Grants AG019695, AG031763, and P30 DK050456 (to the Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD), Grant UL1 TR000135 from the National Center for Advancing Translational Sciences, and Grant 60NANB10D005Z from the National Institute of Standards and Technology.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVF
- abdominal visceral fat
- BMI
- body mass index
- CV
- coefficient of variation
- DHT
- dihydrotestosterone
- E1
- estrone
- E2
- 17 β-estradiol
- HOMA-IR
- homeostasis model assessment insulin resistance index
- IAL
- IALGGLLFPASNLR
- MS
- mass spectrometric
- NIBSC
- National Institute for Biological Standards and Control
- TAF
- total (AVF plus sc) abdominal fat.
References
- 1. Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175(1):113–120 [DOI] [PubMed] [Google Scholar]
- 2. LeBlanc ES, Nielson CM, Marshall LM, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94(9):3337–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naessen T, Sjogren U, Bergquist J, Larsson M, Lind L, Kushnir MM. Endogenous steroids measured by high-specificity liquid chromatography-tandem mass spectrometry and prevalent cardiovascular disease in 70-year-old men and women. J Clin Endocrinol Metab. 2010;95(4):1889–1897 [DOI] [PubMed] [Google Scholar]
- 4. Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010;95(6):2790–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biswas M, Hampton D, Turkes A, Newcombe RG, Aled RD. Reduced total testosterone concentrations in young healthy South Asian men are partly explained by increased insulin resistance but not by altered adiposity. Clin Endocrinol (Oxf). 2010;73(4):457–462 [DOI] [PubMed] [Google Scholar]
- 6. Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol. 2010;316(1):79–85 [DOI] [PubMed] [Google Scholar]
- 7. Orwoll ES, Nielson CM, Labrie F, et al. Evidence for geographical and racial variation in serum sex steroid levels in older men. J Clin Endocrinol Metab. 2010;95(10):E151–E160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vanbillemont G, Bogaert V, De Bacquer D, et al. Polymorphisms of the sex hormone-binding globulin gene contribute to the interindividual variation of sex steroid hormone blood levels in young, middle-aged and elderly men. Clin Endocrinol (Oxf). 2008;70(2):303–310 [DOI] [PubMed] [Google Scholar]
- 9. Giton F, Fiet J, Cornu JN, et al. Serum sex steroids measured in middle-aged European and African-Caribbean men using gas chromatography-mass spectrometry. Eur J Endocrinol. 2011;165(6):917–924 [DOI] [PubMed] [Google Scholar]
- 10. Lakshman KM, Kaplan B, Travison TG, et al. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab. 2010;95(8):3955–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooper CS, Taaffe DR, Guido D, Packer E, Holloway L, Marcus R. Relationship of chronic endurance exercise to the somatotropic and sex hormone status of older men. Eur J Endocrinol. 1998;138(5):517–523 [DOI] [PubMed] [Google Scholar]
- 12. Leifke E, Gorenoi V, Wichers C, Von Zur MA, Von BE, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf). 2000;53(6):689–695 [DOI] [PubMed] [Google Scholar]
- 13. Yasui T, Tomita J, Miyatani Y, et al. Associations of adiponectin with sex hormone-binding globulin levels in aging male and female populations. Clin Chim Acta. 2007;386(1–2):69–75 [DOI] [PubMed] [Google Scholar]
- 14. Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab. 1996;81(5):1821–1826 [DOI] [PubMed] [Google Scholar]
- 15. Xita N, Tsatsoulis A. Genetic variants of sex hormone-binding globulin and their biological consequences. Mol Cell Endocrinol. 2010;316(1):60–65 [DOI] [PubMed] [Google Scholar]
- 16. Forest MG, Lecoq A, David M, Pugeat M. Effects of human chorionic gonadotropin, androgens, adrenocorticotropin hormone, dexamethasone and hyperprolactinemia on plasma sex steroid-binding protein. Ann NY Acad Sci. 1988;538:214–234 [DOI] [PubMed] [Google Scholar]
- 17. Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67(3):460–464 [DOI] [PubMed] [Google Scholar]
- 18. Lee IR, Dawson SA, Wetherall JD, Hahnel R. Sex hormone-binding globulin secretion by human hepatocarcinoma cells is increased by both estrogens and androgens. J Clin Endocrinol Metab. 1987;64(4):825–831 [DOI] [PubMed] [Google Scholar]
- 19. Vanbillemont G, Lapauw B, De Naeyer H, Roef G, Kaufman JM, Taes YE. Sex hormone-binding globulin at the crossroad of body composition, somatotropic axis and insulin/glucose homeostasis in young healthy men. Clin Endocrinol (Oxf). 2012;76(1):111–118 [DOI] [PubMed] [Google Scholar]
- 20. Bonnet F, Cephise FL, Gautier A, et al. Role of sex steroids, intra-hepatic fat and liver enzymes in the association between SHBG and metabolic features. Clin Endocrinol (Oxf). 2013;79(4):517–522 [DOI] [PubMed] [Google Scholar]
- 21. Tchernof A, Labrie F, Belanger A, et al. Relationships between endogenous steroid hormone, sex hormone-binding globulin and lipoprotein levels in men: contribution of visceral obesity, insulin levels and other metabolic variables. Atherosclerosis. 1997;133(2):235–244 [DOI] [PubMed] [Google Scholar]
- 22. Gyllenborg J, Rasmussen SL, Borch-Johnsen K, Heitmann BL, Skakkebaek NE, Juul A. Cardiovascular risk factors in men: the role of gonadal steroids and sex hormone-binding globulin. Metabolism. 2001;50(8):882–888 [DOI] [PubMed] [Google Scholar]
- 23. Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91(1):345–347 [DOI] [PubMed] [Google Scholar]
- 24. Simo R, Barbosa-Desongles A, Saez-Lopez C, Lecube A, Hernandez C, Selva DM. Molecular mechanism of TNFα-induced down-regulation of SHBG expression. Mol Endocrinol. 2012;26(3):438–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gannage-Yared MH, Khalife S, Semaan M, Fares F, Jambart S, Halaby G. Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotropic axis in healthy non-diabetic elderly men. Eur J Endocrinol. 2006;155(1):167–176 [DOI] [PubMed] [Google Scholar]
- 26. Cunningham SK, McKenna TJ. Evaluation of an immunoassay for plasma sex hormone-binding globulin: comparison with steroid-binding assay under physiological and pathological conditions. Ann Clin Biochem. 1988;25:360–366 [DOI] [PubMed] [Google Scholar]
- 27. Chiu CL, Randall S, Molloy MP. Recent progress in selected reaction monitoring MS-driven plasma protein biomarker analysis. Bioanalysis. 2009;1(4):847–855 [DOI] [PubMed] [Google Scholar]
- 28. Zhi W, Wang M, She JX. Selected reaction monitoring (SRM) mass spectrometry without isotope labeling can be used for rapid protein quantification. Rapid Commun Mass Spectrom. 2011;25(11):1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cekan SZ. Biases in the assays of steroids and their binding proteins. J Steroid Biochem. 1987;27(1–3):95–98 [DOI] [PubMed] [Google Scholar]
- 30. Taieb J, Mathian B, Millot F, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49(8):1381–1395 [DOI] [PubMed] [Google Scholar]
- 31. Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJ, III, Riggs BL. Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int. 2008;19(10):1465–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh RJ. Validation of a high throughput method for serum/plasma testosterone using liquid chromatography tandem mass spectrometry (LC-MS/MS). Steroids. 2008;73(13):1339–1344 [DOI] [PubMed] [Google Scholar]
- 33. Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–384 [DOI] [PubMed] [Google Scholar]
- 34. Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD. Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab. 2007;92(9):3626–3632 [DOI] [PubMed] [Google Scholar]
- 35. Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659 [DOI] [PubMed] [Google Scholar]
- 36. Veldhuis JD, Erickson D, Mielke K, Farhy LS, Keenan DM, Bowers CY. Distinctive inhibitory mechanisms of age and relative visceral adiposity on GH secretion in pre- and postmenopausal women studied under a hypogonadal clamp. J Clin Endocrinol Metab. 2005;90(11):6006–6013 [DOI] [PubMed] [Google Scholar]
- 37. Brenta G, Schnitman M, Gurfinkiel M, et al. Variations of sex hormone-binding globulin in thyroid dysfunction. Thyroid. 1999;9(3):273–277 [DOI] [PubMed] [Google Scholar]
- 38. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299 [DOI] [PubMed] [Google Scholar]
- 39. Vikan T, Schirmer H, Njolstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur J Endocrinol. 2010;162(4):747–754 [DOI] [PubMed] [Google Scholar]


