Abstract
Background
A persistent debate in psychiatry concerns whether schizophrenia and bipolar disorder are the clinical realizations of discrete versus shared etiological processes.
Methods
We linked the Multi-Generation Register, containing information about children and their parents of all Swedes, and the Hospital Discharge Register, covering all public psychiatric inpatient hospitalizations in Sweden. We identified 9,009,202 unique individuals in more than 2 million nuclear families. Risks for schizophrenia, bipolar disorder and their co-morbidity were calculated for biological and adoptive parents, offspring, full siblings and half-siblings of probands with the diseases. A multivariate generalized linear mixed model was used to estimate genetic and environmental contributions to liability for schizophrenia, bipolar disorder, and their co-morbidity.
Findings
There were increased risks of both schizophrenia and bipolar disorder to first degree relatives of probands with either disorder. Half-sibs had a significantly increased risk, albeit substantially lower than the full-siblings. When relatives of probands with bipolar disorder were analysed, increased risks for schizophrenia were present for all relationships, including offspring adopted away. Heritability for schizophrenia was 64% and for bipolar disorder 59%. Shared environmental effects were small but significant for both disorders. The co-morbidity between the disorders was primarily (63%) due to additive genetic effects common to both disorders.
Interpretation
Similar to molecular genetic studies, we found compelling evidence that schizophrenia and bipolar disorder partially share a common genetic etiology. These results challenge the current nosological dichotomy between schizophrenia and bipolar disorder, and are consistent with a reappraisal of these disorders as distinct diagnostic entities.
One of the most persistent debates in psychiatry concerns the etiological relationship between schizophrenia and bipolar disorder. The extreme positions are that schizophrenia and bipolar disorder are the clinical realizations of entirely different versus identical etiological processes. A number of intermediate and more complex positions can also be envisioned whereby, for example, a shared etiological risk factor causes some proportion of each disorder. A common etiology has been suggested by molecular genetic studies1, by the existence of an intermediate phenotype (schizoaffective disorder) sharing diagnostic features of both disorders, and by evidence that similar endophenotypes (e.g., brain white matter density)2 are associated with both disorders. On the other hand, the evidence from genetic epidemiological studies is mixed in part because of limited sample sizes3,4.
Genomewide linkage screens have shown some degree of overlap between schizophrenia and bipolar disorder5,6. One meta-analysis found genomic regions in common7 although a more inclusive meta-analysis concluded that there was “no overlap of the highest-ranking regions for the two disorders”8,9 and a third “mega-analysis” (meta-analysis using genotype data from the individual studies) did not show strong overlap10. However, overlap can occur by chance. Assume there are three 500 marker genome scans for schizophrenia and three for bipolar disorder. Under a simple simulation model (α=0.05, 10,000 simulations) with no true genetic effects, the absence of positional overlap between any schizophrenia scan and any bipolar disorder scan is uncommon (7%), a single overlap would occur frequently (18%), and multiple overlaps would predominate (75%). Therefore, given that there are 20 schizophrenia genomewide linkage scans9 and 18 bipolar disorder scans8, substantial overlap across studies is to be expected purely by chance. An additional difficulty is that individuals with schizoaffective disorder are generally classified as affected in genetic studies of each syndrome8,9.
A sizeable body of literature has investigated the genetic association of markers in candidate genes in both schizophrenia and bipolar disorder11–13. These studies provide increasing evidence for an overlap in genetic susceptibility between the disorders and even the possibility of specific relationships between genotype and type of pathology1, although interpretations should be drawn with caution14
Studies based on diagnostic resemblance in familial relationships can be used to understand the sources of overlap between two disorders. One twin study is usually cited as supporting the overlap between schizophrenia and bipolar disorder although its conclusions are qualified by the small sample size and the investigation of “mania”, not bipolar disorder per se15. A small Finnish study of 26 twins with bipolar disorder did not identify any co-twin with schizophrenia16. High-quality, “modern” family studies have provided relatively consistent evidence that schizophrenia and bipolar disorder do not overlap3,4. One review stated, “there is no increased risk for bipolar disorder among first-degree relatives of schizophrenia probands … nor is there increased risk for schizophrenia among first-degree relatives of bipolar disorder probands”5. However, in a large population-based study conducted in Denmark, risk of bipolar disorder was associated with a history of schizophrenia in parents and siblings17.
Given the persistent uncertainties about the relationship between schizophrenia and bipolar disorder, we have used population-based registries covering the entire Swedish population in order to investigate the degree of genetic overlap between these two disorders.
Methods
National Registers
We linked two Swedish national registers, using the unique, individual Swedish national registration number which was introduced in 1947 and is assigned at birth.
The Multi-Generation Register contains information about first-degree relatives18. The register contains entries for an “index person” along with their biological and adoptive parents. To be included in the register, an index person had to be registered in Sweden between 1/1/1961 and 12/31/2002 and to have been born between 1/1/1932 and 12/31/2002. Paternity was assumed to be the husband of the mother at the time of birth or “by acknowledgement” for unwed mothers.
The Hospital Discharge Register covers all public psychiatric inpatient hospitalizations in Sweden since 1973. Each record contains the admission and discharge dates, the main discharge diagnosis, and up to eight secondary diagnoses assigned by the treating physician according to the ICD-system19–21. We obtained information on all psychiatric admissions between 1973 and 2004.
Classification of schizophrenia and bipolar disorder
Schizophrenia
Patients with schizophrenia were defined as individuals identified in the Hospital Discharge Register having at least two inpatient hospitalizations with a discharge diagnosis of schizophrenia (ICD-8 295, ICD-9 295, ICD-10 F20, with latent schizophrenia, 295.5 and 295F, excluded.). The criterion of at least two inpatient hospitalizations was chosen to increase diagnostic precision and provided in a previous study nearly identical estimates of familial risks to those from the literature22.
Bipolar disorder
In a similar manner, we defined bipolar disorder as ≥2 inpatient hospitalizations for a core bipolar diagnosis (296 and F31).
Both diseases were diagnosed using a non-hierarchical diagnostic structure. Thus, an individual could have both schizophrenia and bipolar disorder, conditioned on that the person has been diagnosed at least twice in each disease. To avoid possible misclassification between schizophrenia and bipolar disorder, admissions with the diagnoses of schizoaffective disorder (ICD codes 295.7, 295H, F25.X) were not considered; 3,563 individuals were excluded because of this.
Statistical Analysis
Familial risks
The risk of disease in relatives of a proband with disease was compared to the risk among relatives of 5 unaffected subjects matched by gender and year of birth for each member in the pair (for adoptive relationships the subjects were matched using 5 year age-band intervals). In order to allow equal follow-up time, we additionally required that the relatives of probands and controls were alive and had not been admitted to psychiatric care in Sweden by the date the proband was first hospitalized. The data were analyzed in a conditional logistic regression model using the PROC TPHREG procedure in SAS version 9.1.323. As several possibly correlated pairs of relatives from each family could be included in an analysis, a robust sandwich estimator was used to adjust for the correlated data when calculating the confidence intervals.
Genetic and environmental effects
In order to separate genetic and environmental contributions to liability for schizophrenia and bipolar disorder, we analyzed outcomes from different family types: nuclear, paternal half-sibling and maternal half-sibling families. To reduce complexity in families with half-siblings, the marriages of the index parent were limited to two spouses. This excluded 8% of the half-sibling families. When more than two children were available, we analyzed only the oldest two full siblings from a nuclear family and the oldest two half-sibling from a half-sibling family. Our data include 1,984,182 nuclear families, 172,073 paternal half-sibling families, and 161,409 maternal half-sibling families.
A multivariate generalized linear mixed model (GLMM) with probit link was used24. The probability P for an individual having a disease is determined by a latent risk variable R, corresponding to the area under the standard normal curve – i.e., a small value of R indicates a small probability. Our model specifies R as the sum of several effects, including family-member type (father, mother or children), additive genetic (Asz, Abp), adult shared environmental (Fsz, Fbp) and childhood shared environment (Csz, Cbp) effects along with a common non-shared environmental effect for both schizophrenia and bipolar disorder (E). For schizophrenia and bipolar disorder, our models are
Fsz = βszfIf + βszmIm + βszcIc + Asz + Fsz + Csz + E
Rbp = βbpfIf + βbpmIm + βbpcIc + Abp + Fbp + Cbp + E
where If, Im and Ic are indicator variables for father, mother and children, βf, βm and βc are fixed parameters associated with disease prevalence for respective family members. The random effects A, F, C, and E are assumed to be normally distributed with mean zero and a unique variance component for each term. Thus we have variance components . The non-shared effect E is normal with mean zero and variance .
For each disorder we assume the random effects are independent between families, but dependent within families with the correlations given in Table 1. The model includes cross-disease covariances: σszbpa is the additive-genetic covariance of schizophrenia and bipolar effect, and similarly σszbpf and σszbpc are the adult and childhood environmental effects. All parameters were estimated simultaneously using the maximum likelihood method25, where the likelihood is computed using a fast Monte Carlo integration of the random effects26.
Table 1.
Expected genetic and environmental correlations in disease liability.
| Characteristic | Type of family | |||||
|---|---|---|---|---|---|---|
| Full Sibling | Half siblings | |||||
| Sibling correlation |
Spouse correlation |
Parent- offspring correlation |
Sibling correlation |
Spouse correlation |
Parent- offspring correlation |
|
| Additive genetic effects (A) | 0.5 | 0 | 0.5/01 | 0.25 | 0 | 0.5/01 |
| Adult shared environment (F, maternal and paternal) | 0 | 1 | 0 | 0 | 1 | 1 |
| Childhood shared environment (C) | 1 | 0 | 0 | 1/02 | 0 | 0 |
Note:
Expected correlation is 0.5 between parent - biological offspring and 0 for parent - non-biological offspring
Expected correlations is 1 for maternal half siblings and 0 for paternal half siblings
Variance and covariance parameters are reported as proportional contributions to the liability to disease. Confidence intervals for estimates are obtained using a likelihood-based procedure25, corrected for uncertainty due to Monte Carlo integration. A more thorough description of the bivariate model is provided elsewhere27.
The study was supported by the Swedish Council for Working Life and Social Research and the Swedish Research Council.
Results
We identified 9,009,202 unique individuals of known parentage, where both parents were alive and living in Sweden after 1973. There were 35,985 individuals who met the criteria for schizophrenia and 40,487 individuals for bipolar disorder. Of those with schizophrenia, 2,543 also had bipolar disorder (RR=16.4 95% CI=15.1–17.7).
We have previously presented the recurrence risks for schizophrenia22. Table 2 presents these results for parent-offspring and sibling relationships, and extends our prior work by considering different types of half-sibling and adoptive relationships, along with the parallel results for bipolar disorder and for the familial aggregation of the co-morbidity between the disorders.
Table 2.
Recurrence risks for schizophrenia and bipolar disorders and co-morbidity in parent-offspring and siblings.
| Proband | Relation to proband |
Risk for schizophrenia when proband has schizophrenia |
Risk for bipolar disorder when proband has bipolar disorder |
Risk for schizophrenia when proband has bipolar disorder |
Risk for bipolar disorder when proband has schizophrenia |
||||
|---|---|---|---|---|---|---|---|---|---|
| Relative Risk |
95% CI | Relative Risk |
95% CI | Relative Risk |
95% CI | Relative Risk |
95% CI | ||
| Parent | Offspring | 9.9 | 8.5–11.6 | 6.4 | 5.9–7.1 | 2.4 | 2.1–2.6 | 5.2 | 4.4–6.2 |
| Sibling | Sibling | 9.0 | 8.1–9.9 | 7.9 | 7.1–8.8 | 3.9 | 3.4–4.4 | 3.7 | 3.2–4.2 |
| Sibling | Maternal half-sibling | 3.6 | 2.3–5.5 | 4.5 | 2.7–7.4 | 1.4 | 0.7–2.6 | 1.2 | 0.6–2.4 |
| Sibling | Paternal half-sibling | 2.7 | 1.9–3.8 | 2.4 | 1.4–4.1 | 1.6 | 1.0–2.7 | 2.2 | 1.3–3.8 |
| Adoptive relationships | |||||||||
| Biological parent | Adopted away offspring | 13.7 | 6.1–30.8 | 4.3 | 2.0–9.5 | 4.5 | 1.8–10.9 | 6.0 | 2.3–15.2 |
| Sibling | Adopted away biological sibling | 7.6 | 0.7–87.8 | - | 3.9 | 0.2–63.3 | 5.0 | 0.3–79.9 | |
| Adoptive parent | Adoptee | - | 1.3 | 0.5–3.6 | 1.5 | 0.7–3.5 | - | ||
| Sibling | Non-biological sibling | 1.3 | 0.1–15.1 | - | - | 2.0 | 0.1–37.8 | ||
Similar to our previous publication22, there were increased risks of schizophrenia to first degree relatives; 9.9 (8.5–11.6) for parent-offspring and 9.0 (8.1–9.9) for full siblings. Half-sibs had a significantly increased risk, albeit substantially lower than the full-siblings. Adopted children whose biological parents have schizophrenia and biological siblings growing up in different families also had an increase in risk of being diagnosed with the same disease. There were no cases where both an adoptive parent and an adoptee had a diagnosis of schizophrenia.
The first-degree relatives of individuals with bipolar disorder had somewhat lower risks for the disease as compared to the results for schizophrenia. Otherwise, the familial risks were of similar magnitudes. Adopted children whose biological parents had bipolar disorder had a more than four-fold risk of bipolar disorder.
When the proband had bipolar disorder, there was a markedly increased risk for a diagnosis of schizophrenia among their first degree relatives. The risk was 2.4 (2.1–2.6) for parent-offspring and 3.9 (3.4–4.4) for full sibling relationships. Half-siblings also had an increased risk. When adoptive parents had a diagnosis of bipolar disease, the relative risk for the adoptees to have a diagnosis of schizophrenia was 1.5 (0.7–3.5). Similar results were found when we investigated the risk for bipolar disorder in relatives of probands with schizophrenia (last column of Table 2). There were no noticeable sex-differences in the familial risks (data not shown).
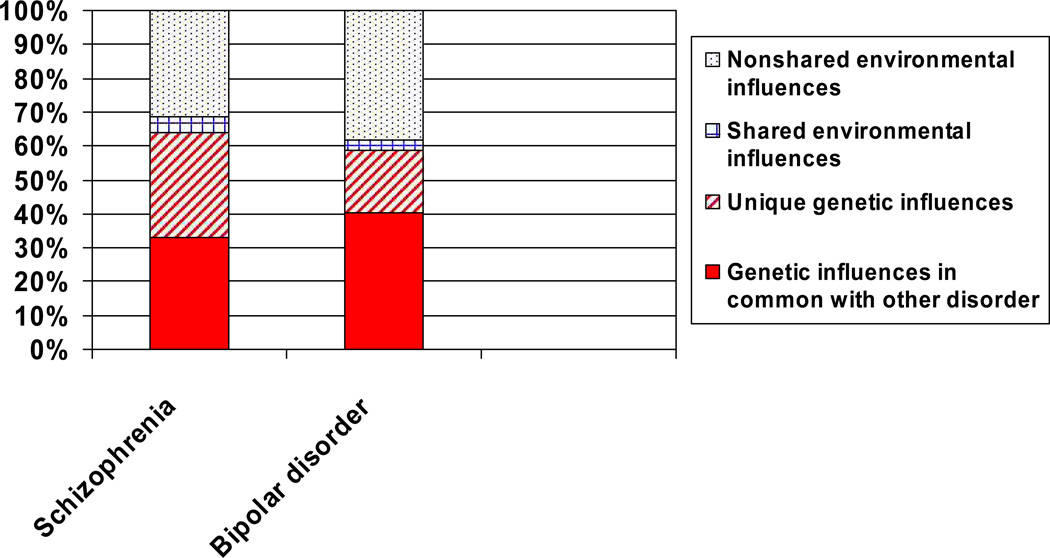
Heritability for schizophrenia was estimated to be 64% (Table 3, Figure 1). For bipolar disorder this estimates was 59%. Shared environmental effects were small but statistically significant for both disorders.
Table 3.
Estimates of proportion of genetic and environmental effects for liability to schizophrenia, bipolar disorder, and their co-morbidity in the child generation from multivariate generalized linear mixed model (GLMM).
| Measure | Parameter estimates (95% Confidence interval) | ||
|---|---|---|---|
| Additive genetic (A) | Childhood shared environmental (C) |
Nonshared environmental (E) |
|
| Non-hierarchical diagnoses | |||
| - Schizophrenia | 64.3 (61.7–67.5) | 4.5 (4.4–7.4) | 31.1 (25.1–33.9) |
| - Bipolar disorder | 58.6 (56.4–61.8) | 3.4 (2.3–6.2) | 38.0 (32.0–41.2) |
| - Co-morbidity | 63.4 (62.0–64.9) | 5.9 (4.0–6.8) | 30.6 (28.7–32.3) |
| Hierarchical diagnoses | |||
| - Schizophrenia | 64.3 (61.5–72.2) | 2.5 (1.9–6.6) | 33.2 (21.2–36.7) |
| - Bipolar disorder | 55.1 (50.8–60.7) | 2.8 (1.9–7.2) | 42.1 (32.1–47.3) |
| - Co-morbidity | 91.7 (85.2–100.0) | 8.3 (0.6–8.3) | - |
Note:
When hierarchical diagnoses are used, nonshared environmental effects did not contribute to co-morbidity because an individual could not be diagnosed with both disorders.
Figure 1.
Amount of variance accounted for by genetic, shared environmental and nonshared environmental influences for schizophrenia and bipolar disorder.
Note: Red denotes genetic influences and blue shared and nonshared environmental influences. Solid red: Genetic variance for disorder (schizophrenia/bipolar disorder) that is shared with the other disorder. Hatched red: Genetic variance for disorder (schizophrenia/bipolar disorder) that is not shared with the other disorder.
The co-morbidity between the disorders was primarily (63%) due to additive genetic effects common to both disorders. The genetic correlation (rg) was 0.60. Unique genetic effects for schizophrenia, not in common with bipolar disorder, accounted for 48% of the genetic variance in schizophrenia (Figure 1). For bipolar disorder this figure was 31%.
The use of a non-hierarchical definition of syndromes might be questioned. We therefore investigated the sibling risk where both probands and siblings with co-morbid schizophrenia and bipolar disorder were excluded. The risk for bipolar disorder in siblings of probands with schizophrenia were still significantly increased (Relative risk = 2.7; 95% confidence interval 2.3–3.1). Further, we also performed the model-fitting analyses using hierarchical diagnoses, where individuals with ≥2 schizophrenia diagnoses were classified as schizophrenic (and not bipolar disorder even if they have had 2 or more such diagnoses). As can be seen in the lower panel of Table 3, estimates were almost exactly the same, except for that nonshared environmental effects did not contribute to co-morbidity (because an individual could not be diagnosed with both disorders).
Discussion
In this population-based study of over 2 million Swedish families, we found evidence of a substantial genetic relationship between schizophrenia and bipolar disorder. All classes of biological relatives of probands with bipolar disorder had a significantly increased risk for schizophrenia, and the genetic correlation (i.e., the correlation between the genetic effects influencing the liabilities for the two disorders) was .60. Further, adopted children whose biological parents had one of the disorders had significant increase in risks for the other disorder. These results agree with previous molecular genetic studies1, as well as twin15 and family17 data in suggesting a common genetic influences in schizophrenia and bipolar disorder, even though it is at odds with some family studies which have used clinicians to review the personal interview and hospital and out-patient records from the course of illness3,4. It is well-known that patients with one diagnosis sometimes evolve into the other. If this development of psychotic diseases is due to misclassification at the first diagnoses rather than to the fact that the individual has had both disorders, our approach of using non-hierarchical diagnoses would upwardly bias the relationship between schizophrenia and bipolar disorder. However, when we analyzed sibling risks without co-morbidity there was still a highly significant increase in risk. Further, in the analyses using hierarchical diagnoses, estimates were almost exactly the same. Thus, we believe that the cumulative evidence suggests that there are common genetic etiologies between schizophrenia and bipolar disorder. Nonetheless, a considerable proportion of the genetic variance was not in common with the other disorder both for schizophrenia and bipolar disorder. Thus, there probably exist sets of genes associated with the risk for both diseases as well as sets of sets of genes associated with the risk unique for only one of the diseases, which should be considered in future research as well as clinical settings.
Heritability for schizophrenia was 64%, somewhat lower than a meta-analysis of twin studies that found a heritability of 81%28. Further, the heritability for bipolar disorder was estimated at 59%, again lower than previous estimates based on twins of around 80%16. A disadvantage of twin studies is that they have relatively low power to estimate heritability for rare diseases. At the same time, they are less sensitive to age and nonadditive genetic effects than family studies. Regardless, both types of studies clearly show that both schizophrenia and bipolar disease are substantially heritable with heritabilities explaining somewhere between 60–80% of the liabilities.
There were small but significant shared environmental influences on both disorders as well as their co-morbidity, accounting for 3–6% of the variance and covariance, which corroborate the results from the meta analysis of twin studies on schizophrenia28. Twin studies have limited power to detect shared environmental effects, especially for binary traits29, and we suggest that caution is warranted when drawing to definite conclusion also for other psychiatric conditions when limited sample sizes are used.
We also note that nonshared environmental effects contributed to the co-morbidity (around 30%). In contrast to nonshared environmental effects estimated in the univariate analyses, the estimate of the co-morbidity due to nonshared environmental effects should be free from measurement error. We can here only speculate on the possible mechanism, but perinatal effects might be a likely candidate30.
The strength of our data is the national coverage of all inpatient treatment facilities including care in psychiatric as well as somatic clinics. In common with most developed nations, there has been a reduction in inpatient psychiatric care in Sweden over the past two decades. However, a recent study showed that while the total number of days spent in psychiatric beds in Sweden between 1994 and 2003 fell dramatically, the number of admissions scarcely changed31. A limitation of the current study is the use of unstandardized diagnoses made by different clinicians with a range of orientations. This is inherent problem with population based studies of this kind. Nevertheless, validation studies confirm a very low number of false positive diagnoses32,33. There was 94% agreement between register diagnoses of schizophrenia and research diagnoses based on semi-structured interviews and medical records33. The validity of a discharge diagnosis of bipolar disorder has not been examined, but is likely high as for schizophrenia considering a conservative and restrictive diagnostic culture for psychoses in Sweden. A small number of cases may have been treated entirely outside hospital. Another limitation with the bipolar diagnoses is that the more rigid ICD-10 definitions were only available for the last seven years of this 31-year study.
Diagnostic bias could have occurred if the diagnostic judgments by physicians were influenced by knowledge of family psychiatric history. If so, this would likely reduce the variation across diagnoses. Our data on adoptive relationship are not prone to such bias.
Cases were individuals with narrow diagnoses of schizophrenia and bipolar disorder. By restricting our analyses to at least two admissions, we enhanced specificity of the register diagnoses. Further, we chose a very conservative approach by excluding schizoaffective disorder, which is symptomatically intermediate between schizophrenia and bipolar disorder. Thus, we likely reduced misclassification and reduced overestimation of shared etiological effects.
In conclusion, similar to recent results from molecular genetic studies1, we have found compelling evidence that schizophrenia and bipolar disorder partially share common genetic etiologies, which challenges the nosological dichotomy between schizophrenia and bipolar disorder. These results should inform the diagnostic classification used in linkage and association studies and encourage further search for endophentypic traits shared between the two disorders. Within clinical practice, the underlying structure of psychosis and knowledge of their common etiology might be beneficial for treatment considerations and development of psychosis medication.
Acknowledgement
This study was funded by the Swedish Council for Working Life and Social Research and the Swedish Research Council.
Footnotes
Conflict of interest: None
References
- 1.Owen MJ, Craddock N, Jablensky A. The genetic deconstruction of psychosis. Schizophr Bull. 2007;33(4):905–911. doi: 10.1093/schbul/sbm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIntosh AM, Job DE, Moorhead TW, Harrison LK, Lawrie SM, Johnstone EC. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biol Psychiatry. 2005;58(3):254–257. doi: 10.1016/j.biopsych.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 3.Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study. IV. Affective illness, anxiety disorders, and alcoholism in relatives. Arch Gen Psychiatry. 1993;50(12):952–960. doi: 10.1001/archpsyc.1993.01820240036005. [DOI] [PubMed] [Google Scholar]
- 4.Maier W, Lichtermann D, Minges J, et al. Continuity and discontinuity of affective disorders and schizophrenia. Results of a controlled family study. Arch Gen Psychiatry. 1993;50(11):871–883. doi: 10.1001/archpsyc.1993.01820230041004. [DOI] [PubMed] [Google Scholar]
- 5.Berrettini W. Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C Semin Med Genet. 2003;123(1):59–64. doi: 10.1002/ajmg.c.20014. [DOI] [PubMed] [Google Scholar]
- 6.Tsuang MT, Taylor L, Faraone SV. An overview of the genetics of psychotic mood disorders. J Psychiatr Res. 2004;38(1):3–15. doi: 10.1016/s0022-3956(03)00096-7. [DOI] [PubMed] [Google Scholar]
- 7.Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7(4):405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- 8.Segurado R, Detera-Wadleigh SD, Levinson DF, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: Bipolar disorder. Am J Hum Genet. 2003;73(1):49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McQueen MB, Devlin B, Faraone SV, et al. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005;77(4):582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter CJ. eIF2B and oligodendrocyte survival: where nature and nurture meet in bipolar disorder and schizophrenia? Schizophr Bull. 2007;33(6):1343–1353. doi: 10.1093/schbul/sbm007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallin MD, Lasseter VK, Avramopoulos D, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77(6):918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlis RH, Purcell S, Fagerness J, et al. Family-based association study of lithium-related and other candidate genes in bipolar disorder. Arch Gen Psychiatry. 2008;65(1):53–61. doi: 10.1001/archgenpsychiatry.2007.15. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan PF. Spurious genetic associations. Biol Psychiatry. 2007;61(10):1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159(4):539–545. doi: 10.1176/appi.ajp.159.4.539. [DOI] [PubMed] [Google Scholar]
- 16.Kieseppa T, Partonen T, Haukka J, Kaprio J, Lonnqvist J. High concordance of bipolar I disorder in a nationwide sample of twins. Am J Psychiatry. 2004;161(10):1814–1821. doi: 10.1176/ajp.161.10.1814. [DOI] [PubMed] [Google Scholar]
- 17.Mortensen PB, Pedersen CB, Melbye M, Mors O, Ewald H. Individual and familial risk factors for bipolar affective disorders in Denmark. Arch Gen Psychiatry. 2003;60(12):1209–1215. doi: 10.1001/archpsyc.60.12.1209. [DOI] [PubMed] [Google Scholar]
- 18.Statistics Sweden. Multi-generation register 2005 – A description of contents and quality. Örebro: Statistics Sweden; 2006. [Google Scholar]
- 19.World Health Organization. International Classification of Diseases. Geneva: WHO; 1967. [Google Scholar]
- 20.World Health Organization. International Classification of Diseases. Geneva: WHO; 1978. [Google Scholar]
- 21.World Health Organization. International Classification of Diseases. Geneva: WHO; 1992. [Google Scholar]
- 22.Lichtenstein P, Bjork C, Hultman CM, Scolnick E, Sklar P, Sullivan PF. Recurrence risks for schizophrenia in a Swedish National Cohort. Psychol Med. 2006;36(10):1417–1425. doi: 10.1017/S0033291706008385. [DOI] [PubMed] [Google Scholar]
- 23.SAS Institute Inc. SAS/STAT® Software: Version 9. Cary, NC: SAS Institute, Inc; 2004. [Google Scholar]
- 24.Pawitan Y. In all likelihood: statistical modelling and inference using likelihood. Oxford: Oxford University Press; 2001. [Google Scholar]
- 25.Pawitan Y, Reilly M, Nilsson E, Cnattingius S, Lichtenstein P. Estimation of genetic and environnmental factors for binary traits using family data. Statistics in Medicine. 2004;(23):449–465. doi: 10.1002/sim.1603. [DOI] [PubMed] [Google Scholar]
- 26.Gentz A. Numerical computation of multivariate normal probabilities. Journal of Computational and Graphical Statistics. 1992;1:141–149. [Google Scholar]
- 27.Yip BH, Bjork C, Lichtenstein P, Hultman CM, Pawitan Y. Covariance component models for multivariate binary traits in family data analysis. Stat Med. 2007 doi: 10.1002/sim.2996. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60(12):1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 29.Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24(3):239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- 30.Hultman CM, Sparen P, Takei N, Murray RM, Cnattingius S. Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. Bmj. 1999;318(7181):421–426. doi: 10.1136/bmj.318.7181.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arvidsson H, Ericson BG. The development of psychiatric care after the mental health care reform in Sweden. A case register study. Nord J Psychiatry. 2005;59(3):186–192. doi: 10.1080/08039480510023061. [DOI] [PubMed] [Google Scholar]
- 32.Dalman C, Broms J, Cullberg J, Allebeck P. Young cases of schizophrenia identified in a national inpatient register--are the diagnoses valid? Soc Psychiatry Psychiatr Epidemiol. 2002;37(11):527–531. doi: 10.1007/s00127-002-0582-3. [DOI] [PubMed] [Google Scholar]
- 33.Ekholm B, Ekholm A, Adolfsson R, et al. Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry. 2005;59(6):457–464. doi: 10.1080/08039480500360906. [DOI] [PubMed] [Google Scholar]