Abstract
Crosslinking soft tissue has become more common in tissue engineering applications, and recent studies have demonstrated that soft tissue mechanical behavior can be directly altered through crosslinking. Using a recently reported test method that shears adjacent disc lamella, the effect of genipin crosslinking on interlamellar shear resistance was studied using in vitro bovine disc annulus.
Specimens of adjacent lamella were dissected from 4 discs taken from 3 fresh frozen bovine tails. These specimens were paired and soaked in either 50mM EPPS Phosphate (ph9) with 20mM genipin at 37°C for 4 hours or in 50mM EPPS Phosphate (ph9) of which twelve specimens (6 per treatment) were successfully tested and analyzed.
Crosslinked specimens were noted to have significantly higher yield force per width (59%), peak force per width (70%), and resilience (69%) compared to sham treated controls, supporting the hypothesis that genipin crosslinking increases the resistance to interlamellar shear of the annulus interface. Additionally, a possible dependency may exist between the interlamellar shear strength and neighboring lamella because of the bridging fiber network previously described by other investigators.
Keywords: disc, lamella, interfacial shear, lamellar shear, crosslinking, genipin
Introduction
The gross anatomy of the annulus fibrosus consists of concentric lamella composed of parallel collagen fibers with orientation that alters from adjacent lamella (Coventry et al., 1945). Tear like defects in the annulus are classified usually by location and direction of the tear, as in rim lesion (RL), concentric tear (CT), or radiating tear (RT) (Vernon-Roberts et al., 2007). Such tears are common in adult human discs and strongly correlated to age. CT tears, comprised primarily of delamination of adjacent lamellae, are the most common and the first to appear (Osti et al., 1990; Vernon-Roberts et al., 2007).
Mechanically, annular tears decrease motion segment stiffness in bending, flexion, and torsion (Fazzalari et al., 2001; Thompson et al., 2000) and CT tears may affect other types of tears due to increased stress near the structural defect (Goel et al., 1995; Osti et al., 1990) but a direct connection between annular tears and pain has only been weakly established. In Thompson's study all cadaver donors were described as having “no history of back pain” and Fazzalari's study utilized animals. Osti (Osti et al., 1990) postulated that pain provocation during discography was “closely related to the presence of tears extending to the outer lamellae of the annulus fibrosus”. Freemont (Freemont et al., 1997) demonstrated that painful discs had more sensory innervation and deeper innervation than pain free discs and aged and pain free discs.
Recently Weiler (Weiler et al., 2002) discussed a connection between annulus tearing/degradation and the presence of matrix metalloproteinases and cytokines that may affect pain. While the presence of sensory innervation near a lesion where shearing stresses are increased (Goel et al., 1995) seems a reasonable mechanism for pain generation, it has yet to be proven true. Nevertheless, tears in the annulus is evidence of structural overload, and presents a structural failure meeting Adams (Adams and Roughley, 2006) proposed definition for a degenerated disc.
In the intervertebral disc, the use of genipin crosslinking has been shown to alter several mechanical parameters which may be advantageous in treating degenerative discs and back pain. For example, studies have demonstrated increased circumferential stiffness (Chuang et al., 2007), yield stress, and resilience (Slusarewicz et al., 2011) of annular tissue. Such effects also extend to the intervertebral joint behavior including changes in axial neutral zone parameters (Barbir et al., 2010; Yerramalli et al., 2007), flexion-extension neutral zone parameters (Hedman et al., 2006; Kirking et al., 2013), and maximum disc bulge during axial loading (Slusarewicz et al., 2011). It was hypothesized that changes in the disc mechanical behavior with cross linking would also extend to an increased resistance against shearing of the interlamellar interface. If true, then exogenous crosslinking of the annulus may have potential therapeutic benefits for the treatment or prevention of disc tears; and along with increasing the circumferential tensile strength of the annulus, decreasing disc bulge, and stabilizing the intervertebral joint, cross linking may be useful in the treatment and prevention of lower back pain.
Recently, Gregory (Gregory et al., 2011) described a method of testing the interface strength between disc lamellae, observing that the shear stresses that lead to debonding are concentrated at the edge of the bonded interface. The described test presents a useful tool for understanding interlamellar tears and their propagation. Using this method, the primary objective of the present study was to test the hypothesis that genipin cross linking would increase the force necessary to debond the interlamellar interface when subjected to shear loading.
Methods
Adjacent musculature was removed from three fresh frozen bovine tails and the spines stored wrapped in plastic. While frozen, the caudal disc locations were identified, cut along the mid sagittal plane, and excised from the endplate. The nucleus was removed and the annulus samples placed in 0.15M PBS to soak overnight at 4°C. The free swelling enlarged the lamella making identification and dissection of the lamella interface easier. After swelling, specimens were refrozen and then while still frozen hand-dissected to isolate a single interlamella interface (Figure 1) from the outer half of the annulus. Additional adjacent lamellae were maintained on both sides of the interlamellar interface of the specimen. Samples were treated for 4 hours at 37°C in 50mM EPPS Phosphate buffer (ph9) (sham) or 50mM EPPS Phosphate (ph9) with 20mM genipin at 37°C (crosslink treated) and then stored overnight at 4C in their respective solutions.
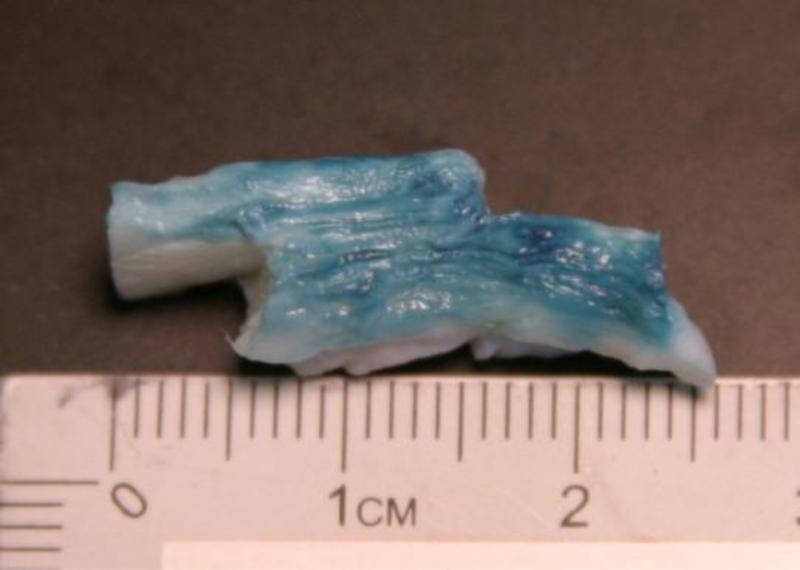
Figure 1.
Bovine annulus specimens after dissection to isolate a single interlamellar interface. Note presence of multiple lamella on either side of interface. Surface was colored with blue dye to enhance lamella boundaries.
One disc per tail was dissected into four specimens producing 12 specimens from 3 tails. A second disc was taken from one tail yielding three additional samples resulting in 15 total samples. To reduce interspecimen variability, samples were paired with two sham and two genipin treated samples from each disc, and each sham/genipin pair further grouped by size. Specimen interface width, thickness and length were measured using calipers after treatment and immediately before testing.
Mechanical testing was conducted using a Test Resources R-1000 frame and 100N load cell. Custom rake fixtures were used to clamp the specimens (Figure 2). The location of the interlamellar lap joint boundaries were marked on the lamina plane and joint midpoint was similarly indicated on the side of the specimen. Two digital cameras monitored the specimens and from the images, stretch of the joint was calculated using the boundary marks. The force - displacement output of the R-1000 was synchronized with the stretch by tracking the clamp positions. Three preconditioning cycles were performed at 1%/sec to 10% strain and testing was carried out at 2%/sec (Gregory et al., 2011). No attempt was made to test the effects of genipin at differing strain rates.
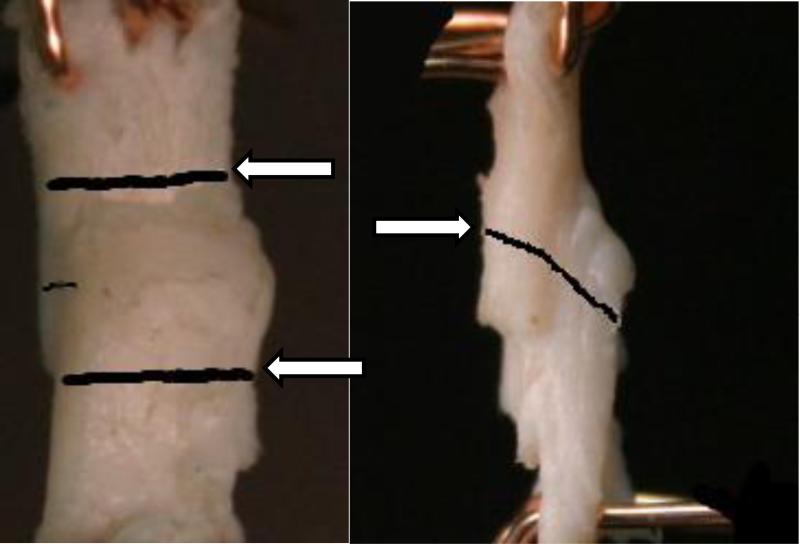
Figure 2.
Lamellar interface plane view (left) showing interface boundaries tracked during the experiment (arrows) and side view (right) showing marking indicating midpoint of interface.
Using Octave, stiffness was calculated by fitting a line (Figure 3) through the linear region of the force – displacement curve (normalized by lap joint width to compare directly with Gregory (Gregory et al., 2011)). Yield force was taken where the measured data deviated from the linear fit by 2%. Peak force was taken as the largest force measured. Work was calculated as the area under the force - displacement curve up to yield and to peak. Statistical significance was tested using the Wilcoxon Rank Sum test on the paired differences with Stata R11.
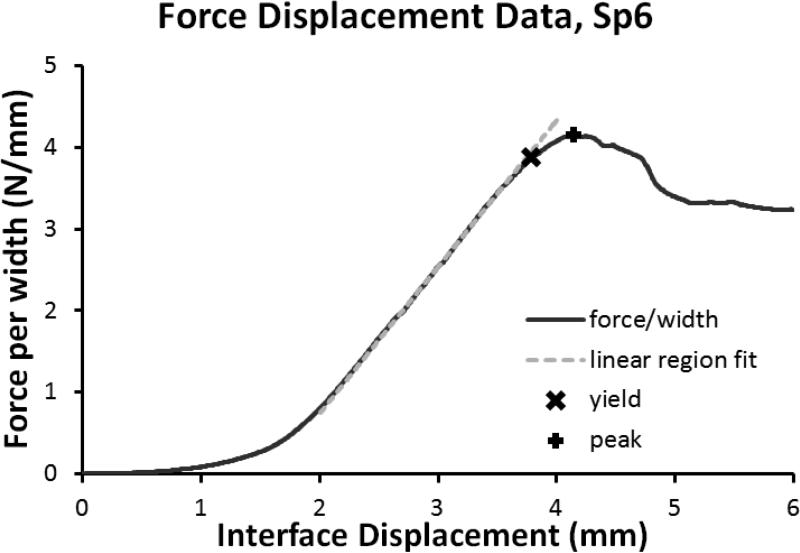
Figure 3.
Example of the force displacement data with the yield point, peak point, and linear region stiffness.
Results
Of the fifteen specimens six sham and six genipin treated specimens were successfully tested. These specimens all failed by debonding of the interlamellar interface. As the crosshead displaced, specimens would stretch as observed by continuity of the indicator mark across the midpoint of the interface. At peak force, the lamella adjacent to the interface would slip but not completely debond. As the displacement continued, the slipping would increase and the force would decrease.
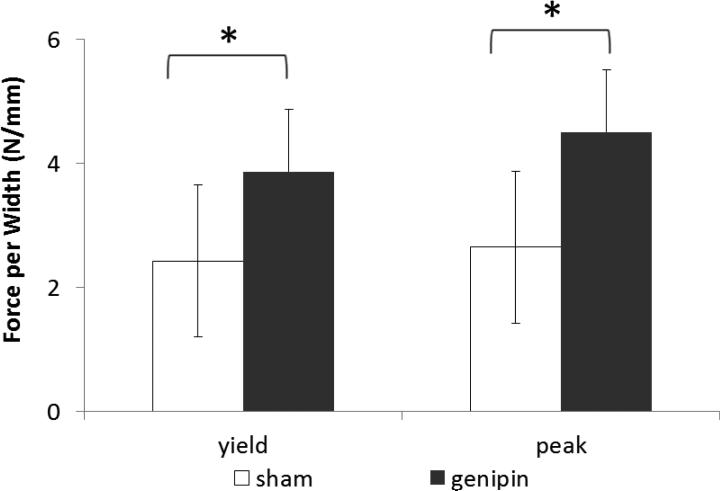
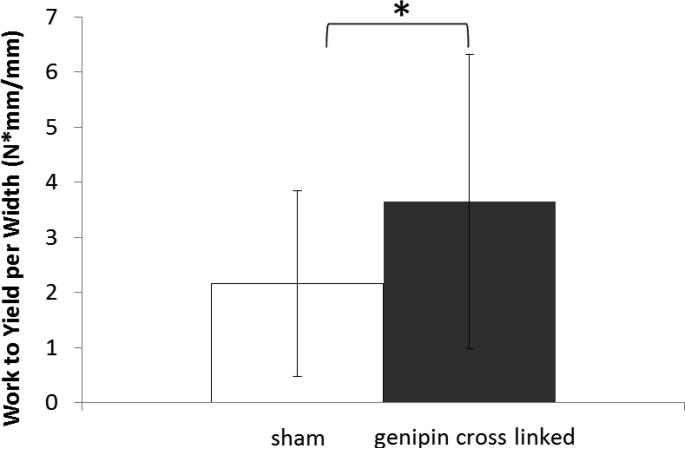
The mean yield force per width was 2.43 N/mm for sham specimens (Table 1), 3.87 N/mm for genipin specimens (59% greater than sham specimens), and the paired difference between the groups was 1.81 N/mm (Figure 4) which was statistically significant (p<.018). The mean peak force per width was 2.65 N/mm for sham specimens, 4.50 N/mm for genipin specimens (70% greater than sham specimens), and the paired difference between the groups was 2.23 N/mm which was statistically significant (p<.018). The mean work to yield was 2.16 J/mm for sham specimens, 3.65 J/mm for genipin specimens (69% greater), and the paired difference between the groups was 2.38 J/mm (Figure 5) which was statistically significant (p<.018).
Table 1.
The shear loading properties of the bovine lamellar interface after sham or genipin crosslink treatment. Units are as follows: forces are in Newtons, force per width in N/mm, work per width in J/mm, stiffness in N/mm/mm, and stretch in mm/mm. Abbreviations are sd=standard deviation and paired difference (Pd. Dif.)
| Parameter | sham mean | sham sd | genipin mean | genipin sd | Pd. Dif. mean | Pd. Dif. sd | Pd. Dif. p value |
|---|---|---|---|---|---|---|---|
| Yield Force | 14.9 | 9.25 | 19.7 | 5.55 | 7.17 | 5.70 | 0.028 |
| Yield Force per Width | 2.43 | 1.23 | 3.87 | 1.01 | 1.81 | 0.87 | 0.018 |
| Stretch at Yield | 1.21 | 0.09 | 1.23 | 0.13 | 0.07 | 0.12 | 0.270 |
| Work to Yield per Width | 2.16 | 1.69 | 3.65 | 2.67 | 2.38 | 1.97 | 0.018 |
| Peak Force | 16.2 | 9.56 | 22.9 | 6.80 | 9.20 | 5.05 | 0.018 |
| Peak Force per Width | 2.65 | 1.23 | 4.50 | 1.01 | 2.23 | 0.81 | 0.018 |
| Stretch at Peak | 1.26 | 0.15 | 1.27 | 0.21 | 0.04 | 0.27 | 0.734 |
| Work to Peak per Width | 3.09 | 1.43 | 5.78 | 5.79 | 4.32 | 5.75 | 0.128 |
| Linear Region Stiffness | 1.46 | 0.23 | 2.30 | 1.26 | 0.71 | 1.18 | 0.176 |
Figure 4.
Interlamellar interface force per width. * Denotes statistically significant difference (p<0.05) of the paired differences.
Figure 5.
Interlamellar interface work to yield. * Denotes statistically significant difference (p<0.05) of the paired differences
Discussion
The purpose of this study was to determine if crosslinking treatment of the annulus fibrosus would increase the load necessary to debond the interlamellar interface. The significant increases in yield force per width, peak force per width, and work to yield supports this hypothesis. Additionally, the crosslink treated samples demonstrated a larger mean stiffness than controls, but the observed difference was not statistically significant, nor was the slight observed increase in stretch to yield and in stretch to peak.
The data from this study suggests that the genipin soaking treatment was very effective at increasing the disc's resistance to interlamellar debonding. In addition to increasing the force to yield, the magnitude of the force to yield after genipin treatment was greater than the mean peak force of the controls. Further, this increase did not appear to be associated with an increase in brittleness as the percent increase in peak force (70%) was larger than the percent increase in force to yield (59%).
The percent increase in force to yield (59%) during shear loading determined in this study was identical to the 59% increase in yield stress previously reported for circumferential tensile testing of genipin soaked annulus tissue (Slusarewicz et al., 2011); however, the 70% increase in peak force during shear loading was much greater than the increase in ultimate stress of 9% during tensile loading. This difference in ultimate failure could result from the difference in failure mechanism within the tissue for the two loading types. In shear, cross linking prevented separation of lamella by reinforcing inter-fiber connections where the bond strength of the exogenous cross links were comparable to existing endogenous cross links. To affect ultimate tension stress, the cross links must reinforce the intact fiber where the cross link bond strength was considerably less than the intact fiber.
The current study results demonstrated some marked differences in the sham treated samples compared to Gregory's initial report (Gregory et al., 2011). First, Gregory's ‘load at peak’, which was more similar to our yield force than our peak force, was approximately 8 times smaller than controls in this study. Also, Gregory's reported mean modulus value was nearly 7× smaller than our controls. Finally, we were not able to consistently identify a post peak plateau force, but most specimens did carry some load after peak force.
There are many differences between this study and Gregory's that preclude direct comparison of the results including the difference in species, preloading, specimen hydration, and geometry. However, a major difference between the studies has to do with the total number of lamella present on either side of the interlamellar interface. Gregory stated that their specimens consisted of “two intact adjacent AF layers with single-layer tabular ends” and in Gregory's thesis, similar experiments are described as containing two adjacent lamella approximately 0.36mm thick. Our specimens, in contrast, had on the order of 2 to 10 extra lamellae present on either side.
Initially, the extra lamellae were expected to provide additional strength for clamping, but not affect the interfacial shear strength. While increasing the number of lamella would likely decrease the amount of tension carried by an individual element, because the material is neither rigid nor isotropic, the distribution of tension load and associated bending moments may not be obvious. More importantly, in light of recent works by Schollum (Schollum et al., 2008) and Pezowicz (Pezowicz et al., 2006), it is likely that our specimens may have demonstrated greater resistance to separation due to the inter connectivity of the bridging fibers into the adjacent lamella. The presence of the fiber bridges in this study presents a significant departure from the lap joint interface idealization in Gregory's model: “the assembly behaves as if the two lamellae are riveted together at both ends of the lap but are free to move with respect to each other in the zone between the rivets” (Gregory et al., 2011) and complicates the analytical stress analysis.
Further, with bridging fibers present, the independence of the interface strength to interface length may no longer apply as the number of bridging fibers in the interface may vary with interface length. Similarly, bridging fiber strength may depend on the number of extra lamella. Therefore, the mean force to yield and mean peak force from this study are also presented (Table 2) after normalizing to lap joint area and lap joint volume. Because the genipin treated samples were thinner and narrower, the overall effect of the alternative normalizations was to increase the effect of genipin for both yield and peak force. Future testing of the lamella connections should take into account the interconnectivity of these bridging fibers for proper interpretation of results with implications for in vivo applications.
Table 2.
Dimensions of the lap joint in millimeters and alternative normalizations based on lap joint area and volume with the following units: force per area (N/mm2) and force per volume (N/mm3). Note that SD refers to standard deviation.
| width | length | area | thickness | |||||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | mean | SD | |
| sham | 6.00 | 0.94 | 5.94 | 1.41 | 35.63 | 10.42 | 6.21 | 1.48 |
| genipin | 5.06 | 0.82 | 5.03 | 0.67 | 25.58 | 6.62 | 5.03 | 1.61 |
| yield force per area | peak force per area | yield force per volume | peak force per volume | |||||
|---|---|---|---|---|---|---|---|---|
| mean | SD | mean | SD | mean | SD | mean | SD | |
| sham | 0.39 | 0.167 | 0.43 | 0.183 | 0.07 | 0.038 | 0.07 | 0.043 |
| genipin | 0.74 | 0.095 | 0.86 | 0.128 | 0.14 | 0.015 | 0.16 | 0.021 |
Acknowledgements
The authors gratefully acknowledge support from the NIH R44AR055014 and the Kentucky SBIR-STTR Matching Funds Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
B. Kirking and T. Hedman disclose that they have or had a financial interest and receive salary and/or stock options from Intralink-Spine and/or Orthopeutics LP (parent company to Intralink-Spine). J. Criscione discloses no financial interest.
Contributor Information
Bryan Kirking, Orthopeutics LP and University of Kentucky Department of Neurosurgery and Biomedical Engineering.
Thomas Hedman, Orthopeutics LP and University of Kentucky Department of Neurosurgery and Biomedical Engineering.
John Criscione, Texas A&M University Department of Biomedical Engineering.
References
- Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- Barbir A, Michalek AJ, Abbott RD, Iatridis JC. Effects of Enzymatic Digestion on Compressive Properties of Rat Intervertebral Discs. J Biomech. 2010;43:1067–1073. doi: 10.1016/j.jbiomech.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang SY, Odono RM, Hedman TP. Effects of exogenous crosslinking on in vitro tensile and compressive moduli of lumbar intervertebral discs. Clin Biomech (Bristol, Avon) 2007;22:14–20. doi: 10.1016/j.clinbiomech.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Coventry M, Ghormley R, Kernohan J. The Intervertebral Disc: Its Microscopic Anatomy And Pathology Part I. Anatomy, Development, and Physiology. The Journal of Bone & Joint Surgery. 1945;27:8. [Google Scholar]
- Fazzalari NL, Costi JJ, Hearn TC, Fraser RD, Vernon-Roberts B, Hutchinson J, Manthey BA, Parkinson IH, Sinclair C. Mechanical and pathologic consequences of induced concentric anular tears in an ovine model. Spine (Phila Pa 1976) 2001;26:2575–2581. doi: 10.1097/00007632-200112010-00010. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O'Brien J, Jayson MIV. Nerve ingrowth into diseased intervertebral disc in chronic back pain. The Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- Goel VK, Monroe BT, Gilbertson LG, Brinckmann P. Interlaminar shear stresses and laminae separation in a disc. Finite element analysis of the L3-L4 motion segment subjected to axial compressive loads. Spine (Phila Pa 1976) 1995;20:689–698. [PubMed] [Google Scholar]
- Gregory DE, Veldhuis JH, Horst C, Wayne Brodland G, Callaghan JP. Novel lap test determines the mechanics of delamination between annular lamellae of the intervertebral disc. J Biomech. 2011;44:97–102. doi: 10.1016/j.jbiomech.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Hedman TP, Saito H, Vo C, Chuang SY. Exogenous cross-linking increases the stability of spinal motion segments. Spine (Phila Pa 1976) 2006;31:E480–485. doi: 10.1097/01.brs.0000224531.49174.ea. [DOI] [PubMed] [Google Scholar]
- Kirking BC, Toungate JK, Hedman TP. The dose response relationship between intervertebral disc flexion-extension neutral zone metrics and injected genipin concentration. Journal of applied biomaterials & functional materials. 2013:0. doi: 10.5301/JABFM.5000151. [DOI] [PubMed] [Google Scholar]
- Osti OL, Vernon-Roberts B, Fraser RD. 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976) 1990;15:762–767. doi: 10.1097/00007632-199008010-00005. [DOI] [PubMed] [Google Scholar]
- Pezowicz CA, Robertson PA, Broom ND. The structural basis of interlamellar cohesion in the intervertebral disc wall. Journal of anatomy. 2006;208:317–330. doi: 10.1111/j.1469-7580.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schollum ML, Robertson PA, Broom ND. ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part I: a microscopic investigation of the translamellar bridging network. Spine (Phila Pa 1976) 2008;33:2702–2710. doi: 10.1097/BRS.0b013e31817bb92c. [DOI] [PubMed] [Google Scholar]
- Slusarewicz P, Zhu K, Kirking B, Toungate J, Hedman T. Optimization of protein crosslinking formulations for the treatment of degenerative disc disease. Spine (Phila Pa 1976) 2011;36:E7–13. doi: 10.1097/BRS.0b013e3181cc3de9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Pearcy MJ, Downing KJ, Manthey BA, Parkinson IH, Fazzalari NL. Disc lesions and the mechanics of the intervertebral joint complex. Spine (Phila Pa 1976) 2000;25:3026–3035. doi: 10.1097/00007632-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Vernon-Roberts B, Moore RJ, Fraser RD. The natural history of age-related disc degeneration: the pathology and sequelae of tears. Spine (Phila Pa 1976) 2007;32:2797–2804. doi: 10.1097/BRS.0b013e31815b64d2. [DOI] [PubMed] [Google Scholar]
- Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerramalli CS, Chou AI, Miller GJ, Nicoll SB, Chin KR, Elliott DM. The effect of nucleus pulposus crosslinking and glycosaminoglycan degradation on disc mechanical function. Biomech Model Mechanobiol. 2007;6:13–20. doi: 10.1007/s10237-006-0043-0. [DOI] [PubMed] [Google Scholar]